Abstract
Objective
To investigate the efficacy and safety of targeted delivery of autologous bone marrow mononuclear cells (BMMCs), which are highly enriched with mesenchymal stem cells (BMMSCs), via medial circumflex femoral artery in the treatment of osteonecrosis of the femoral head (ONFH).
Methods
62 patients (78 hips) with ONFH were recruited in this study. All of these patients were treated with BMMCs perfusion via medial circumflex femoral artery. The concentrated BMMCs (30–60 ml) were gained from autologous bone marrow (100–200 ml) harvested from anterior iliac crest and then were intra-arterially perfused into the femoral head. Ficat stage was used to classify the radiological stage of ONFH. Harris hip score was used to evaluate the clinical symptoms of osteonecrosis. Ficat stage and Harris hip scores were assessed at onset of treatment at 6, 12, 24, 36, 48 and 60 months after the initial treatment. Total hip arthroplasty (THA) was also assessed as an endpoint at each follow-up.
Results
A follow-up on the patient was done at the end of five years, and 92.31% (72 of 78) of hips achieved a satisfactory clinical result while only 6 hips (7.69%) progressed to clinical failure and required THA. Radiological progression was noted in 34 of 78 hips (43.59%); the overall rate of collapse was 38.24% (26 of 68 hips) in stage-I and stage-II hip combinations and 12.5% (2 of 16)in stage-I hips and 46.15% (24 of 52) in stage-II hips. The mean time of conversion to THA was 3 years (1 to 5 years) and the average time to collapse were 3.5 years (1–5 years). The mean Harris hip score increased from 59 points at baseline to 75 points at 12 months, 82 points at 24 months, 81 points at 36 months, 79 points at 48 months and 74 points at 60 months. Five years after the treatment, 3 of 10 hips (30%) in stage-III had deteriorated to clinical failure whereas only 3 of 68 hips (4.41%) in stage-I and II combination had progressed to clinical failure (p < 0.05). Kaplan–Meier survival analysis showed a significant difference in the time to failure between the pre-collapse hips (Ficat stage-I and II) and the post-collapse hips (Ficat stage-III) at five years follow-up (Log-rank test; p < 0.01). No complication was found in any patients.
Conclusions
Autologous BMMSC perfusion via the medial circumflex femoral artery can relieve symptoms, improve hip function and delay the progression of ONFH. The clinical outcome is better when it is applied prior to the collapse. This work demonstrates that autologous BMMSC perfusion via the medial circumflex femoral artery is a safe, effective and minimally invasive treatment strategy for early-stage ONFH.
Keywords: Osteonecrosis of the femoral head, Bone marrow, Mesenchymal stem cells, Medial circumflex femoral artery, Intraarterial delivery
Introduction
Osteonecrosis of the femoral head (ONFH) is a devastating disease in orthopedic clinics [1], and is characterized by the death of bone caused by an interruption of the blood supply [2]. The disease mainly influences young patients [3]. Without timely effective treatment, ONFH frequently progresses to femoral head collapse and osteoarthritis, which often results in unbearable pain and immobility [4]. The total hip arthroplasty (THA) is the only treatment option for relieving pain and restoring hip joint function [5]. Due to the young age of many of these patients, THA cannot be expected to last a lifetime, therefore the less invasive treatment modalities should be used to protect the femoral head before collapse [6]. Thus, early intervention is the key for the success of joint preserving procedures [7].
Currently, the early therapy recommendations of ONFH remain controversial, and the exact indications have not yet been established [4]. Increased cell death and altered intra-medullar vascularity have been demonstrated to be the common features of ONFH [8]. The induction of blood vessel regeneration and the construction of a collateral circulation are the most effective ways to break the vicious pathological cycle of vascular disease or necrosis [9]. Bone marrow mesenchymal stem cells (BMMSCs) are multipotent cells that can secrete many growth factors and angiogenic factors [9]. BMMCs are highly enriched with BMMSCs in terms of fibroblast colony-forming units [10]. Nowadays, many researchers try to transplant BMMCs into the femoral head through a decompression tunnel after core depression (CD), and obtain more satisfactory results than CD treatment alone [9–13]. But CD may cause new trauma and increase therapeutic cost. Intravascular infusion of mesenchymal stem cells (MSCs) has been applied to treat with a variety of diseases [14–22]. It has the advantages of minimal invasiveness, convenient operation and less complications [14–17]. Therefore, it might be beneficial to treat early-stage ONFH with the intravascular infusion of MSCs.
Bone marrow is considered as a system with two components: the hematologic part and the stromal system containing the MSCs [13]. Recently, targeted intraarterial delivery has been used as a strategy of intravascular implantation of stem cells [22]. The medial circumflex femoral artery is the main vessel which nourishes the femoral head. In the present studies, we prepared concentrated autologous bone marrow mononuclear cells (BMMCs) from bone marrow harvested from the anterior iliac crest. Subsequently, the collected autologous BMMCs were injected into the femoral head via medial circumflex femoral artery. All patients in this study were treated with targeted intraarterial delivery of concentrated autologous BMMCs. We have analyzed the outcome in 62 patients (78 hips) and compared our findings with previously reported data. In this study, we wish to address the following questions. 1) Do medial circumflex femoral artery perfusion of autologous BMMCs delay the progression of ONFH? 2) Is medial circumflex femoral artery perfusion of autologous BMMCs safe?
Materials and methods
Patient population
The study population consisted of a patient group treated with medial circumflex femoral artery perfusion of BMMCs in our hospital. The study was approved by the Ethics Committee at The First Affiliated Hospital of Zhejiang Chinese Medical University. The diagnosis of ONFH was based on imaging examination (radiograph, CT or MRI). All of these patients had documented ONFH and complained of hip pain due to ONFH. A total of 62 patients (78 hips) with 35 men and 27 women were included in this study. The mean age of these patients at the time of treatment was 36.3 years (22 to 54 years). The baseline characteristics of osteonecrosis are described in Table 1. Etiologic factors were trauma in 12 patients (12 hips), alcohol abuse in 22 patients (27 hips), excessive use of corticosteroid in 21 patients (30 hips) and idiopathic in 7 patients (9 hips). The Ficat stage has been used to classify radiological results [23]. Radiographs of all patients were classified into stage I (16 hips), stage II (52 hips) and stage III (10 hips) according to the Ficat stage system at onset of treatment.
Table 1.
Baseline characteristics of osteonecrosis and indications for femoral artery perfusion of BMMCs.
| Number of hips (%) | |
|---|---|
| Etiologic factors | |
| Trauma | 12 (15.38%) |
| Alcohol | 27 (34.62%) |
| Steroids | 30 (38.46%) |
| Idiopathic | 9 (11.54%) |
| Stage of hip (Ficat classification) | |
| I | 16 (20.51%) |
| II | 52 (66.67%) |
| III | 10 (12.82%) |
Bone marrow aspiration and BMMC isolation
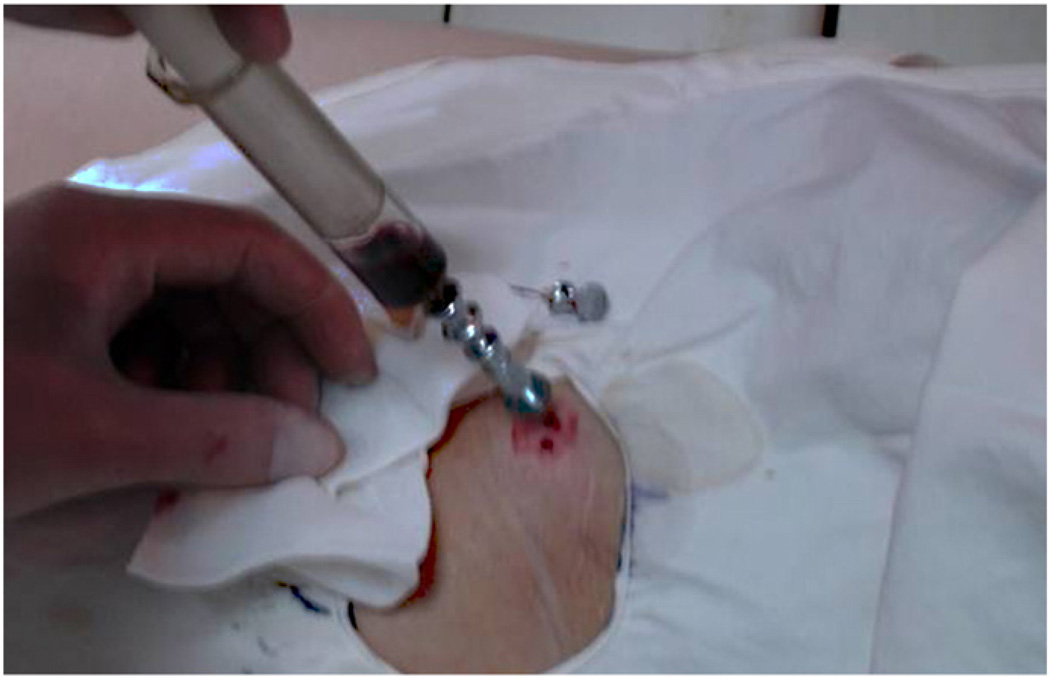
The collection of autologous BMMCs was performed as previously reported [10]. In brief, the bone marrow blood was harvested from bilateral anterior iliac crests using stab incisions under local anesthesia. Four or five perforations were made in the iliac crest by using a beveled metal trocar of 8 cm length and a bore of 1.5 mm. The distance between perforations was approximately 2 cm. The bone marrow blood was then aspirated using a 10 ml syringe that had been flushed with heparin (Fig. 1). The aspirated bone marrow blood was pooled in a plastic bag containing an anticoagulant solution (citric acid, sodium citrate, and dextrose). The original bulk of collected bone marrow blood was 100 ml to 200 ml according to the area of application. The bone marrow components can be stratified in accordance with the density. BMMCs were isolated by density gradient centrifugation with Percoll. After centrifugation, some of the red blood cells (the non-nucleated cells) and the plasma were isolated and removed. Finally, 30–60 ml of concentrated BMMCs were yielded and placed in syringes for injection.
Fig. 1.
A 10 ml syringe that had been flushed with heparin was used to aspirate bone marrow from anterior iliac.
Calculation of BMMC concentration
2–3 ml bone marrow sample of each patient was retained for BMMCs count before and after the centrifugation, respectively. The BMMC number was calculated and recorded.
Autologous BMMC grafting into femoral head
The BMMCs were injected into the femoral head after femoral artery puncture. Femoral artery puncture was done with the Seldinger technique. This procedure is briefly described as follow: Femoral artery puncture was conducted under local anesthesia. The pierced site was a point of 1–3 cm distance below the mid-point of inguinal ligament. Under sterile conditions, a 2 or 3 mm skin incision was made at the level of the femoral artery puncture point, and just opened the dermis. The trocar for puncture was then pushed with a 45° angle to skin by hand into the femoral artery, so that the tip was located between anterior and posterior wall of the femoral artery (Fig. 2A). When the end of the trocar sprayed blood, the needle body was pressed downward to reduce the angle between the trocar and skin. Then, a guide wire was inserted into the femoral artery and reached the heterolateral femoral artery (Fig. 2B). After that, a 5.0 Cobra catheter was introduced into the heterolateral femoral artery under the guidance of the guide wire (Fig. 2C), and Omnipaque was injected into the artery with an infusion speed of 5 ml/s for angiography to find out the medial circumflex femoral artery (Figs. 2D, E). Finally, the prepared autologous BMMCs were perfused into the femoral head through the medial circumflex femoral artery (Figs. 2F, G, H). The position of the Cobra catheter was monitored with fluoroscopy (Fig. 2H).
Fig. 2.
Procedures of BMMC grafting into the femoral head via the medial circumflex femoral artery. (A) A trocar was pushed into the femoral artery by hand; (B) A guide wire was inserted into femoral artery; (C) A 5.0 Cobra catheter was introduced into the heterolateral femoral artery under the guidance of the guide wire; (D, E) Omnipaque was injected into the artery with a infusion speed of 5 ml/s for angiography to find out the medial circumflex femoral artery; (F,G,H) BMMC concentrate was infused into the femoral head through the medial circumflex femoral artery(arrow); (H) The procedures were monitored with fluoroscopy.
Post-treatment care
After treatment, all patients stayed in bed in coordination with skin traction for 3 days. Functional training of hip started the first day after treatment. Partial weight bearing commenced at day 4 after treatment. Full weight bearing was allowed at the beginning of 3 months after treament. Heavy physical labor was avoided for one year after treatment.
Follow-up
Patients were followed up at the onset of treatment and at 6, 12, 24, 36, 48 and 60 months after treatment, respectively.
Clinical assessment
Clinical outcomes of all patients were assessed by two experienced orthopedic surgeons. Harris hip scoring (HHS) system, which gauges patients' pain (44 points), joint function (47 points), deformity of hip joint (4 points) and motion of joint (5 points), was used for the clinical follow-up. At onset of treatment and at each follow-up visit, the Harris hip scores of patients were recorded as 0 to 100 points. Outcomes were graded as excellent when the Harris hip score was greater than 90 points, good when it was between 80 and 89 points, fair when it was between 70 and 79 points, and poor when it was less than 70 points, according to previous reports [24,25]. Conversion to THA was designed as the end point to evaluate the efficacy of the treatment in the current study. It was considered to be a clinical failure when deterioration on the Harris hip score was severe enough to require THA. The hips which did not require THA were regarded as survived hips. Each hip of patients suffering from bilateral hip involvement was examined respectively. Complications of the treatment were registered at each follow-up visit.
Radiological assessment
Anteroposterior radiographs or CT scans of the affected hips were taken at each visit. Due to the high cost, MRI was only done in the case of patient with stage I ONFH. All imaging examinations were analyzed by two experienced radiologist. Radiological progression was decided according to the development of the Ficat stage. Radiological collapse was defined as progression of Ficat stage I or II to Ficat stage III according to a previous report [26]. Finally, our findings were compared with the previously reported data regarding the natural history of ONFH and the efficacy of CD plus autologous BMMSC transplantation.
Statistical analysis
The Fisher's exact test was performed to determine the significant difference in clinical failure rate or radiological progression rate between the groups divided according to the Ficat stages at onset of treatment. The clinical survival was compared between the groups with Kaplan–Meier survival analysis. The log rank test was used as a test of significance. The significance of difference in the changes of HHS between the baseline and each follow-up visit was assessed using Student's t-test. Differences were considered significant when p < 0.05 was obtained. All data was analyzed by SPSS statistical software (version 15.0; SPS, Chicago, USA).
Results
Concentration of BMMCs
The average original volume of bone marrow harvested from each patient was 127.42 ± 44.97 ml, and was concentrated into 44.59 ± 15.74 ml after density gradient centrifugation with Percoll. The concentration of BMMCs in collected bone marrow increased from 14.65 ± 4.68 × 106/ml to 41.86 ± 6.75 × 106/ml after isolation. A final volume of 30 ml concentrated BMMC suspension was intra-arterially injected into the femoral head of each hip. Hence, We have administered average of 1.26 ± 0.5 × 109 BMMCs to each hip with ONFH which contain about 61.4 ± 20.4 × 107/cells of fibroblast colony-forming units.
Clinical outcomes
In this study, we were able to do follow-up studies on all recruited patients. The mean duration of the follow-up was 4.8 years (1 to 5 years) (Table 2). No complications were observed during or after the treatment. Our data showed that 1 of 16 (6.25%) hips in the Ficat stage-I required THA at 5 years, 2 of 52 (3.84%) hips in the Ficat stage-II underwent THA at 2 and 4 years, respectively, and 3 of 10 (30%) hips in the Ficat stage-III received THA at 1, 2 and 4 years, respectively (Table 2 and 3). At the end of 5 years of follow-up, 6 hips (7.69%) had progressed to clinical failure while 72 hips (92.31%) survived (Table 3). The mean time of conversion to THA were 3 years (1 to 5 years) (5 years for stage-I, 3 years (2 to 4 years) for stage-II, 2.3 years (1 to 4 years) for stage-III) (Table 2).
Table 2.
Duration of follow-up, number of clinical failure hips at follow-up and time of conversion to THA for each Ficat stage.
| Ficat stage at onset of treatment |
Mean duration (rang) of follow-up (yrs) |
Follow-up period (yrs) |
Mean time (rang) to THA (yrs) |
||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |||
| I (n = 16) | 5.0 | 0 | 0 | 0 | 0 | 1 | 5 |
| II (n = 52) | 4.9 (2 to 5) | 0 | 1 | 0 | 1 | 0 | 3 (2 to 4) |
| III (n = 10) | 4.2 (1 to 5) | 1 | 1 | 0 | 1 | 0 | 2.3 (1 to 4) |
| Total (n = 78) | 4.8 (1 to 5) | 1 | 2 | 0 | 2 | 1 | 3 (1 to 5) |
Table 3.
Number of hips with THA or survived at the last follow-up for each Ficat stage.
| Ficat stage at onset of treatment | Survival (number; %) | THA (number; %) |
|---|---|---|
| I (n = 16) | 15 (93.75%) | 1 (6.25%) |
| II (n = 52) | 50 (96.16%) | 2 (3.84%) |
| III (n = 10) | 7 (70%) | 3 (30%) |
| Total (n = 78) | 72 (92.31%) | 6 (7.69%) |
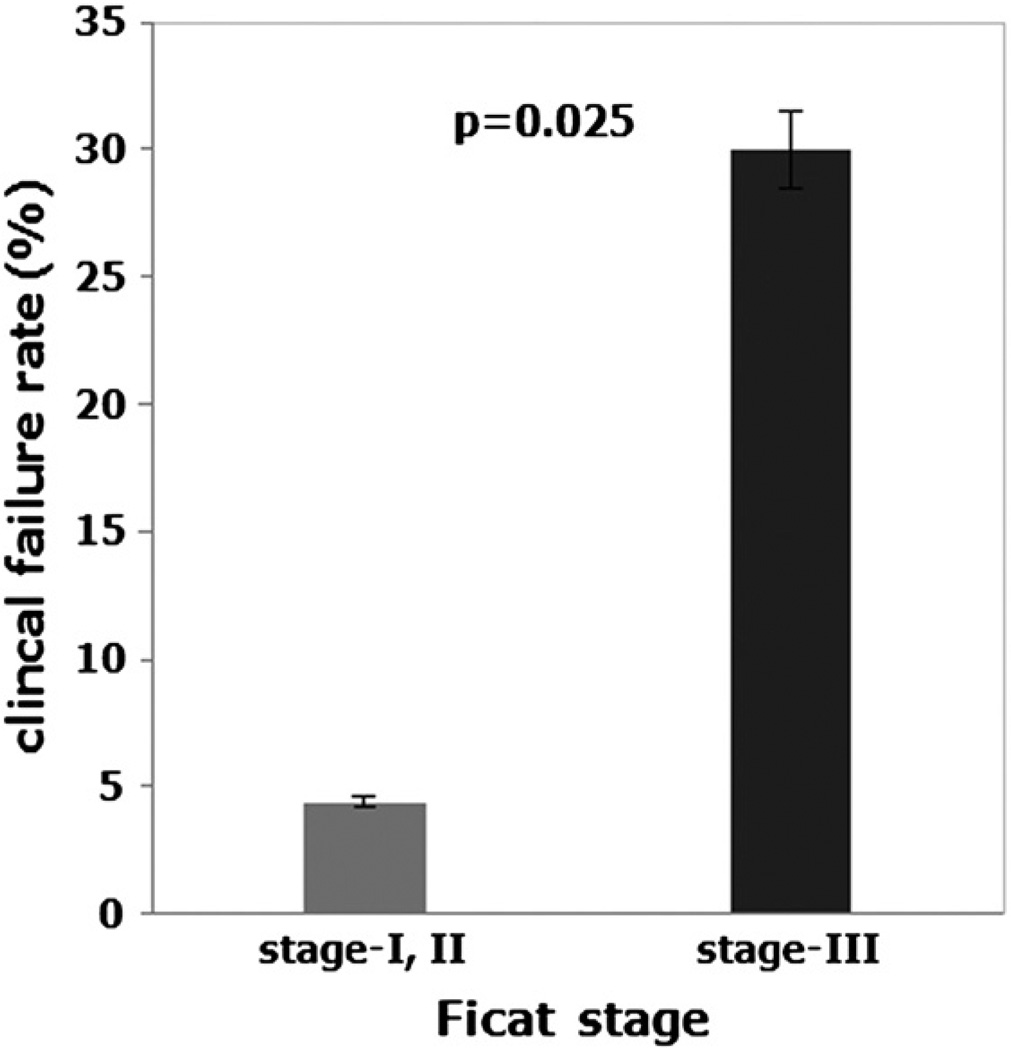
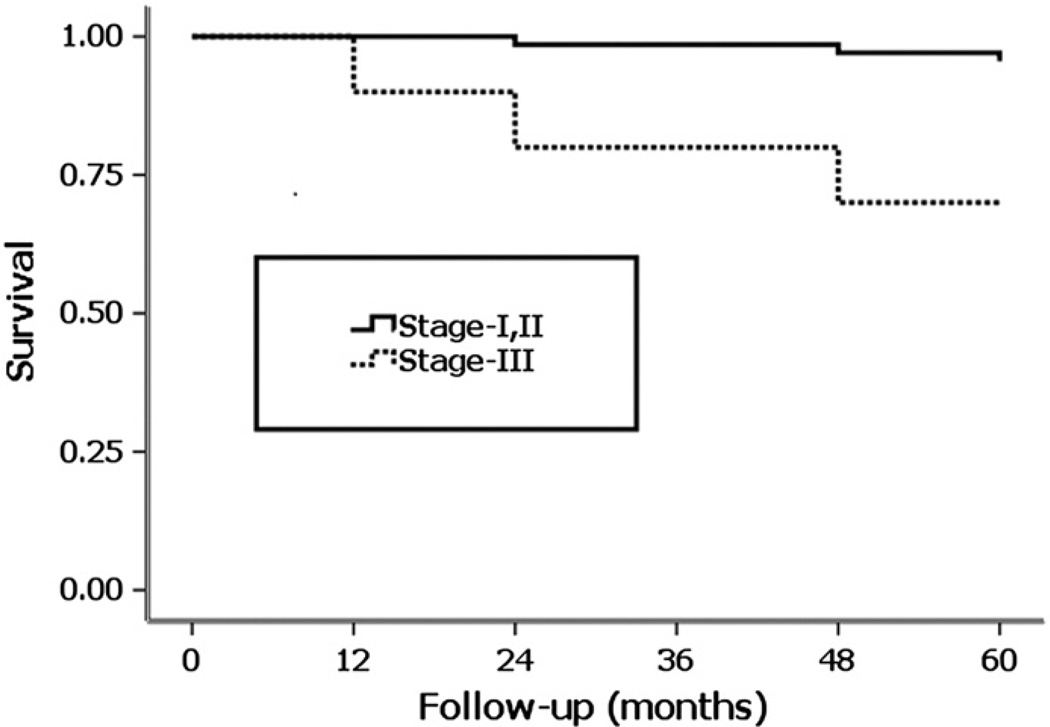
Our procedure achieved better success for Ficat stage-I and II hips when compared with Ficat stage-III hips. The results showed a clinical failure rate of 4.41% (3 of 68 hips) in stage-I and II hips (pre-collapse) combination, while a clinical failure rate of 30% (3 of 10 hips) in stage-III hips (post-collapse) at 5 years. They were statistically different (p = 0.025) (Fig. 3). Kaplan–Meier survival analysis for the stages of ONFH showed a significant difference in the survival time between the pre-collapse hips (Ficat stage-I and II) and the post-collapse hips (Ficat stage-III) at 5 years (Log-rank test; p = 0.002) (Fig. 4). The average survival times were 4.94 years for the stage-I and II hip combinations (5 years for the stage-I hips and 4.92 years for the stage-II hips) and 4.2 years for the stage III hips.
Fig. 3.
Clinical failure rate of hips with ONFH grouped according to Ficat stage at onset of treatment. “Stage-I, II” represents the clinical failure rate of stage-I and II hips (precollapse) taken together, and “Stage-III” represents the clinical failure rate of stage-III hips (postcollapse). The Fisher’s exact test showed a significant difference between the two groups (p = 0.025).
Fig. 4.
Survivorship curves for the hips with ONFH grouped according to the Ficat stage at onset of treatment, with the conversion to THA as the end point. Kaplan–Meier survivorship analysis showed a significant difference between the two groups in the distributions of the time to THA (Log–rank test, p = 0.002).
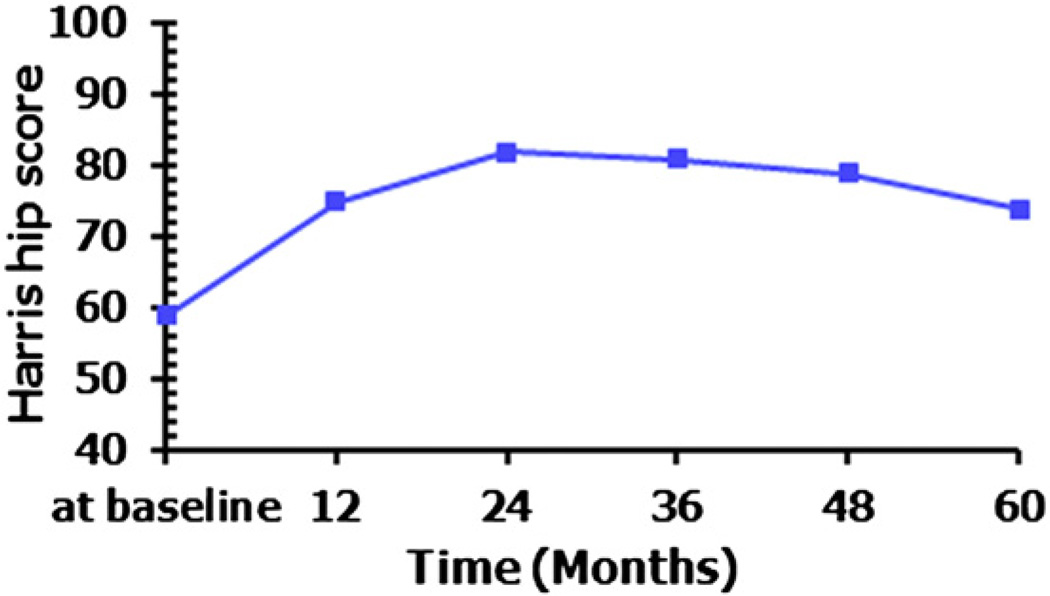
Medial circumflex femoral artery perfusion of BMMCs could improve joint symptoms of the failing hips with respect to the Harris hip score compared to that at baseline. The HHS was examined for all hips at the last follow-up visit. The scores of hips that received THA were obtained at the time just before THA. The mean HHS increased from 59 points at baseline to 75 points at 12 months, 82 points at 24 months, 81 points at 36 months, 79 points at 48 months and 74 points at 60 months (Fig. 5). The mean HHS of each follow-up visit was statistically higher than that of baseline (p < 0.01). However, the HHS began to decline at 36 months (Fig. 5). This may indicate a rebound of joint symptoms, and we may need to treat hips with targeted intraarterial delivery of concentrated autologous BMMCs again.
Fig. 5.
Changes of Harris hip score over follow-up for five years. The mean Harris hip score increased from 59 points at baseline to 75 points at 12 months, 82 points at 24 months, 81ponits at 36 months, 79 points at 48 months and 74 points at 60 months.
Radiological outcomes
At the final follow-up examination, radiological progression was noted in 34 of 78 hips (43.59%). The rates of radiological progression were 37.5% (6 of 16 hips), 46.2% (24 of 52 hips) and 40% (4 of 10 hips) for Ficat stage-I, II and III, respectively (Table 4). The overall rate of progression in hips without collapse (Ficat stage-I and II) was 44.12% (30 of 68 hips). It was worse than that in hips with collapse (Ficat stage-III), but there was no significant statistical difference (Fig. 6). The reason for this phenomenon may be the limited Ficat stage-III cases were involved. The opposite result might have been seen if a larger numbers of hips in Ficat stage-III had been included in the study.
Table 4.
Ficat stage of the hips at the last follow-up and the rate of radiological progression for each Ficat stage.
| Number of hips |
Number of hips |
Rate of radiographic progression |
|---|---|---|
| (Stage at onset of treatment) | (Stage at last visit) | |
| 16 (Stage-I) | 3 (Stage-0) | 37.5% (6/16) |
| 7 (Stage-I) | ||
| 4 (Stage-II) | ||
| 1 (Stage-III) | ||
| 1 (Stage-IV) | ||
| 52 (Stage-II) | 28 (Stage-II) | 46.2% (24/52) |
| 21 (Stage-III) | ||
| 3 (Stage-IV) | ||
| 10 (Stage-III) | 6 (Stage-III) | 40% (4/10) |
| 4 (Stage-IV) |
Fig. 6.
The rates of radiological progression of hips with ONFH grouped according to Ficat stage at onset of treatment. “Stage-I, II” represents the radiological progression rate of stage-I and II hips (precollapse) taken together, and “Stage-III” represents the radiological progression rate of stage-III hips (postcollapse). The Fisher’s exact test showed no significant difference between the two groups (p = 1.00).
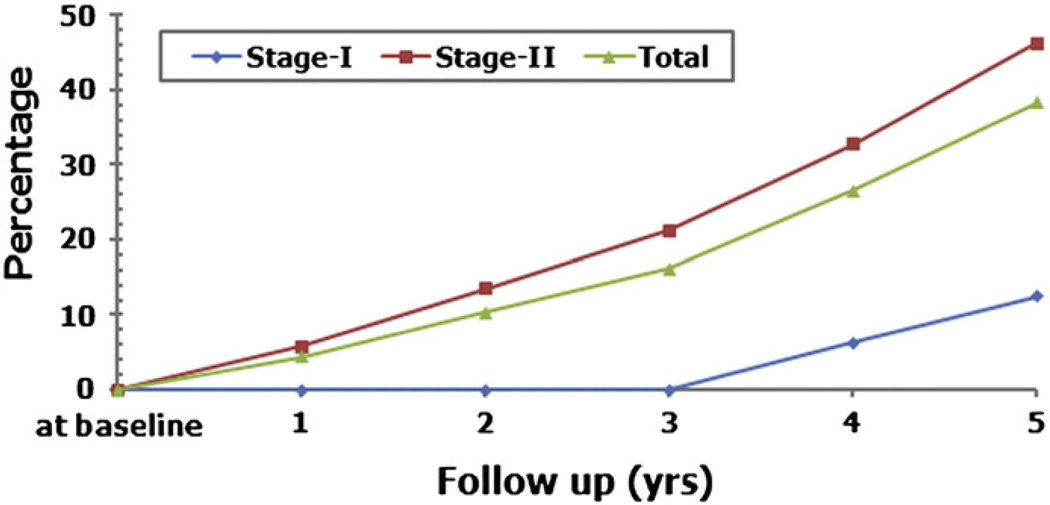
Our procedure can delay the progression to collapse (Fig. 7). At 5 years, 2 of 16 stage-I hips (12.5%) and 24 of 52 stage-II hips (46.15%) progressed to the subchondral fracture stage of osteonecrosis (Table 5). The overall rate of collapse was 38.24% (26 of 68 hips) in stage-I and stage-II hips combination (Table 5). The overall mean time of progression to collapse was 3.5 years (1–5 years) for stage-I and stage-II hips taken together and 4.5 years (4–5 years) for stage-I hips and 3.4 years (1–5 years) for stage-II hips (Table 5). The changes of the collapse rate for each Ficat stage and of the overall collapse rate over the entire follow-up period can be seen in Fig. 8.
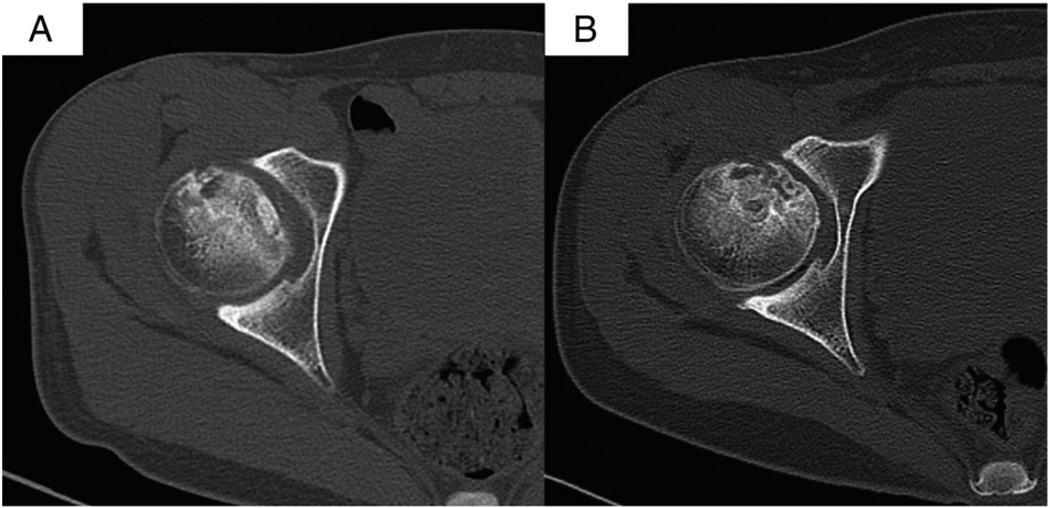
Fig. 7.
CT scans at 12 months after treatment show healing in necrotic region of femoral head, and the femoral head remains intact and round. (A) was obtained before the treatment, (B) was obtained at 12 months after the treatment.
Table 5.
Number of hips progressed to collapse at follow-up, rate of collapse and time to collapse for each Ficat stage.
| Ficat stage at onset of treatment |
Follow-up period (yrs) |
Rate of collapse (%) | Mean time (rang) to collapse (yrs) |
||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |||
| I (n = 16) | 0 | 0 | 0 | 1 | 1 | 12.5 (2/16) | 3.5 (1 to 5) |
| II (n = 52) | 3 | 4 | 4 | 6 | 7 | 46.15 (24/52) | 4.5 (4 to 5) |
| Total (n = 78) | 3 | 4 | 4 | 7 | 8 | 38.24 (26/68) | 3.4 (1 to 5) |
Fig. 8.
The percentage of collapsed hips over follow-up for five years in Ficat stage-I, and II hips.
Discussion
ONFH often leads to impaired hip joint function and is a major cause of disability in young patients [13]. Nevertheless, the exact pathogenesis of ONFH has not been clearly addressed yet, and the better treatment for ONFH remains to be developed [4]. To date, core decompression (CD) is one of the methods widely utilized to treat ONFH [10]. This procedure has been demonstrated to induce reconstruction repair, but usually this repair is incomplete [8]. Decrease of osteogenic progenitors is one of the common features of ONFH [8]. Reduction in the number and function of circulation endothelial progenitor cells has also been observed in patients with ONFH [27]. This phenomenon led to a lack of osteogenesis and revascularization may contribute to the insufficient reconstruction repair after CD. For this reason, local application of stem cells may be one of the solutions [28]. BMMCs are highly enriched with BMMSCs in terms of fibroblast colony-forming units [10]. Nowadays, many researchers try to transplant BMMCs into the femoral head through the decompression tunnel after CD, and obtained more satisfactory results than CD treatment alone [9–13]. However, the strategy has disadvantages of difficult manipulation, creating new trauma and increasing the therapeutic cost [29]. Both intraarterial [22] and intravenous [14–21] deliveries of stem cells have been used in stem cell implantation. The targeted intraarterial delivery of autologous BMMCs could be a minimally invasive strategy for the treatment of ONFH. The medial circumflex femoral artery is the main vessel which nourishes the femoral head. Therefore, the present study assessed the efficacy and safety of autologous BMMC perfusion via medial circumflex femoral artery in the treatment of ONFH.
We compared our findings with previously reported data with respect to the natural history of ONFH. It has been reported that the overall clinical progression rate for ONFH was 77% to 98%, and the radiological progression rate was 68% to 75% at a 3-year follow-up study [26,30,31]. The radiological follow-up of the 559 hips demonstrated a radiological progression rate of 74% [26]. In contrast, our study showed that 92.31% of hips achieved a satisfactory clinical result, while 43.59% of hips progressed radiologically at the end of 5-year study. Previous reports indicate that the rate of collapse of the femoral head were 75% and 80% at two and four years, respectively [26]. However, our study showed a collapse rate of 39.71% (12.5% for stage I and 48.08% for stage II) at the end of 5-year study. This study was designed to assess THA as an endpoint. 64% of the untreated hips requires THA at the end of 2-years [32], compared with our result of 7.69% at the end of 5-years. Mont and Hungerford [33] found that the rates of hips underwent THA were 65%, 69% and 87% for Ficat stage-I, -II and -III disease respectively. In the present study, THA was performed only in 6.25%, 3.84% and 30% of hips for Ficat stage-I, -II and -III disease respectively. The above information suggests that the current treatment approach could favorably alter the natural progression of ONFH.
It was suggested in a review of 21 studies involving 819 hips received non-operative treatment, and that only 22% had a satisfactory clinical result which meant that there was no need of surgical intervention at a mean follow-up of 34 months [33]. Bisphosphonates are now regarded as one of the standard choices in the non-operative treatment of early-stage ONFH [26]. Agarwala et al. [26] applied alendronate in the treatment of Ficat stage-I, -II and -III ONFH. At a mean follow-up of four years, 92.2% achieved a satisfactory result and did not require any surgery. The radiological progression rate was 45%. In the present study, a satisfactory clinical result was found in 92.31% of hips and 43.59% of hips progressed radiologically at a mean follow-up of 4.8 years. These results suggest that our procedure achieved better clinical efficacy as compared to alendronate treatment and other non-operative treatment. Gangji et al. [34] treated early stage ONFH (pre-collapse) with CD plus autologous BMMC implantation and found that only 10% of hips required THA at five years. Wang et al. [13] assessed the effect of the same procedure in ONFH treatment. At a mean follow-up of 27.6 months (range 12–40 months), 6 of 50 (12%) hips with early-stage ONFH (pre-collapse) required THA. According to their protocols, surgical procedures were performed to establish the decompression tunnel. Subsequently, the BMMCs were implanted into the femoral head through the tunnel. This strategy may create new trauma and increase the therapeutic cost. In the present study, THA was performed only in 7.69% of hips with early-stage ONFH (pre-collapse) at a mean follow-up of 4.8 years. Our procedure achieved better success for early stage ONFH (pre-collapse) as compared to CD plus autologous BMMC implantation. However, our procedure has the advantages of being minimally invasive and requiring simple manipulation. Taken together, our novel findings suggest that medial circumflex femoral artery perfusion of autologous BMMCs can effectively postpone surgical intervention, including THA in early-stage of ONFH.
Our novel approach can also improve the Harris hip score in all stages of ONFH (including post-collapse). The mean Harris hip score, which was evaluated at each follow-up visit after the treatment, was higher than that at the onset of treatment (Fig. 5), suggesting that this therapeutic method plays a role in alleviating pain and ameliorating the function, activity and motion of the hip. We also observed an interesting phenomenon that the mean Harris hip score began to decline from three years after treatment (Fig. 5). This finding indicates that there is a deterioration of hips from three years, and the cell-based therapy may be required every three years.
All kinds of treatment options suggest that the best treatment time period is at the subclinical stage of the disease. In this project, we also noticed that the therapeutic effects of our device for stage I and stage II patients (pre-collapse) were superior to that for stage-III patients (post-collapse). In this clinical study, 4.41% of stage-I, -II hips (pre-collapse) received THA compared with 30% of stage-III hips (post-collapse). THA mostly occurred at approximately 5 years and 3 years for staged-I and -II disease respectively compared with 2.3 years for staged-III disease. We also performed the survival analysis, and the result indicates that the survival chances of post-collapse hips (stage-III) were markedly reduced when compared to that of pre-collapse hips (stage-I, -II). These results are consistent with data from literature. Therefore, we also conclude that the effect of stem cell transplantation is limited for late stage ONFH (post-collapse), and the early intervention prior to the collapse (stage-I, -II) is extremely important for joint-preservation.
In spite of its favorable outcome, there have been concerns on the feasibility and safety of arterial perfusion of BMMSCs. Several lines of evidence support the conclusion that BMMSC transplantation is feasible and safe [9–13]. BMMSCs can regulate the secretion of TNF-α, IFN-α, IL-4, and IL-10 and modulate Treg cells to reduce the incidence of graft-versus-host disease [29]. In a phase I clinical trial, no side effects of BMMSC transplantation were found [35]. Li et al. [29] intravenously injected allogeneic BMMSCs into the rabbit model of ONFH and found no local or systematic manifestations of acute and chronic toxic reactions and graft versus host disease during and after BMMSC transplantation. In the present studies, typical complications of BMMC transplantation, such as infections, tumor induction and morbidity at the removal site on the iliac crest were not observed. These findings clearly demonstrate that minimal risks and complications are associated with the BMMC transplantation. In other words, BMMC arterial perfusion is feasible and safe.
The therapeutic efficiency of BMMSC implantation may be closely related to the fate of BMMSCs implanted into femoral head. We did not examine the fate of BMMSCs after transplantation, but there have been reports demonstrating that BMMSCs can migrate into the target tissues and participate in repairing injured tissues via blood circulation after ischemia [36,37]. Sixty percent of the BMMSCs remained in the femoral head 24 h after implantation as shown by the radionuclide labeling [12]. Li et al. [29] analyzed the distribution of BMMSCs in the rabbit model of ONFH after intravenous transplantation. They found that the amounts of BMMSCs in the necrotic femoral head were higher than that in the normal femoral head, liver and lungs. They then concluded that femoral head ischemia or necrosis is able to attract BMMSCs to migrate into and survive in injured femoral heads. Similarly, Yan et al. [7] demonstrated that BMMSCs could survive and expand in the ischemic environment of the femoral head up to 12 weeks after the transplantation, and the numbers of these cells increased significantly with time. In addition, Yan et al. [7] demonstrated that transplanted BMMSCs can differentiate into osteoblast in the osteonecrotic region of ONFH, and hypoxia can stimulate BMMSCs to secrete angiogenic factors resulting in increased angiogenesis which may contribute to the bone repair process.
The limitation of the present work is that it is not a randomized control trial and the cohort is relatively small. Accordingly, prospective randomized controlled studies with large sample size are necessary to verify the therapeutic effects of autologous BMMC perfusion via medial circumflex femoral artery. However, according to our present results, we are optimistic that this novel approach may lead to a successful outcome in ONFH treatment.
Conclusion
In conclusion, this work demonstrates that targeted intraarterial delivery of autologous BMMCs via medial circumflex femoral artery is a safe, effective and minimally invasive treatment strategy for early-stage ONFH. It is capable of relieving symptoms, improving hip function and delaying progression of the disease. The clinical outcome is better when it is applied prior to the collapse. This novel approach is likely to be a favorable intervention choice for the treatment of early-stage ONFH,
Acknowledgments
The authors appreciate the patients and their families. The authors are also grateful for the financial support provided by the National Natural Science Foundation of China (Grant No. 30672702 and No. 30873276 and No. 81273770) and Key Program of Traditional Chinese Medicine of Zhejiang Province, China (Grant No. 2005Z003).
Contributor Information
Qiang Mao, Email: peijiantongzy@126.com.
Hongting Jin, Email: hongtingjin@163.com.
Fei Liao, Email: feiliao@163.com.
Luwei Xiao, Email: xlw@139.com.
Di Chen, Email: di_chen@rush.edu.
References
- 1.Li W, Sakai T, Nishii T, Nakamura N, Takao M, Yoshikawa H, et al. Distribution of TRAP-positive cells and expression of HIF-1alpha, VEGF, and FGF-2 in the reparative reaction in patients with osteonecrosis of the femoral head. J Orthop Res. 2009;27:694–700. doi: 10.1002/jor.20802. [DOI] [PubMed] [Google Scholar]
- 2.Cardozo JB, Andrade DM, Santiago MB. The use of bisphosphonate in the treatment of avascular necrosis: a systematic review. Clin Rheumatol. 2008;27:685–688. doi: 10.1007/s10067-008-0861-9. [DOI] [PubMed] [Google Scholar]
- 3.Lieberman JR, Engstrom SM, Meneghini RM, SooHoo NF. Which factors influence preservation of the osteonecrotic femoral head? Clin Orthop Relat Res. 2012;470:525–534. doi: 10.1007/s11999-011-2050-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alves EM, Angrisani AT, Santiago MB. The use of extracorporeal shock waves in the treatment of osteonecrosis of the femoral head: a systematic review. Clin Rheumatol. 2009;28:1247–1251. doi: 10.1007/s10067-009-1231-y. [DOI] [PubMed] [Google Scholar]
- 5.Johannson HR, Zywiel MG, Marker DR, Jones LC, McGrath MS, Mont MA. Osteonecrosis is not a predictor of poor outcomes in primary total hip arthroplasty: a systematic literature review. Int Orthop. 2011;35:465–473. doi: 10.1007/s00264-010-0979-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marker DR, Seyler TM, McGrath MS, Delanois RE, Ulrich SD, Mont MA. Treatment of early stage osteonecrosis of the femoral head. J Bone Joint Surg Am. 2008;90(Suppl. 4):175–187. doi: 10.2106/JBJS.H.00671. [DOI] [PubMed] [Google Scholar]
- 7.Yan Z, Hang D, Guo C, Chen Z. Fate of mesenchymal stem cells transplanted to osteonecrosis of femoral head. J Orthop Res. 2009;27:442–446. doi: 10.1002/jor.20759. [DOI] [PubMed] [Google Scholar]
- 8.Hernigou P, Zilber S, Filippini P, Rouard H, Methieu G, Poignard A. Bone marrow injection in hip osteonecrosis. Tech Orthop. 2008;23:18–25. [Google Scholar]
- 9.Wen Q, Ma L, Chen YP, Yang L, Luo W, Wang XN. Treatment of avascular necrosis of the femoral head by hepatocyte growth factor-transgenic bone marrow stromal stem cells. Gene Ther. 2008;15:1523–1535. doi: 10.1038/gt.2008.110. [DOI] [PubMed] [Google Scholar]
- 10.Zhao D, Cui D, Wang B, Tian F, Guo L, Yang L, et al. Treatment of early stage osteonecrosis of the femoral head with autologous implantation of bone marrow-derived and cultured mesenchymal stem cells. Bone. 2012;50:325–330. doi: 10.1016/j.bone.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Gao YS, Zhang CQ. Cytotherapy of osteonecrosis of the femoral head: a mini review. Int Orthop. 2010;34:779–782. doi: 10.1007/s00264-010-1009-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gangji V, De Maertelaer V, Hauzeur JP. Autologous bone marrow cell implantation in the treatment of non-traumatic osteonecrosis of the femoral head: five year follow-up of a prospective controlled study. Bone. 2011;49:1005–1009. doi: 10.1016/j.bone.2011.07.032. [DOI] [PubMed] [Google Scholar]
- 13.Wang BL, Sun W, Shi ZC, Zhang NF, Yue DB, Guo WS, et al. Treatment of nontraumatic osteonecrosis of the femoral head with the implantation of core decompression and concentrated autologous bone marrow containing mononuclear cells. Arch Orthop Trauma Surg. 2010;130:859–865. doi: 10.1007/s00402-009-0939-0. [DOI] [PubMed] [Google Scholar]
- 14.Annaloro C, Onida F, Lambertenghi Deliliers G. Autologous hematopoietic stem cell transplantation in autoimmune diseases. Expert Rev Hematol. 2009;2:699–715. doi: 10.1586/ehm.09.60. [DOI] [PubMed] [Google Scholar]
- 15.Rosato E, Pisarri S, Salsano F. Current strategies for the treatment of autoimmune diseases. J Biol Regul Homeost Agents. 2010;24:251–259. [PubMed] [Google Scholar]
- 16.Karussis D, Karageorgiou C, Vaknin-Dembinsky A, Gowda-Kurkalli B, Gomori JM, Kassis I, et al. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch Neurol. 2010;67:1187–1194. doi: 10.1001/archneurol.2010.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Szodoray P, Varoczy L, Szegedi G, Zeher M. Autologous stem cell transplantation in autoimmune and rheumatic diseases: from the molecular background to clinical applications. Scand J Rheumatol. 2010;39:1–11. doi: 10.3109/03009740903030324. [DOI] [PubMed] [Google Scholar]
- 18.Mazo M, Planat-Bénard V, Abizanda G, Pelacho B, Léobon B, Gavira JJ, et al. Transplantation of adipose derived stromal cells is associated with functional improvement in a rat model of chronic myocardial infarction. Eur J Heart Fail. 2008;10:454–462. doi: 10.1016/j.ejheart.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 19.Mazo M, Gavira JJ, Abizanda G, Moreno C, Ecay M, Soriano M, et al. Transplantation of mesenchymal stem cells exerts a greater long-term effect than bone marrow mononuclear cells in a chronic myocardial infarction model in rat. Cell Transplant. 2010;19:313–328. doi: 10.3727/096368909X480323. [DOI] [PubMed] [Google Scholar]
- 20.Mark AL, Sun Z, Warren DS, Lonze BE, Knabel MK, Melville Williams GM, et al. Stem cell mobilization is life saving in an animal model of acute liver failure. Ann Surg. 2010;252:591–596. doi: 10.1097/SLA.0b013e3181f4e479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walker PA, Shah SK, Jimenez F, Gerber MH, Xue H, Cutrone R, et al. Intravenous multipotent adult progenitor cell therapy for traumatic brain injury: pre-serving the blood brain barrier via an interaction with splenocytes. Exp Neurol. 2010;225:341–352. doi: 10.1016/j.expneurol.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walczak P, Zhang J, Gilad AA, Kedziorek DA, Ruiz-Cabello J, Young RG, et al. Dualmodality monitoring of targeted intraarterial delivery of mesenchymal stem cells after transient ischemia. Stroke. 2008;39(5):1569–1574. doi: 10.1161/STROKEAHA.107.502047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ficat RP. Idiopathic bone necrosis of femoral head. Early diagnosis and treatment. J Bone Joint Surg Br. 1985;67:3–9. doi: 10.1302/0301-620X.67B1.3155745. [DOI] [PubMed] [Google Scholar]
- 24.Yoo MC, Chung DW, Hahn CS. Free vascularized fibula grafting for the treatment of osteonecrosis of the femoral head. Clin Orthop Relat Res. 1992;277:128–138. [PubMed] [Google Scholar]
- 25.Sotereanos DG, Plakseychuk AY, Rubash HE. Free vascularized fibula grafting for the treatment of osteonecrosis of the femoral head. Clin Orthop Relat Res. 1997;344:243–256. [PubMed] [Google Scholar]
- 26.Agarwala S, Shah S, Joshi VR. The use of alendronate in the treatment of avascular necrosis of the femoral head: follow-up to eight years. J Bone Joint Surg Br. 2009;91:1013–1018. doi: 10.1302/0301-620X.91B8.21518. [DOI] [PubMed] [Google Scholar]
- 27.Feng Y, Yang SH, Xiao BJ, Xu WH, Ye SN, Xia T, et al. Decreased in the number and function of circulation endothelial progenitor cells in patients with avascular necrosis of the femoral head. Bone. 2010;46:32–40. doi: 10.1016/j.bone.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Cui Q, Botchwey EA. Treatment of precollapse osteonecrosis using stem cells and growth factors. Clin Orthop Relat Res. 2011;469:2665–2669. doi: 10.1007/s11999-010-1738-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li ZH, Liao W, Cui XL, Zhao Q, Liu M, Chen YH, et al. Intravenous transplantation of allogeneic bone marrow mesenchymal stem cells and its directional migration to the necrotic femoral head. Int J Med Sci. 2011;8:74–83. doi: 10.7150/ijms.8.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Merle D’Aubigné R, Postel M, Mazabraud A, Massias P, Gueguen J, France P. Idiopathic necrosis of the femoral head in adults. J Bone Joint Surg Br. 1965;47:612–633. [PubMed] [Google Scholar]
- 31.Patterson RJ, Bichel WH, Dahlin DC. Idiopathic necrosis of the head of femur: a study of fifty-two cases. J Bone Joint Surg Am. 1964;46:267–282. [PubMed] [Google Scholar]
- 32.Lai KA, Shen WJ, Yang CY, Shao CJ, Hsu JT, Lin RM. The use of alendronate to prevent early collapse of femoral head in patients with non traumatic osteonecrosis: a randomized clinical study. J Bone Joint Surg Am. 2005;87:2155–2159. doi: 10.2106/JBJS.D.02959. [DOI] [PubMed] [Google Scholar]
- 33.Mont MA, Hungerford DS. Non-traumatic avascular necrosis of the femoral head. J Bone Joint Surg Am. 1995;77-A:459–474. doi: 10.2106/00004623-199503000-00018. [DOI] [PubMed] [Google Scholar]
- 34.Gangji V, Toungouz M, Lambermont M, Bastianelli E, Hauzeur J. Treatment of osteonecrosis of the femoral head with implantation of autologous bone-marrow cells: five- year follow-up. Bone. 2007;40:S46–S47. [Google Scholar]
- 35.Liu L, Sun Z, Chen B, Han Q, Liao L, Jia M, et al. Ex vivo expansion and in vivo infusion of bone marrow-derived Flk-1 + CD31-CD34- mesenchymal stem cells: feasibility and safety from monkey to human. Stem Cells Dev. 2006;15:349–357. doi: 10.1089/scd.2006.15.349. [DOI] [PubMed] [Google Scholar]
- 36.Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 37.Yagi H, Soto-Gutierrez A, Parekkadan B, Kitagawa Y, Tompkins RG, Kobayashi N, et al. Mesenchymal stem cells: mechanisms of immunomodulation and homing. Cell Transplant. 2010;19:667–679. doi: 10.3727/096368910X508762. [DOI] [PMC free article] [PubMed] [Google Scholar]