Abstract
Background:
Dermatoscopy is a new noninvasive tool for the diagnosis of various skin and hair disorders. Dermatoscopy of alopecia areata (AA) shows various specific features, which may aid in confirming the diagnosis.
Aims:
The aim of this study was to determine the various clinical patterns and the dermatoscopic features of AA.
Materials and Methods:
A total of 75 patients of AA were evaluated with a dermatoscope (magnification ×25 and ×60).
Results:
The mean age of onset of AA was 23.58 years. Males were more commonly affected. Scalp was most commonly involved. Patchy alopecia was the most common pattern observed. 10 patients showed concomitant nail changes. The dermatoscopic features included yellow dots (YDs) in 43 (57.33%) patients, black dots (BDs) in 63 (84%) cases, broken hairs (BHs) in 28 (37.33%) cases, short vellus hair (SVH) in 51 (68%) patients and tapering hair (TH) in 14 (18.67%) cases.
Conclusion:
The most common dermatoscopic finding observed was BDs, followed by SVHs, YDs, BH and TH.
Keywords: Alopecia areata, black dots, dermatoscopy, short vellus hairs, yellow dots
INTRODUCTION
Alopecia areata (AA) is a chronic, organ specific autoimmune disease, probably mediated by autoreactive T cells, which affects the hair follicles and sometimes the nails.[1] There is a high frequency of a family history of AA in affected persons, ranging from 10% up to 42% of cases.[2]
Clinically, the disease manifests as patchy alopecia, reticulate alopecia, ophiasis, ophiasis inversus (sisaphio), alopecia totalis or alopecia universalis.[3] A new subtype of AA is acute diffuse and total alopecia of the female scalp characterized by rapid progression of diffuse alopecia of the female scalp, marked female predominance and a favorable prognosis.[4] Nail changes (diffuse fine pitting, longitudinal ridging, thin and brittle fingernails and toenails and trachyonychia) may be seen in 3-30% of patients.[3]
Dermatoscopy is a noninvasive technique allowing rapid and magnified in vivo observation of the skin with the visualization of morphologic features often imperceptible to the naked eye.[5] AA is usually diagnosed based on clinical appearance. Dermatoscopy may be useful for differential diagnosis of difficult cases, in particular in diffuse AA or in patients with total hair loss by the presence of cadaverized hairs (black dots [BDs]), exclamation mark hairs (tapering hairs [TH]), broken hairs (BHs), yellow dots (YDs) and clustered short vellus hairs (SVHs) in the hair loss areas.[3,6]
Our aim was to study the various clinical and the dermatoscopic patterns of AA.
MATERIALS AND METHODS
This study was carried out in 75 patients of AA visiting dermatology Outpatient Department of a tertiary care center in North Karnataka between November 2012 and July 2013. Institutional ethical clearance was obtained. The clinical diagnosis of AA was established after a detailed history taking including past and family history and clinical examination. Relevant investigations like 10% KOH preparation, biopsy was carried out in select cases.
Grading of AA was done using the following scale: Scalp hair loss: S0, no hair loss; S1, <25% hair loss; S2, 26-50% hair loss; S3, 51-75% hair loss; S4, 76-99% hair loss; S5, 100% hair loss. Body hair loss: B0, no body hair loss; B1, some body hair loss; B2, 100% body (excluding scalp) hair loss. Nail involvement: N0, no nail involvement; N1, some nail involvement.
A digital dermatoscope (of magnification ×25 and ×60) was used for the evaluation.
RESULTS
A total of 75 patients were included in the study. There were 54 males and 21 females. The male to female ratio was 2.57:1. The mean age of the patients at presentation was 26.94 years, youngest being 10 months and oldest being 68 years. Most of the patients were in the age group of 15-30 years. The age distribution of the patients is shown in Graph 1.
Graph 1.
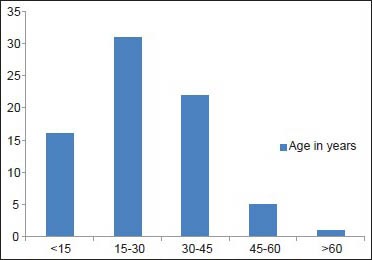
Alopecia areata patients categorized based on age
The mean age of onset was 23.58 years. The mean duration of all cases was 14.86 months. Mean duration of 1st episode was 6.6 months (1 week-3 years). The mean duration of recurrent cases was 7.23 years (1 year-27 years). The mean duration of the current episode in recurrent cases was 5.7 months (1 week-3 years). 9 patients (12%) had a family history of AA.
A total of 46 patients had their first episode of AA at presentation (Males 32, females 14). 29 patients had recurrent episodes of AA (males 22, females 7).
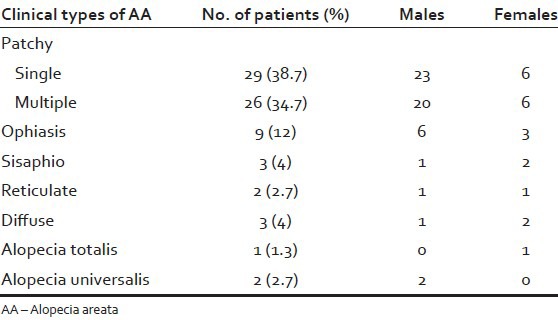
Patchy alopecia was the most common pattern of AA seen in 55 (73.3%) patients (43 males, 12 females). 29 patients (23 males, 6 females) had a single patch and 26 patients (20 males, 6 females) had multiple patches. The other patterns observed were: Ophiasis - 9 cases (6 males, 3 females), Sisaphio - 3 cases (1 male, 2 females), Reticulate - 2 cases (1 male, 1 female), diffuse - 3 cases (1 male, 2 females), alopecia totalis in 1 female and alopecia universalis in 2 males [Table 1].
Table 1.
Clinical types of AA
55 patients showed involvement of the scalp (34 males - 62.96%, 21 females - 100%) which included: Occiput - 27, frontal and vertex - 19 each, temporal - 7 and parietal - 5. The other sites involved were - Beard 13 (13 males - 24.07%), moustache 8 (8 males - 14.81%), eyebrows 8 (4 males - 7.4%, 4 females - 19.04%), eyelashes 2 (1 male - 1.85%, 1 female - 4.76%), limbs 2 (2 males - 3.7%) and alopecia universalis 2 (2 males - 3.7%) [Graph 2].
Graph 2.
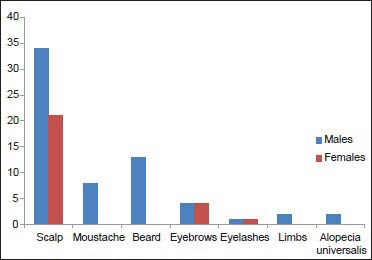
Sites of involvement in alopecia areata in both sexes
A single site was involved in 59 patients (scalp only - 46, beard only - 9, moustache only - 3, eyebrows only - 1). 11 patients had involvement of 2 sites and >2 sites were involved in 5 patients.
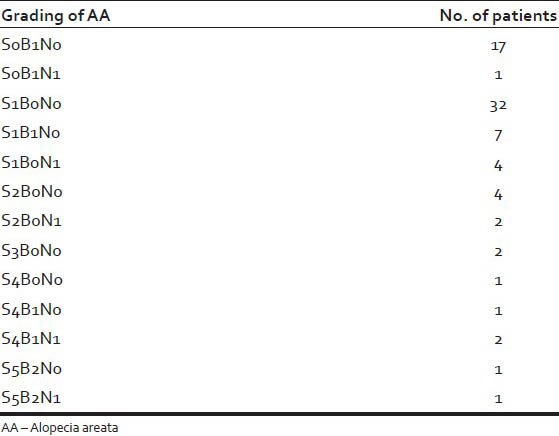
The grading of AA is tabulated in Table 2.
Table 2.
Grading of alopecia areata
10 patients had concomitant nail changes. These included pitting in 7 patients, ridging in 1 patient and striate leuconychia in 3 patients.
The dermatoscopic features seen in our patients were as follows: YDs-43 (57.33%), BDs - 63 (84%), BH - 28 (37.33%), SVH - 51 (68%) and TH - 14 (18.67%) [Figures 1-5]. All these dermatoscopic features were seen in 7 patients whereas 2 patients had none of the above features. The dermatoscopic features in various patterns of AA is tabulated in Table 3.
Figure 1.
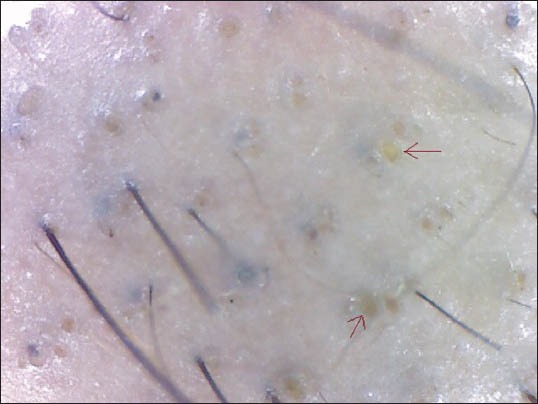
Yellow dots
Figure 5.
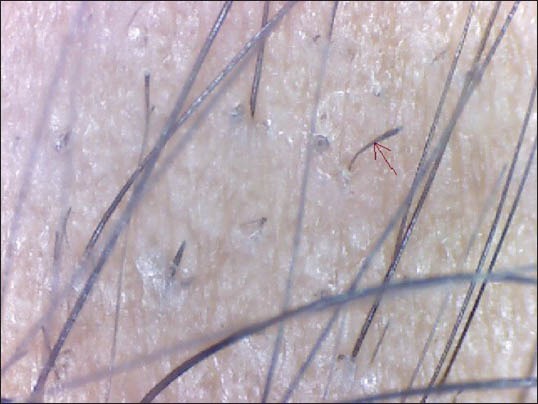
Tapering hairs
Table 3.
Dermoscopic features in various patterns of AA
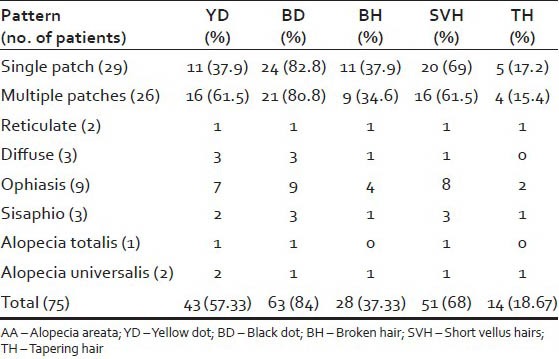
Figure 2.
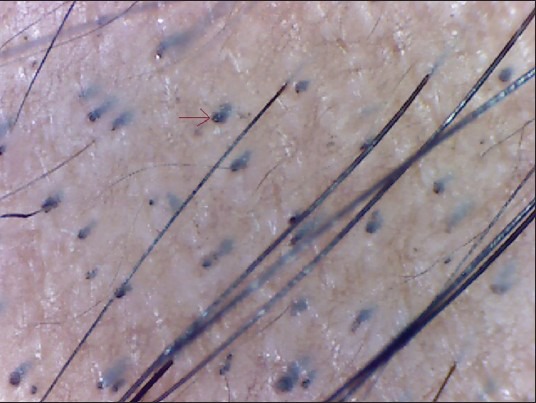
Black dots
Figure 3.
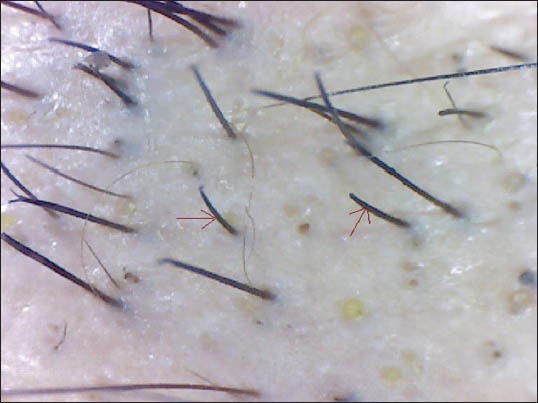
Broken hairs
Figure 4.

Short vellus hairs
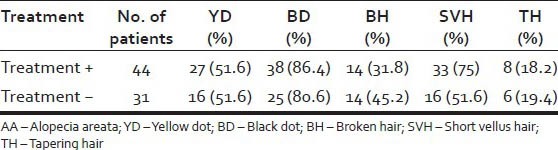
44 patients (58.67%) had received some form of treatment at presentation, whereas 31 patients (41.33%) had not taken any treatment. 75% of treated patients showed presence of SVH at presentation in contrast to 51.6% of untreated patients. The various dermoscopic features in patients with and without treatment is tabulated in Table 4.
Table 4.
Dermoscopic features based on treatment
DISCUSSION
AA is a common condition characterized by a patchy loss of hair without atrophy.[7] The onset is within the first three decades of life although it can start at any age. The sex incidence is probably equal.[1] In our study, males were most commonly affected than females with a male: female ratio of 2.57:1. This was consistent with the study done by Mane et al.,[3] whereas the study conducted by Inui et al.,[8] showed an increased female preponderance. The mean age of onset of AA was 23.58 years which was consistent with the previous studies.[3,8]
Patchy alopecia, being the most common pattern of AA in our study, was seen in 73.3% of patients. The study by Inui et al.[8] and Mane et al.,[3] also noted patchy alopecia as the most common pattern involving 46.7% and 87.7% of patients respectively.
Dermoscopy has been established as an indispensable tool in the diagnosis and follow-up of hair disorders. In AA, dermoscopy of active disease shows YDs, dystrophic hairs, as well as cadaverized (BDs) and exclamation mark hairs.[9]
YDs are a powerful new tool in the diagnosis of hair loss diseases initially proposed by Ross et al.[8,10] YDs are marked by a distinctive array of yellow to yellow-pink, round or polycyclic dots that vary in size and are uniform in color. They represent distention of the affected follicular infundibulum with keratinous material and sebum. In AA, degenerating follicular keratinocytes probably constitute the bulk of the YD.[10] In a study conducted by Inui et al.,[8] YDs were seen in 63.7% (191/300) of cases in contrast to Ross et al.'s study where 94.8% cases with AA had YDs (55/58 cases).[10] Mane et al.,[3] reported an incidence of 81.8% among 66 patients. The incidence of YDs in our study was 57.3%. The low incidence in our study may be attributed to the skin color of our patients which might make the YDs difficult to perceive.
THs (Exclamation marks hairs) are characterized by wider diameter in the distal shaft and thinner diameter in the proximal shaft. This pattern marks presence of the lymphocytic inflammatory infiltrate affecting the hair bulb and thus, producing thinner hair shaft.[9] THs were seen in 31.7% (95/300) cases of AA by Inui et al.[8] and 12.1% (8/66) cases by Mane et al.[3] We noted THs in 14 (18.67%) cases which was consistent with the previous studies.
BHs, considered to be dystrophic hairs produced by the least severely affected follicles in AA, are clinical markers of the disease activity and severity.[8] Inui et al.,[8] demonstrated BHs in 45.7% (137/300) of alopecia cases. 55.4% patients had BH in the study conducted by Mane et al.[3] BHs were seen in 28 patients (37.33%) in our study.
BDs (formerly “cadaverized hairs”) which represent pigmented hairs broken or destroyed at scalp level are characteristic of black haired individuals.[8,11] This sign is not seen in white population due to the hair color and cuticle resistance. BDs are remnants of exclamation mark hairs or BHs. These are a sensitive marker not only for disease activity, but also for the severity of AA.[8] In our study, BDs were seen in 84% of cases (63/75). Inui et al.,[8] demonstrated BDs in 44.3% (133/300) cases of AA. 67.7% (44/66) patients studied by Mane et al. had BDs.[3]
The presence of SVHs indicates the nondestructive nature of AA, allowing hair regrowth whether or not it results in completely mature hair shafts The pigmented hairs of Asians facilitate the detection of SVHs by dermoscopy.[8] These are seen as new, thin and unpigmented hairs within the patch.[3] The regrowth of SVHs can be seen after treatment in dermoscopy even when the recovered hairs are hardly perceived by the naked eye.[8] Short hypopigmented vellus hairs are characteristic of remitting disease.[5] In our study, 51 out of 75 patients (68%) demonstrated the presence of SVHs. Our findings were consistent with the previous studies conducted by Inui et al.,[8] where SVHs were observed in 72.7% (218/300) of cases. Mane et al.,[3] demonstrated SVHs in 40.9% of patients. The higher incidence of SVH in our study was probably due to the various forms of treatment already received by 58.67% of patients before their first visit to our center.
Inui et al. demonstrated that for diagnosis of AA, YDs and SVHs were the most sensitive markers. BDs, THs and BHs are specific for AA and indicate strong disease activity. BDs and YDs correlated positively with the severity of AA. SVHs correlated negatively with disease activity as well as severity of AA.[8]
CONCLUSION
Our study showed an increased male preponderance of AA. The mean age of onset of AA was 23.58 years. Patchy alopecia was the most common pattern. Scalp was more commonly involved. The most common dermoscopic finding observed was BDs, followed by SVHs, YDs, BHs and THs.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Paus R, Oslen EA, Messenger AG. Hair growth disorders. In: Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. Fitzpatrick's Dermatology in General Medicine. 7th ed. New York: McGraw Hill; 2008. pp. 753–77. [Google Scholar]
- 2.Madani S, Shapiro J. Alopecia areata update. J Am Acad Dermatol. 2000;42:549–66. [PubMed] [Google Scholar]
- 3.Mane M, Nath AK, Thappa DM. Utility of dermoscopy in alopecia areata. Indian J Dermatol. 2011;56:407–11. doi: 10.4103/0019-5154.84768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Inui S, Nakajima T, Itami S. Significance of dermoscopy in acute diffuse and total alopecia of the female scalp: Review of twenty cases. Dermatology. 2008;217:333–6. doi: 10.1159/000155644. [DOI] [PubMed] [Google Scholar]
- 5.Micali G, Lacarrubba F, Massimino D, Schwartz RA. Dermatoscopy: Alternative uses in daily clinical practice. J Am Acad Dermatol. 2011;64:1135–46. doi: 10.1016/j.jaad.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 6.Rudnicka L, Rakowska A, Olszewska M. Trichoscopy: How it may help the clinician. Dermatol Clin. 2013;31:29–41. doi: 10.1016/j.det.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 7.Wadhwa SL, Khopkar U, Nischal KC. Hair and scalp disorders. In: Valia RG, Valia AR, editors. IADVL Textbook of Dermatology. 3rd ed. Mumbai: Bhalani Publishing House; 2010. pp. 1368–72. [Google Scholar]
- 8.Inui S, Nakajima T, Nakagawa K, Itami S. Clinical significance of dermoscopy in alopecia areata: Analysis of 300 cases. Int J Dermatol. 2008;47:688–93. doi: 10.1111/j.1365-4632.2008.03692.x. [DOI] [PubMed] [Google Scholar]
- 9.de Moura LH, Duque-Estrada B, Abraham LS, Barcaui CB, Sodre CT. Dermoscopy findings of alopecia areata in an African-American patient. J Dermatol Case Rep. 2008;2:52–4. doi: 10.3315/jdcr.2008.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ross EK, Vincenzi C, Tosti A. Videodermoscopy in the evaluation of hair and scalp disorders. J Am Acad Dermatol. 2006;55:799–806. doi: 10.1016/j.jaad.2006.04.058. [DOI] [PubMed] [Google Scholar]
- 11.Rudnicka L, Olszewska M, Rakowska A, Slowinska M. Trichoscopy update 2011. J Dermatol Case Rep. 2011;5:82–8. doi: 10.3315/jdcr.2011.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]


