Abstract
Providing transplantation opportunities for patients with incompatible live donors through kidney paired donation (KPD) is an important strategy for easing the crisis in organ availability. KPD is can overcome the barriers when the only living potential donors are deemed unsuitable owing to an incompatibility of blood type, of human leukocyte antigen cross-match, or both. In KPD, the incompatibility problems with two donor recipient pairs can be solved by exchanging donors. In the absence of well-organized deceased donor program, or transplantation with desensitization protocol and ABO incompatible transplantation, living donor KPD promises hope to the growing number of patients suffering from end-stage renal disease in India. We report our first successful three-way KPD transplantation from India. In an era of organ shortage, this approach is relevant to encourage wider participation from KPD donors and transplant centers to prevent commercial transplantation.
Keywords: Kidney paired donation, living donor, renal transplantation, three-way
Introduction
Many potential kidney transplant recipients are unable to receive a live donor transplant due to cross-match or blood type incompatibility. Kidney paired donation (KPD) increases access to live donor transplantation.[1] From its first realization as an exchange of kidneys between two incompatible donor/recipient pairs, KPD has expanded to include compatible pairs, non-directed donors, three-way and larger exchanges, and living/deceased donor exchanges.[2]
Our center has been exploring KPD as a modality for facilitating living donor (LD) transplantation. In a single-center report (n = 70), we showed acceptable incidences of acute rejection, patient/graft survival rates over 10 years.[3] Here, we report our first successful three-way KPD transplantation resulting in transplantation of a highly sensitized patient and hard to match patient with AB donor.
Case Report
All potential donors and recipients were informed about risks and benefits of KPD prior to initiating evaluation. Sufficient time was given consider donation preferences, discuss options with their family and the donor evaluation team, and attenuate feelings of pressure or coercion if KPD is presented later. All three pairs of recipients and donors were allowed to meet each other before transplantation.
Patient profiles
Tables 1 and 2 describe the patient and donor profiles.
Table 1.
Demographic and HLA data
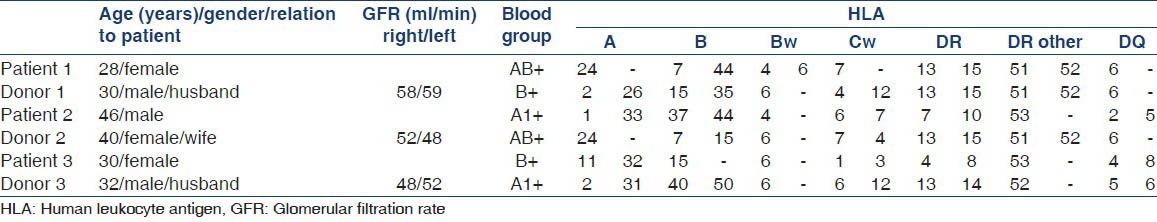
Table 2.
Immunological data 3 days prior to transplantation
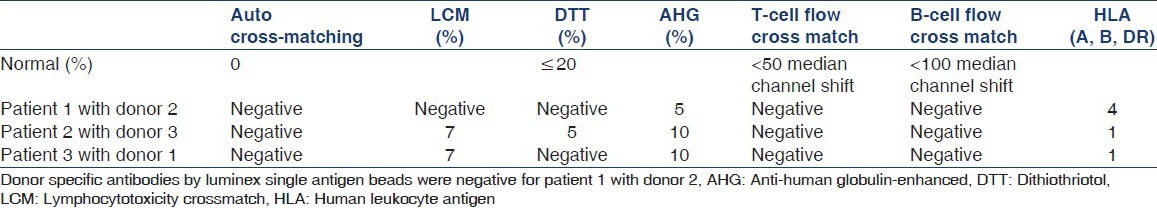
Patient 1 was hard-to-match with broad human leukocyte antigen (HLA) sensitization and had positive anti-human globulin-enhanced complement-dependent cytotoxicity cross matches a value of 90% with the donor, her husband. She was hepatitis C virus positive so desensitization treatment was not considered. Patient 2 was hard-to-match due to AB donor and a recipient blood type combination. Patient 3 with B blood group was ABO incompatible with his healthy willing donor of A1 blood group.
Three way KPD was planned of patient 1 with donor 2, patient 2 with donor 3, and patient 3 with donor 1. All donors were subjected to a diethylenetriamine pentaaceticacid renal scan before transplantation and all displayed satisfactory glomerular filtration rate (>40 ml/min on each side). All three pairs of recipients were cytomegalovirus (CMV) IgG positive.
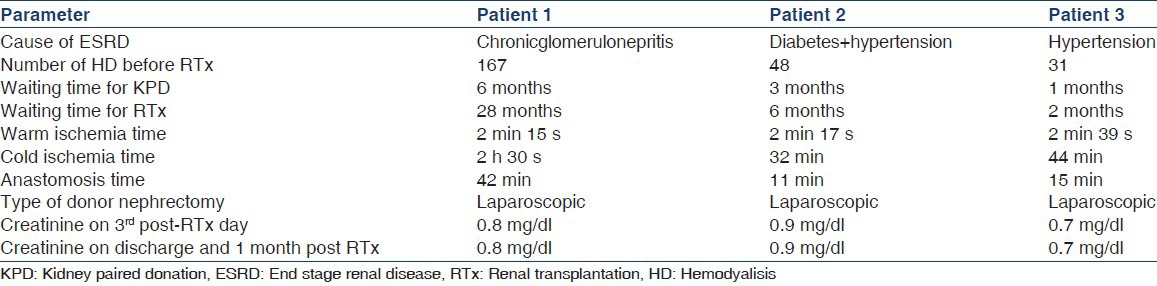
Table 3 describes pre-transplantation and surgical data and outcome.
Table 3.
Pre-transplantation and surgical data and outcome
Kidney transplantation
All surgeries were carried out on the same day by two transplant teams. All donors had single renal artery and single renal vein on the left side and underwent laparoscopic left donor nephrectomy. Immunosuppressive therapy constituted induction with methylprednisolone 500 mg for 3 days + rabbit-anti thymocyte globulin (r-ATG) (1.5 mg/kg single dose) along with calcineurin inhibitor based triple immunosuppression for maintenance therapy. Patient number 1 was hepatitis C virus positive so ATG induction was not given. Cold ischemia time was 2 h 30 s, 32 min and 44 min respectively for patient 1, 2 and 3. All patients received prophylaxis against CMV, fungal, and Pneumocystis jiroveci pneumonia infection.
All patients showed immediate graft function and normalization of serum creatinine. None of the patients had rejection and all had stable graft function on discharge and at 1month after transplantation.
Discussion
Live donation offers superior outcomes and is the most readily expandable source of kidneys for transplantation. ABO incompatibility and HLA sensitization represent the two greatest barriers to improving live donation rates. KPD offers a relatively low-cost option for subverting the incompatible barrier.[4]
KPD is feasible, successful, and if applied to larger donor pools, capable of expanding access to renal transplantation.[3] We have earlier shown similar graft and patient survival and rejection rates of KPD versus living related donor kidney transplantation LRDKTx over 2 years.[5]
Three-way KPDs were first reported in the USA in 2005. Simulations demonstrated that the match rates in KPD pools could be significantly improved by using the algorithms that allowed three-way donations. Three-way exchanges are more challenging either within or between institutions. This is because it is generally accepted that the donor operations should all be started simultaneously to reduce the chance that one of the transplants do esnotprogress (e.g. donor reneging).[5]
KPD can be carried out by any center that performs LD renal transplantation. Lack of awareness; counseling and participation in KPD may significantly disadvantage patients within compatible donors. Broader implementation of KPD across a wide number of centers has the potential to lead to more than 1000 additional live donor renal transplants every year.[6] It is cost effective, feasible, and crucial to properly serve transplant candidates with healthy but in compatible LD.[7] Recent study results are valuable for encouraging participation of KPD pairs and transplant centers in national KPD program.[8] In developing countries such as India, the costs of antibody removal protocols, and risk of infections in ABO incompatible renal transplantation make KPD program attractive.[8,9]
Future research should identify factors influencing donor and recipient willingness and preferences for entering KPD as compatible pairs and identify the perceived barriers to KPD participation among potential donors and recipients, and evaluate strategies for removing these barriers. Transplant centers should work together towards national KPD program and frame a uniform acceptable allocation policy.
Conclusion
Three-Way KPD is a valuable source of kidneys for renal transplantation. Encouraging the use of this approach to expand the donor pool would be important specially in low-income countries where deceased donor program is in infantile stage and transplantation with desensitization protocol and ABO incompatible transplantation are prohibitive due to economic constrains, risk of infections and lack of availability in all transplant centers.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Bingaman AW, Wright FH, Jr, Kapturczak M, Shen L, Vick S, Murphey CL. Single-center kidney paired donation: The Methodist San Antonio experience. Am J Transplant. 2012;12:2125–32. doi: 10.1111/j.1600-6143.2012.04070.x. [DOI] [PubMed] [Google Scholar]
- 2.Gentry S, Segev DL. Living donor kidney exchange. Clin Transpl. 2011:279–86. [PubMed] [Google Scholar]
- 3.Kute VB, Gumber MR, Patel HV, Shah PR, Vanikar AV, Modi PR, et al. Outcome of kidney paired donation transplantation to increase donor pool and to prevent commercial transplantation: A single-center experience from a developing country. Int Urol Nephrol. 2012 doi: 10.1007/s11255-012-0323-9. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 4.Montgomery RA. Living donor exchange programs: Theory and practice. Br Med Bull. 2011;98:21–30. doi: 10.1093/bmb/ldr008. [DOI] [PubMed] [Google Scholar]
- 5.Kute VB, Gumber MR, Vanikar AV, Shah PR, Patel HV, Engineer DP, et al. Comparison of Kidney Paired Donation Transplantations with Living Related Donor Kidney Transplantation: Implications for National Kidney Paired Donation Program. Ren Fail. 2013 doi: 10.3109/0886022X.2013.773914. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 6.Massie AB, Gentry SE, Montgomery RA, Bingaman AA, Segev DL. Center-Level Utilization of Kidney Paired Donation. Am J Transplant. 2013 doi: 10.1111/ajt.12189. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Melcher ML, Blosser CD, Baxter-Lowe LA, Delmonico FL, Gentry SE, Leishman R, et al. Dynamic challenges inhibiting optimal adoption of kidney paired donation: Findings of a consensus conference. Am J Transplant. 2013;13:851–60. doi: 10.1111/ajt.12140. [DOI] [PubMed] [Google Scholar]
- 8.Kute VB, Vanikar AV, Shah PR, Gumber MR, Patel HV, Modi PR, et al. Facilitators to national kidney paired donation program. Transpl Int. 2013 doi: 10.1111/tri.12078. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 9.Jha V. Post-transplant infections: An ounce of prevention. Indian J Nephrol. 2010;20:171–8. doi: 10.4103/0971-4065.73431. [DOI] [PMC free article] [PubMed] [Google Scholar]


