Abstract
It has been postulated that there is a link between age related degenerative diseases and cancer. The TNF-related apoptosis-inducing ligand (TRAIL) has been shown to selectively kill tumor cells by binding to pro-apoptotic and anti-apoptotic receptors. Our aim was to study the levels of anti-apoptotic receptor (DcR1) in age related macular degeneration (AMD) and controls. AMD patients (115) were classified into two groups: Dry and Wet AMD. Wet AMDs were further classified into occult, predominant classic and minimal classic. 61 healthy individuals were recruited as normal controls. After normalization with total protein, DcR1 levels were analyzed by ELISA. Mann Whitney U-statistic was used for analysis of DcR1 ELISA results. We have observed DcR1 levels in serum sample which were significantly lower in AMD patients as compared to controls (p = 0.001). On the other hand, we did not find difference in DcR1 levels between wet and dry AMD. The present study defines the plausible role of DcR1 in AMD pathology signifying a new therapeutic target for AMD.
Age is the most important risk factor for neurodegenerative disorders including age-related macular degeneration (AMD)1. It is a leading cause of irreversible vision loss in elderly in both the developed as well as developing world. The disease is broadly classified according to the severity of disease. It has been reported that about two million individuals are affected with AMD in United Kingdom2 and statistics on AMD in India show frequency varies from 2.7% early AMD to 0.6% late AMD in South India to 4.7% in North India3. The major hallmark characteristic of early AMD is the presence of drusen. Drusen are tiny accumulations of extracellular material which accumulate between the retinal pigment epithelium and Bruch's membrane of the eye. The drusen consists of lipoproteins (especially Lipoprotein E), complement system components (Complement factor H, complement factor B and complement factor C etc.), apolipoproteins (apolipoproteins B48 and B100), clusterin and some exosome molecules [CD63 (Cluster of Differentiation 68), LAMP2 (Lysosome-associated membrane protein 2), and CD81(Cluster of Differentiation 81) etc)4. There are two types of AMD i.e. Dry AMD and Wet AMD. Dry AMD is marked by drusen or depigmentation caused by products of the photoreceptors and retinal pigment epithelium (RPE). Wet AMD is caused due to the growth of abnormal blood vessels below the retina and RPE, which results in subretinal bleeding and consequent scar formation occluding vision. This may affect any one or both of the eyes. Patients with dry AMD may progress into wet AMD. Several genetic and environmental factors have already been associated with AMD. Among the genetic factors, the genes which are involved in cell apoptosis and its regulation have not been much investigated in AMD. However, apoptosis plays a major role in pathology of AMD5. In this study, we measured the expression level of DcR1 in serum of AMD patients. Previously, many genetic studies have confirmed the role of various genes in AMD by use of whole blood from AMD patients of similar sample size6,7,8,9. The disease progression is characterized by impairment of regulatory processes like apoptosis, chronic inflammation, increase in cell numbers, invasiveness etc. Moreover, there are some common risk factors and underlying molecules for AMD and cancer like smoking, complementary factor H (CFH) with inflammation being main mediator in the progression of diseases pathology10. TNF-related apoptosis-inducing ligand (TRAIL) acts as antitumor agent, which induces apoptosis in cancer cells. TRAIL is a cytokine and can bind with four receptors i.e. Death receptor 4 (DR4) and Death receptor 5 (DR5) (pro-apoptotic) and with anti-apoptotic (DcRs), Death receptor 1 (DcR1) and Death receptor 2 (DcR2)11. By binding with DR4 or DR5 receptors TRAIL can induce apoptosis by caspase-8 dependent manner which further activates the effector caspases like caspases-3, 6,7 etc. DcR1 is a GPI-anchored member of the tumor necrosis factor receptor (TNFR) super family which is also known as CD263, TRID and TRAIL-R3. It is not expressed in all tumors. It includes a transmembrane domain and an extracellular TRAIL binding domain. It does not contain a functional death domain. It acts as TRAIL decoy receptor by reducing the apoptosis. The TRAIL's binding to DcR1 or DcR2 stimulate the NFk-β leading to activation of transcription genes antagonizing apoptotic mechanisms or promoting inflammation. Decoy receptors after binding to TRAIL inhibit the TRAIL-induced apoptosis by inhibiting binding to proapoptotic receptors i.e. DR4 and DR5.
In this study, we hypothesized that the lower levels of DcR1 in serum of AMD patients may provide an environment conducive for degenerative processes. TRAIL binding and therefore, DcR1 mediated anti-apoptotic process may not be active which may lead to photoreceptor degeneration leading to AMD.
Results
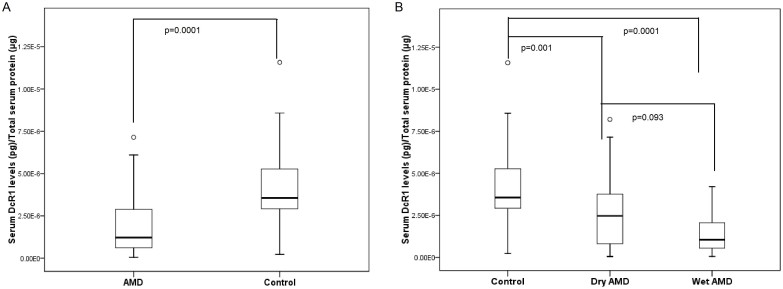
Summary statistics of main variables has been reported in Table 1. The serum concentration of DcR1 is shown in Table 2. DcR1 serum levels were skewed therefore, Kruskal-Wallis test was applied for statistical comparison. The serum concentration of DcR1 was found to be lower in AMD cases as compared to controls (Table 2, Figure 1A, p = 0.0001). Difference was not significant between serum concentration of DcR1 in dry and wet AMD (Table 2, Figure 1B, p = 0.093). The levels of dry and wet AMD were significantly lower as compared to normal controls (Figure 1B, p = 0.001 & 0.0001 respectively). To estimate the predictive value of DcR1, serum levels of DcR1 were again segregated into minimal classic, predominant classic and occult AMD. No significant difference was found between the wet AMD subgroups (Table 2). While adjusting the risk factors to AMD an independent analysis was carried out. Important risk factors like smoking, alcohol, food habits, diabetes and heart diseases were analyzed to examine their association with DcR1. However, no significant difference observed between AMD smokers versus AMD never smokers, alcohol consumers versus never alcohol consumers, vegetarian versus non vegetarian, diabetic versus non diabetic, AMD with heart disease versus AMD without heart disease and Male versus female (Table 2). When multivariate analysis was carried out with adjustment for age, smoking, alcohol, food habits, diabetes and heart disease, we found significant difference between AMD and controls (OR = 11.181, p = 0.001).
Table 1. Demographic characteristics of Controls and AMD patients.
| Variables | AMD | Controls |
|---|---|---|
| Total | 115 | 61 |
| Wet AMD | 84 | ---- |
| Dry AMD | 31 | ---- |
| Sporadic Cases | 105 | ---- |
| Familial Cases | 10 | ---- |
| Duration of disease¥ | 23 ± 2.6 (M) | ---- |
| Smokers | 50 | 11 |
| Non Smokers | 65 | 44 |
| Alcoholic | 37 | 17 |
| Non-alcoholic | 78 | 38 |
| Vegetarian | 61 | 31 |
| Non-vegetarian | 54 | 24 |
| Age | 64.97 ± 7.1 | 60.38 ± 13.2 |
| Heart Disease | 16 | ---- |
| No Heart Disease | 60 | 55 |
| Male | 75 | 40 |
| Female | 40 | 21 |
Demographic and clinical details of subjects. Age, Age of onset; M, Months; ¥ Duration of disease is the interval between appearance of first symptom of AMD and collection of sample. Values are mean ± SD.
Table 2. Comparison of serum DcR1 levels between control, AMD and their subtypes along with odds ratio (adjusted for covariates).
| Mann Whitney Statistic | Unadjusted p value | Multivariate analysis adjusted for, age smoking, alcohol, food habits, diabetes and heart disease | ||||
|---|---|---|---|---|---|---|
| Subjects | Mean Rank | Z -Value | p-value | Odd Ratio | CI (95%) | p- value |
| AMD | 48.12 | 5.762 | 0.0001* | 11.181 | 2.67–46.7 | 0.001 |
| Control | 86.32 | |||||
| Dry | 41.85 | |||||
| Wet | 33.29 | 1.681 | 0.093 | 0.296 | 0.049–1.790 | 0.182 |
| Minimal Classic | 17.00 | |||||
| Predominant Classic | 12.11 | 0.867 | 0.386 | * | * | * |
| Occult | 20.05 | 0.611 | 0.541 | * | * | * |
| Alcoholic | 38.38 | |||||
| Non Alcoholic | 35.56 | 0.538 | 0.591 | 0.286 | 0.021–3.892 | 0.347 |
| Smokers | 37.40 | |||||
| Non Smokers | 35.86 | 0.308 | 0.758 | 0.457 | 0.053–3.962 | 0.477 |
| Vegetarian | 38.19 | |||||
| Non Vegetarian | 34.71 | 0.704 | 0.481 | 0.964 | 0.165–5.638 | 0.968 |
| Heart Disease | 28.00 | |||||
| No heart Disease | 25.57 | 0.445 | 0.671 | 0.529 | 0.044–6.309 | 0.614 |
| Diabetic | 33.62 | |||||
| No Diabetes | 35.93 | 0.370 | 0.711 | 1.727 | 0.246–12.136 | 0.583 |
| Male | 37.09 | |||||
| Female | 35.52 | 0.308 | 0.758 | 0.182 | 0.011–2.99 | 0.233 |
*Odds ratio can not be computed (with reference category as minimal classic) because of the constant values.
Figure 1.
(A) Serum levels of DcR1 in AMD and controls. (B) Serum levels of DcR1 in dry, wet and normal controls. pg, picogram; μg, microgram.
The association between levels of DcR1 in AMD and age have been computed using Chi square (χ2 = 3.929, p = 0.141), which shows that there is no significant association between age and DcR1 levels. However, the association between severity of AMD and levels of DcR1 was significant (χ2 = 5.982, p = 0.014). The prediction equation for AMD, by considering DcR1 levels as independent variable, shows that 74.4% of the cases have been correctly classified with model authenticity of 74.4% and close confidence intervals for ROC curve. The area under ROC was 0.75 (p = .0001) with standard error of 0.044 and confidence interval of 0.660–0.834 (Figure 2).
Figure 2. Receiver operating characteristic (ROC) obtained from binary logistic regression model which generates significant predictors of AMD.
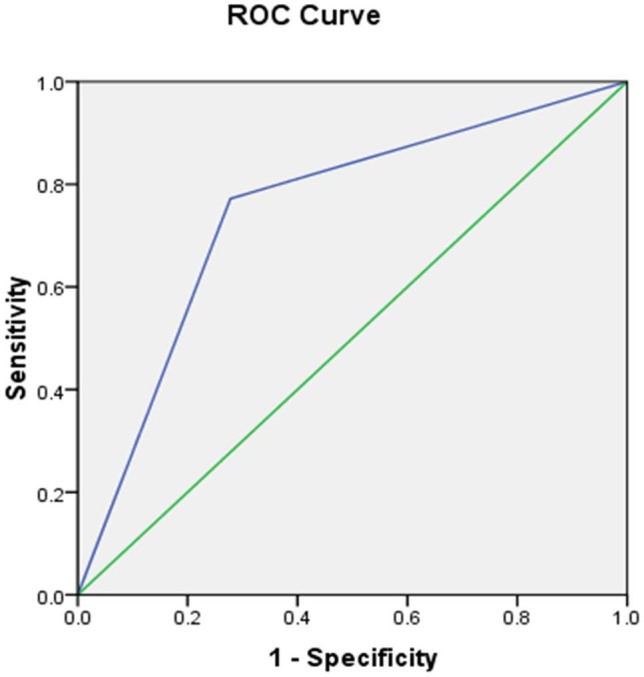
Area under the curve is reported to be 75%.
Discussion
In a number of diseases, apoptosis plays a major role in development of disease pathology like AMD and retinitis pigmentosa. AMD causes a gradual loss of central vision due to the death of photoreceptors in the retina especially in central part of retina called the macula. The study was performed to determine whether the difference in the levels of serum DcR1 are associated with AMD. We showed that the DcR1 expression decreased significantly in patients of AMD as compared to controls irrespective of other environmental factors that indicates the noon redundant role of DcR1 mediated processes independent of these factors. Additionally, we also did not find the considerable difference in serum DcR1 levels in between dry AMD and wet AMD indicating apoptosis as a common phenomenon in both types of AMD.
In pathological processes of many degenerative diseases, apoptosis and necrosis play an important role. Histochemical and ultrastructural analysis shows that during AMD, RPE cells overlying drusen degenerate because of necrosis12. Initiation of necrosis in cells results in morphological modifications, membrane rupture which may lead to release of cytoplasmic contents from the cell. Necrotic material from these cells is believed to activate the complement, inducing inflammation in local tissue. Krabb et al has also shown that histones, which are released during necrosis, also constitute drusen13 leading to RPE atrophy which further results in geographic atrophy. There are several studies which have simultaneously implicated the role of apoptotic processes in degeneration of photoreceptors, retinal pigment epithelium (RPE) and inner nuclear layer (INL) in AMD14. It has already been reported that reduced expression of DcR1 was associated with sensitivity to TRAIL in many cell lines of cancers15. In carcinomas, it has also been examined that lack of expression of the DcRs may provide them extra susceptibility to apoptosis induced by TRAIL16.
However, apoptotic action mediated by TRAIL has been shown to be exerted by participation of p53 thus exacerbating anti-tumor activity by down regulating the DcR1 expression in colon cancer17. Moreover, it has been found to be overexpressed in the p53 mutated cell line. Recently, Ambati et al have also examined the role of p53 in retinal angiogenesis and have found disruption of p53 transcriptional network can abolish the anti-angiogenic activity of Nutlin3 (drugs commonly used in cancer). Even though the group did not show any relation with DcR1 or DcR2 receptors the role of DcR1 in apoptotic process could be examined in the context of p53 processes18. Several mouse models for retinal degeneration have been established by genetic manipulations in several retinal related genes and also created by light injury of retinal layers in which apoptosis contributes towards photoreceptor cell death19,20,21. Human retinitis pigmentosa, serous retinal detachment and pathologic myopia are characterized by similar pathologic photoreceptor apoptosis22,23. In glaucoma retinal ganglion cells also undergo apoptosis24. Additionally, it was also shown that the number of TUNEL-positive cells in the retinal pigment epithelium, choroid, outer nuclear layer, and inner nuclear layer are significantly more in AMD eyes as compared to control eyes, signifying that these cells may possibly die by apoptosis12. Therefore, these studies have revealed that main cause of photoreceptor death in retinal and other diseases presumably regulated by TRAIL signaling. Due to low levels of DcR1, TRAIL has increased affinity to bind with TRAILR1 or TRAILR2 which results in receptor oligomerization and initiation of apoptosis. Enhanced apoptosis can result in tissue degeneration, a feature of AMD, while reduced apoptosis results in accumulation of immune cells. Therefore, cellular protection protocols engage a delicate balance in pro- apoptotic and anti-apoptotic factors. Apoptosis involves two major signaling pathways: the extrinsic death pathway and intrinsic death pathway. Extrinsic pathway is initiated through apoptotic signal transduction cascades mediated by members of TNFR while intrinsic death pathway is mediated by pro-apoptotic and anti-apoptotic Bcl2 family proteins at the mitochondria25.
We did not find any difference in the levels of DcR1 for dry and wet AMD and the difference was not found to be significant for wet AMD subtypes. However, after categorizing DcR1 levels (below and above median) the association with severity of AMD was found to be significant but it was not significant with age. While analyzing the other risk factors to AMD, no association of DcR1 levels was observed. Death of photoreceptor cells is the main cause in the pathogenesis of AMD. We have attempted to predict AMD based on DcR1 levels (below and above median) using logistic regression, which showed 74.4% model predictivity and AUC is 0.75. The moderate value of AUC may be used to diagnose AMD patients with very less standard error. TNF-related apoptosis inducing ligand stimulates apoptosis and DcR1 is believed to act as dominant-negative receptor for TNF-related apoptosis sensitising ligand. Due to low levels of DcR1, the levels of TNF-related apoptosis inducing ligand may be enhanced resulting in apoptosis mediated AMD. The present study highlights that the lower levels of DcR1 may be associated with AMD however additional mechanistic studies can shed more light on the putative mechanism.
Conclusion
Conclusively, this study substantiates the role of apoptosis mediated by DcR1 receptor. A bigger study is imperative. An effective method of neuroprotection based on targeting of DcR1 could potentially supplement the current treatment strategies for this disorder.
Methods
Study population
A signed informed consent was taken from each participant. Individuals with AMD were recruited from advanced eye centre, PGIMER, Chandigarh, India. We included 176 cases which contained 115 AMD samples (75 male and 40 females) and 61 normal healthy controls (40 male and 21 females) after obtaining a signed informed consent as per inclusion exclusion criteria. The unrelated attendants like spouses who accompanied AMD patients to Eye clinic were recruited as controls. The samples were collected at the same site using same procedure by the same individual. Ethical clearance was taken from the Ethics Committee of the Institute vide letter No Micro/10/1411.
Inclusion and exclusion criteria
Inclusion criteria included the age of 50 years or older with the diagnosis of age related macular degeneration defined by choroidal neovascularization and/or dry AMD with at least five drusen in one eye. The controls were of 50 years or older without drusen and without any AMD diagnostic criteria. Exclusion criteria excluded the retinal diseases in the outer retinal layers and/or photoreceptors (other than AMD) like central serous retinopathy, high myopia, diabetic retinopathy, retinal dystrophies, uveitis, vein occlusion and other problems that precluded satisfactory stereo fundus photography. These situations contain occluded pupils due to ocular diseases like cataracts, opacities and synechiae9.
Diagnosis of AMD
A retina consultant examined the patients for best corrected visual acuity (BCVA), intraocular pressure, slit lamp biomicroscopy (SLB) of anterior and posterior segment with 90D lens and indirect ophthalmoscopy for peripheral fundus examination. All patients underwent fluorescein fundus-angiography (FFA) and optical coherence-tomography (OCT). AMD diagnosis was based on ophthalmoscopic and FFA findings10.
Demographic profile
The demographic detail was taken by a trained interviewer after getting signed consent form using a risk factor questionnaire9. The detail (age, sex, race, cigarette smoking, food habit, alcohol intake, diabetes and heart diseases) was self reported by participants. Smokers were districted as those having smoked at least three cigarettes per day or 54-boxes for at least 6 contiguous months and were separated further into never smokers and smokers. Non-vegetarian patients were distincted as those consuming meat, chicken,or fish for at least 6 contiguous months and alcohol consumer patients were defined as those having rum, whiskey, wine or homemade alcohol for at least 6 contiguous months. Heart diseases were determined based on the participant's answers to whether a physician had ever told them about this finding and whether they had ever taken medicine for this situation10.
Serum separation
Blood samples were collected from all individuals for carrying out ELISA. About 4.0 ml of blood was collected in a serum separator tube (BD Biosciences, USA) and was left for 30 minutes at room temperature to allow it to clot and after centrifugation at 3000-rpm for 30 minutes, serum was separated. The samples were labeled, coded and stored to study the levels by established procedures described below.
Total protein estimation
Total serum proteins for normalization of DcR1 levels from ELISA was done by Bradford assay. The procedure was carried out according to manufacturer's recommendations. In double distilled water, serum samples were diluted upto 1500 times. The standard curve was generated by using protein Bovine Serum Albumin (BSA). Standard protein BSA and samples were mixed in ratio 4:1 with coomassie brilliant blue G – 250 dye (Bradford reagent) followed by incubation at room temperature for about 15 minutes on shaker. The absorbance was taken at 595 nm in 680XR model of Microplate reader (Biorad, Hercules, CA, USA). Standard curve was generated with quadratic fit or linear models11.
ELISA
ELISA for DcR1 was performed according to the manufacturer's protocol (Abcam, Cat no ab46018). Sample serum assays were performed in duplicate. Standard DcR1 was assayed over a concentration range of 10000–312.5 pg/ml in duplicates for each experiment. The assay recognizes both natural and recombinant human DcR1 and the sensitivity of assay was <147 pg/ml. Appropriate blanks were also incorporated into experimental design. Normalization was done with total protein.
Statistical analysis
The assumption of normality was tested with the help of Normal Quantile plot (Q-Q plot) and it was observed that data was not normally distributed. Mann-Whitney U-test was, therefore, applied for comparing the two groups. For comparing more than two groups, Kruskal Wallis one-way analysis of variance (ANOVA) followed by post-hoc was applied for multiple comparisons. The p ≤ 0.05 was considered significant. The measure R2 (Coefficient of determination) was used to determine the goodness of standard curve fit for ELISA and total protein. All statistical analysis such as linear regression, quadratic fit and test of significance were performed with statistical product and service solutions (SPSS; IBM SPSS Statistics 20.0, Chicago, Illinois, USA) 20.0 software. Receiver operating characteristic (ROC) curve, which is defined as a plot of true positive rate (test sensitivity) versus false positive (1-specificity) rate for different cut-off points of DcR1 levels was drawn for predicted model. Area under the ROC curve is considered as an effective measure of inherent validity of a diagnostic test.
Author Contributions
A.A. conceptualized the study and framework. N.K.S. and A.A. conducted the study, data analysis interpreted the results and wrote the manuscript. S.K.S., A.K.B., N.J. and P.K.G. contributed to the data analysis and prepared some figures. A.G., S.P. and R.D.S. contributed to the interpretation and reviewed the manuscript. All authors reviewed the manuscript.
Acknowledgments
The study was carried out at Department of Neurology, PGIMER, Chandigarh, India. We acknowledge Indian Council of Medical research, India for providing funds (45/11/2010-HUM/BMS). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Brown R. C., Lockwood A. H. & Sonawane B. R. Neurodegenerative Diseases, An Overview of Environmental Risk Factors. Environ Health Perspect 113, 1250–1256 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen C. G., Fletcher A. E., Donoghue M. & Rudnicka A. R. How big is the burden of visual loss caused by age related macular degeneration in the United Kingdom? Br J Ophthalmol. 87, 312–317 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azad R., Chandra P. & Gupta R. The economic implications of the use of anti-vascular endothelial growth factor drugs in age-related macular degeneration. Indian Journal of Ophthalmology. 55, 441–443 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A. L. et al. Autophagy and exosomes in the aged retinal pigment epithelium, Possible relevance to drusen formation and age-related macular degeneration. PLoS ONE 4, e4160 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya S., Chaum S., Johnson D. A. & Johnson L. R. Age-Related Susceptibility to Apoptosis in Human Retinal Pigment Epithelial Cells Is Triggered by Disruption of p53–Mdm2 Association. Invest. Ophthalmol. 53, 8350–8366 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N. K. et al. Association between CFH Y402H polymorphism and age related macular degeneration in North Indian cohort. PLoS ONE 8, e70193 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N. K. et al. Single Nucleotide Polymorphism and Serum Levels Of VEGFR2 are Associated With Age Related Macular Degeneration. Curr Neurovasc Res. 9, 256–65 (2012). [DOI] [PubMed] [Google Scholar]
- Sharma N. K. et al. New Biomarker for Neovascular Age Related Macular Degeneration, Eotaxin-2. DNA Cell Biol. 31, 1618–1627 (2012). [DOI] [PubMed] [Google Scholar]
- Anand A. et al. Single Nucleotide Polymorphisms in MCP-1 and Its Receptor Are Associated with the Risk of Age Related Macular Degeneration. PLoS ONE 7, e49905 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R. et al. Risk alleles in CFH and ARMS2 and the long-term natural history of age-related macular degeneration, the Beaver Dam Eye Study. JAMA Ophthalmol. 131, 383–92 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone W. R., Frew A. J. & Smyth M. J. The TRAIL apoptotic pathway in cancer onset, progression and therapy. Nature 8, 782–798 (2008). [DOI] [PubMed] [Google Scholar]
- Hageman G. S. et al. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch's membrane interface in aging and age-related macular degeneration. Prog. Retin. Eye Res. 20, 705–732 (2001). [DOI] [PubMed] [Google Scholar]
- Crabb J. W. et al. Drusen proteome analysis: an approach to the etiology of age-related macular degeneration. Proc. Natl. Acad. Sci. 99, 14682–14687 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunaief J. L., Dentchev T., Ying G. & Milam A. H. The Role of Apoptosis in Age-Related Macular Degeneration. Arch Ophthalmol. 120,1435–1442 (2002). [DOI] [PubMed] [Google Scholar]
- Ozoren N. & El-Deiry W. S. Cell surface death receptor signaling in normal and cancer cells. Semin Cancer Biol. 13, 135–147 (2003). [DOI] [PubMed] [Google Scholar]
- Yamanaka T. et al. Chemotherapeutic agents augment TRAIL-induced apoptosis in human hepatocellular carcinoma cell lines. Hepatology 32, 482–490 (2000). [DOI] [PubMed] [Google Scholar]
- Toscano F. et al. P53-mediated upregulation of DcR1 impairs oxaliplatin/TRAIL-induced synergistic anti-tumour potential in colon cancer cells. Oncogene 27, 4161–71(2008). [DOI] [PubMed] [Google Scholar]
- Chavala S. H. et al. Retinal angiogenesis suppression through small molecule activation of p53. J Clin Invest. 123, 4170–4181 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang B. et al. Retinal degeneration mutants in the mouse. Vision Res. 42, 517–25 (2002). [DOI] [PubMed] [Google Scholar]
- Chang B. et al. Age-related retinal degeneration (arrd2) in a novel mouse model due to a nonsense mutation in the Mdm1 gene. Hum Mol Genet. 17, 3929–41 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawes N. L. et al. Retinal degeneration 6 (rd6): a new mouse model for human retinitis punctata albescens. Invest Ophthalmol Vis Sci. 41, 3149–57 (2000). [PubMed] [Google Scholar]
- Li Z. Y. & Milam A. H. Apoptosis in retinitis pigmentosa. Anderson RELaVail MMHollyfield JGeds.Degenerative Diseases of the Retina. New York, NY Plenum Press 1–8 (1995).
- Xu G. Z., Li W. W. & Tso M. O. Apoptosis in human retinal degenerations. Trans Am Ophthalmol Soc. 94, 411–430 (1996). [PMC free article] [PubMed] [Google Scholar]
- Kerrigan M. S., Zack D. J., Quigley H. A., Smith S. D. & Pease M. E. TUNEL-positive ganglion cells in human primary open-angle glaucoma. Arch Ophthalmol. 115, 1031–1035 (1997). [DOI] [PubMed] [Google Scholar]
- Johnstone R. W., Ruefli A. A. & Lowe S. W. Apoptosis, a link between cancer genetics and chemotherapy. Cell 108, 153–164 (2002). [DOI] [PubMed] [Google Scholar]