Abstract
Whole-genome duplication events have driven to a large degree the evolution of angiosperm genomes. Although the majority of redundant gene copies after a genome duplication are lost, subfunctionalization or gene balance account for the retention of gene copies. The Arabidopsis 80S ribosome represents an excellent model to test the gene balance hypothesis as it consists of 80 ribosomal proteins, all of them encoded by genes belonging to small gene families. Here, we present the isolation of mutant alleles of the APICULATA2 (API2) and RPL36aA paralogous genes, which encode identical ribosomal proteins but share a similarity of 89% in their coding sequences. RPL36aA was found expressed at a higher level than API2 in the wild type. The loss-of-function api2 and rpl36aa mutations are recessive and affect leaf development in a similar way. Their double mutant combinations with asymmetric leaves2-1 (as2-1) caused leaf polarity defects that were stronger in rpl36aa as2-1 than in api2 as2-1. Our results highlight the role of combined haploinsufficiency and purifying selection in the retention of these paralogous genes in the Arabidopsis genome.
Ancient genome duplication (polyploidization) events have played a central role in the evolution of angiosperm genomes1. Several models account for the fate of individual genes after a duplication occurs. The redundant gene copies might be inactivated and lost or, alternatively, they might be retained in the genome by different evolutionary processes. In the duplication-degeneration-complementation model, subfunctionalization (i.e. the distribution of the ancestral functions among the duplicated genes) accounts for the evolutionary maintenance of duplicate gene copies2. Duplicated genes might also be maintained, retaining the same (redundant) function and expression pattern, when the duplication leads to a new balance between the products of dosage-dependent genes (gene balance hypothesis)3,4.
The availability of complete plant genome sequences has revealed the presence of recent and ancient whole-genome duplication events in the evolutionary history of numerous plant species. Three rounds of polyploidization, followed by extensive chromosomal rearrangements and gene loss to reach the present diploid state, have been documented in the genome of Arabidopsis thaliana (hereafter, Arabidopsis)5. The phenomenon by which individual genes are lost from one of the two homeologous chromosome pairs after a whole-genome duplication event is called fractionation6. Somewhat counter-intuitively, it has been demonstrated that fractionation is a biased phenomenon, with a majority of genes being preferentially lost from one of the homeologous chromosomes7. Interestingly, some genes appear to have escaped fractionation, so that two or more gene copies resulting from duplication events have persisted in the genome8. While retention of some gene pairs might be attributed to subfunctionalization, the gene balance hypothesis accounts for the retention in other cases. Indeed, the retained genes have been found to preferentially belong to certain over-represented functional categories7, which typically comprise proteins that are dosage-dependent or form part of multisubunit complexes, often with regulatory roles, suggesting that haploinsufficiency has played a major role in their retention. Loss-of-function mutations at haploinsufficient loci are typically dominant because the level of gene function in a heterozygote is below the threshold to produce a wild-type phenotype.
The eukaryotic ribosome represents an excellent model for testing the gene balance hypothesis, as it consists of a large subunit (60S) with 46 ribosomal proteins and 3 ribosomal RNA (rRNA) molecules (25S, 5.8S and 5S), and a small subunit (40S) with 33 ribosomal proteins and a single 18S rRNA molecule9. The structure of the prokaryotic and eukaryotic ribosomes has been determined at an atomic-level resolution10,11,12,13,14,15. Prokaryotic and eukaryotic ribosomes share a general macroorganization but they differ in size and number of ribosomal proteins and rRNA molecules. In Arabidopsis, all cytosolic ribosomal proteins are encoded by small gene families, each with 2 to 7 members16. Although 249 genes encoding ribosomal proteins have been annotated in the Arabidopsis genome, a maximum of 80 different ribosomal proteins has been identified in isolated 80S ribosomes17,18,19.
Here, we report the isolation of mutant alleles of two genes, APICULATA2 (API2) and its closest paralog (i.e. homologous gene resulting from a duplication event) in the Arabidopsis genome, At3g23390. These genes encode two identical ribosomal proteins, RPL36aB and RPL36aA, despite they carry nucleotide substitutions at 34% of their codons. Loss-of-function mutations at each of these unlinked loci are recessive and affect leaf development in a similar way. We found that the api2 and rpl36aa mutations represent a rare example of non-allelic non-complementation, indicating that two (out of the four) functional gene copies present in a diploid plant are not sufficient to produce a wild-type phenotype. We demonstrate that both paralogous genes are expressed in the same tissues and show that both loci behave as a single haploinsufficient unit with four alleles (combined haploinsufficiency) on which natural selection has acted to filter out deleterious non-synonymous substitutions (i.e. nucleotide substitutions that change the protein sequence), a process known as purifying selection.
Results
Cloning and molecular characterization of the api2 mutation
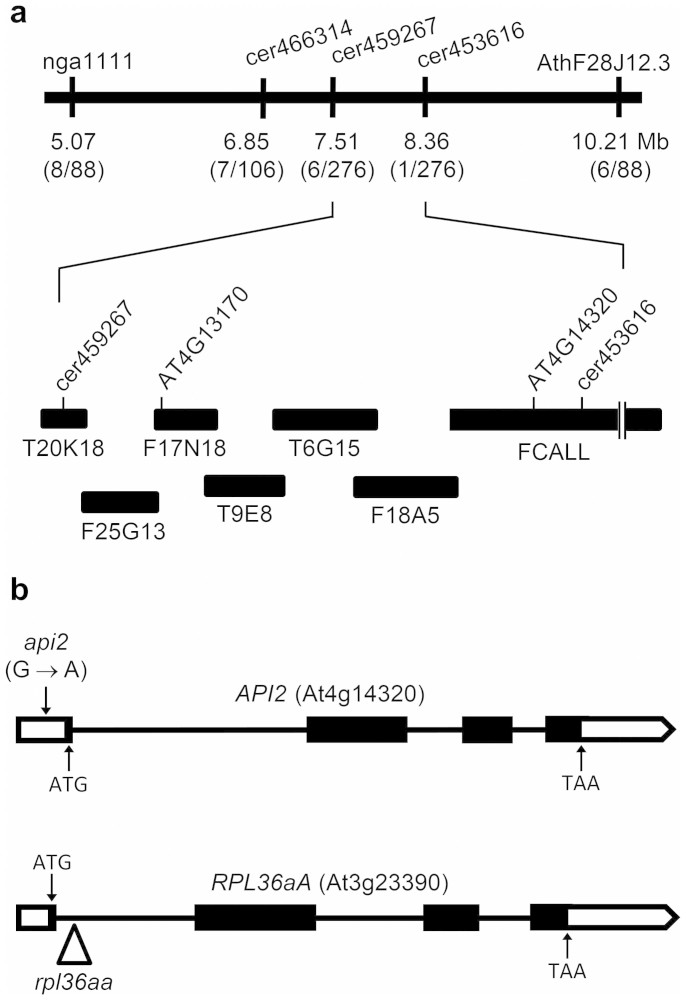
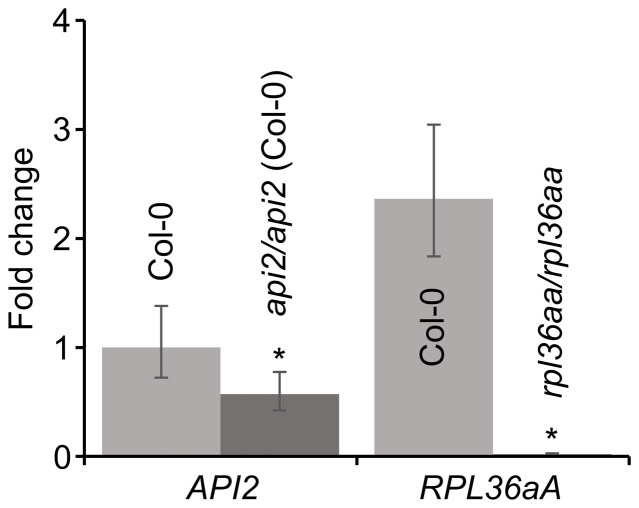
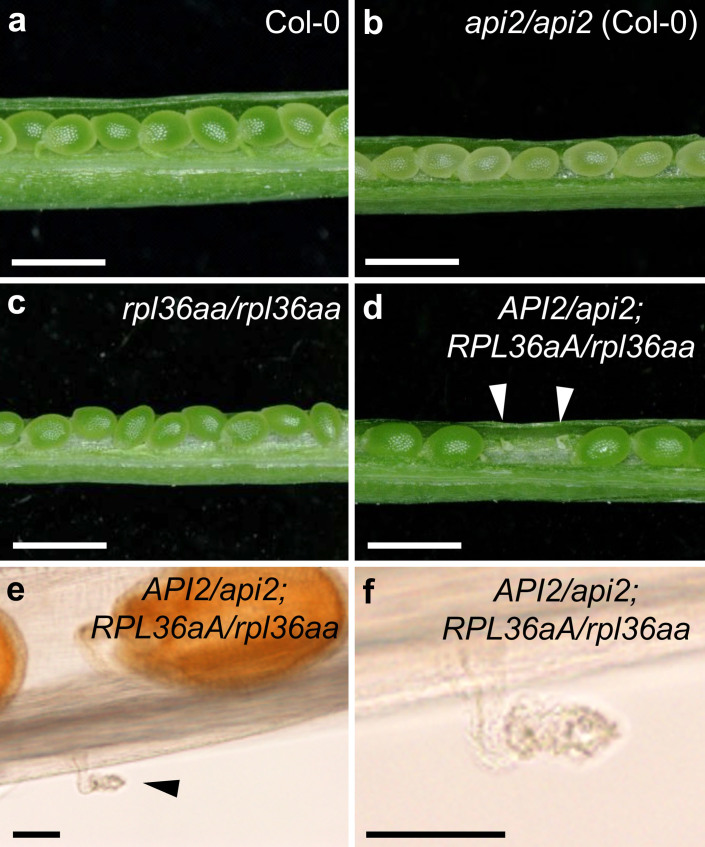
In a large-scale screen for ethylmethane sulfonate (EMS)-induced mutants with abnormal leaf shape, we previously isolated 42 mutants with a pointed-leaf phenotype. These mutants were assigned to phenotypic classes dubbed Apiculata (Api), Denticulata (Den) and Angusta (Ang)20,21. The Api class comprises recessive alleles of seven different genes, named API1 to API720. Here, we focus on the API2 gene, which was initially defined by a single mutant allele, api2. To identify API2 at the molecular level, we undertook a positional approach. We first mapped the api2 mutation to an 850-kb interval on chromosome 4, flanked by two insertion-deletion (InDel) markers, cer459267 and cer453616 (Figure 1a), encompassing 248 annotated genes. Because the api2/api2 mutant has pointed leaves (Figure 2b, d, g, i), and is very similar in phenotype to other mutants affected in genes that encode ribosomal proteins22,23,24,25,26,27, we focused on the only two genes within the interval that encode subunits of the cytosolic ribosome, At4g13170 and At4g14320. After sequencing the transcriptional unit of both genes, we found a single G-to-A transition mutation in the 5′ untranslated region (UTR) of the At4g14320 gene (Figure 1b and Supplementary Figure S1), also known as RPL36aB17. This mutation creates an out-of-frame ATG codon upstream of the normal translation start site of RPL36aB transcripts (Supplementary Figure S1). We used real-time qRT-PCR to measure the relative RPL36aB expression level in rosettes collected 14 days after stratification (das), and found a 0.57-fold decrease in api2/api2 plants compared with the wild type (Figure 3). To determine whether the lesion in RPL36aB is responsible for the mutant phenotype, we followed a transgenic approach to complement the api2 mutation. The phenotype of api2/api2 plants expressing a 35Spro:RPL36aB transgene was indistinguishable from wild type in 4 independent lines (Figure 2l, o), indicating that loss of RPL36aB function causes the mutant phenotype, either by the observed down-regulation of transcript levels or because the api2 mutation interferes with normal translation initiation.
Figure 1. Positional cloning of the API2 gene and molecular nature and position of the api2 and rpl36aa mutations.
(a) A mapping population of 138 F2 plants derived from an api2/api2 × Col-0 outcross was used to define a candidate interval of 850 kb on chromosome 4. The names and map positions of the molecular markers used are indicated. The number of recombinant chromosomes found and the total of chromosomes analyzed are shown in parentheses. Black boxes represent BAC clones. (b) Structure of the API2 and RPL36aA paralogous genes, with indication of the nature and position of the api2 and rpl36aa mutations. A triangle indicates the position of a T-DNA insertion. Boxes and lines between boxes indicate exons and introns, respectively. Open boxes correspond to the 5′ and 3′ untranslated regions. The predicted translation start (ATG) and stop (TAA) codons are indicated.
Figure 2. Phenotypes and phenotypic rescue of the api2 and rpl36aa mutants.
(a–e), (l–n) Rosettes and (f–j), (o–q) first-node leaves from (a), (f) Ler, (b), (g), (d), (i) api2/api2 (b), (g) in a Ler background and (d), (i) in a Col-0 background, (c), (h) Col-0, (e), (j) rpl36aa/rpl36aa, (l), (o) API2/api2;RPL36aA/rpl36aa, (m), (p) api2/api2;35Spro:RPL36aB and (n), (q) rpl36aa/rpl36aa;35Spro:RPL36aA plants. Arrowheads indicate marginal teeth in mutant pointed leaves. (k) Adult plants. All plants are in a Col-0 background, the only exceptions being (b), (g) the api2 mutant and (a), (f) the Ler wild type. Pictures were taken (a–j), (l–q) 14 and (k) 35 das. Scale bars indicate (a–j), (l–q) 2 mm and (k) 3 cm.
Figure 3. Expression of the API2 and RPL36aA genes in the Col-0 wild type and the api2/api2 and rpl36aa/rpl36aa mutants.
Real-time qRT-PCR analysis of the expression of the API2 and RPL36aA genes was performed in api2/api2 and rpl36aa/rpl36aa vegetative leaves. Relative expression levels are shown, determined by the comparative CT method, and normalized with the expression of the 18S rRNA housekeeping gene. Error bars indicate the interval delimited by 2−(ΔΔCT ± SD). Asterisks indicate ΔCT values significantly different from those of Col-0 in a Mann-Whitney U test (p < 0.01; n = 9).
Two paralogous genes encode the RPL36a protein in Arabidopsis
The Arabidopsis genome contains two RPL36a paralogous genes that encode identical proteins, RPL36aA (At3g23390) and the above-mentioned RPL36aB (hereafter, API2)17. We also searched for mutant alleles of RPL36aA, and identified a publicly available T-DNA insertion line (SALK_148438) that, when homozygous for the insertion, exhibited a pointed-leaf phenotype identical to that caused by the api2 mutation in the Col-0 genetic background (Figure 2d, e, i, j). We refer to this line as rpl36aa/rpl36aa. The presence of the T-DNA insertion in the first intron of RPL36aA was confirmed by PCR genotyping (Figure 1b). The inflorescences of rpl36aa/rpl36aa and api2/api2 plants were shorter than those of wild-type plants (Figure 2k). We used real-time qRT-PCR to measure the relative RPL36aA expression level in rpl36aa/rpl36aa rosettes collected 14 das, and only found a residual expression level (Figure 3), suggesting that rpl36aa is a null allele. To demonstrate that the insertion causes the defects observed in the rpl36aa line, we also followed a transgenic approach to complement its mutant phenotype. Four independent rpl36aa/rpl36aa;35Spro:RPL36aA transgenic lines exhibited a wild-type leaf phenotype (Figure 2m, p), confirming that the mutant phenotype results from the inactivation of RPL36aA. We concluded that identical recessive leaf mutant phenotypes occur when either API2 or RPL36aA are inactivated.
API2 and RPL36aA are dosage-dependent genes
Because the API2 and RPL36aA proteins have identical amino acid sequences (Supplementary Figure S2), they are expected to perform identical functions in the ribosome, offering a unique opportunity to ask questions about the functional redundancy and haploinsufficiency of paralogous ribosomal proteins, with no interference due to the divergence of their sequences. The api2/api2 and rpl36aa/rpl36aa single mutants are viable, suggesting that both genes contribute quantitatively to a global pool of RPL36a ribosome subunits and that two functional copies of any one gene can alleviate the effects of having two inactive copies of its paralog. We hypothesized that the simultaneous loss of one copy of each locus should also lead to a mutant phenotype similar to those of api2/api2 or rpl36aa/rpl36aa single mutants. We crossed the single mutants and found that all the doubly heterozygous plants (API2/api2;RPL36aA/rpl36aa) in the F1 progeny exhibited a pointed-leaf phenotype identical to those of the api2/api2 and rpl36aa/rpl36aa homozygous parentals (Figure 2n, q). This non-allelic non-complementation involving hypomorphic28 (partial loss-of-function) alleles of API2 and RPL36aA requires that both genes play redundant functions and are co-expressed in at least some cells.
In order to investigate the effects of carrying less than two wild-type alleles, we genotyped 169 mutant plants from an F2 family segregating mutant alleles of both loci. We did not find plants with a single functional copy of either gene (API2/api2;rpl36aa/rpl36aa or api2/api2;RPL36aA/rpl36aa) or lacking wild-type copies (api2/api2;rpl36aa/rpl36aa), despite these genotypes are collectively expected to appear in 31.25% (5 out of 16) of the F2 plants. Interestingly, the progeny of self-pollinated API2/api2;RPL36aA/rpl36aa plants segregated an excess of aborted ovules (Figure 4), with a 13% increase relative to the single mutants and the wild type.
Figure 4. Aborted ovules in API2/api2;RPL36aA/rpl36aa siliques.
(a–d) Dissected mature siliques from (a) Col-0, (b) api2/api2 (Col-0), (c) rpl36aa/rpl36aa and (d) API2/api2;RPL36aA/rpl36aa plants. (e), (f) Detail of an aborted ovule in an API2/api2;RPL36aA/rpl36aa cleared silique. Arrowheads in (d) and (e) indicate aborted ovules. Scale bars indicate (a–d) 1 mm and (e), (f) 100 μm.
To test any male- or female-gametophytic lethality, we performed reciprocal crosses between doubly heterozygous API2/api2;RPL36aA/rpl36aa and Col-0 plants. When we pollinated API2/api2;RPL36aA/rpl36aa pistils with Col-0 wild-type pollen, we observed an average 23% excess of aborted ovules in the siliques relative to the values obtained in control crosses, indicating female gametophytic lethality. Reciprocally, we also pollinated Col-0 pistils with pollen from API2/api2;RPL36aA/rpl36aa plants. If the four possible types of haploid male gametophytes are equally transmitted to the progeny, then one fourth of the F1 plants should have the same genotype (API2/api2;RPL36aA/rpl36aa) and exhibit the same mutant phenotype as their male parental. However, all 140 F1 plants studied showed a wild-type phenotype, a result that indicates that the api2 rpl36aa gametophytes are not viable.
RPL36a genes are subjected to strong purifying selection
The identical protein sequences encoded by different RPL36a paralogous genes might occur as a consequence of their recent evolutionary origin; alternatively, they might be explained by concerted evolution (e.g. gene conversion) or by strong purifying selection29. The coding regions of both API2 and RPL36aA transcripts consist of 105 codons. A pairwise alignment of the API2 and RPL36aA coding sequences uncovered 36 nucleotide substitutions, each affecting a different codon (Supplementary Figure S1). All these substitutions were synonymous, affecting the third base of a codon in 35 cases and the first base only in 1 case (Supplementary Figure S2). Because the affected codons were scattered along the sequence, this level of divergence is unlikely to have arisen as a consequence of concerted evolution or a recent duplication. We determined the rates of synonymous (dS) and non-synonymous substitutions (dN) from a pairwise alignment of the API2 and RPL36aA coding sequences. The low value of the dN/dS ratio (Table 1) is a signature of strong purifying selection, and is in line with the values obtained for other pairs of paralogous genes encoding identical ribosomal proteins.
Table 1. Selected ribosomal protein paralogs and their synonymous and non-synonymous substitution rates.
| Paralog pairs | AGI gene codes | Protein length (aa)* | Amino acid sequence identity (%) | Nucleotide sequence identity (%) | dS | dN | dN/dS |
|---|---|---|---|---|---|---|---|
| API2, RPL36aA | At4g14320, At3g23390 | 105 | 100 | 89 | 1.1338 | 0.0011 | 0.0010 |
| RPS18A, RPS18C | At1g22780, At4g09800 | 152 | 100 | 88 | 1.1036 | 0.0056 | 0.0051 |
| RPS28A, RPS28B | At3g10090, At5g03850 | 64 | 100 | 88 | 0.9059 | 0.0009 | 0.0010 |
| RPL21A, RPL21D | At1g57860, At1g57660 | 164 | 100 | 99 | 0.0339 | 0.0000 | 0.0010 |
| RPL38A, RPL38B | At2g43460, At3g59540 | 69 | 100 | 90 | 0.7566 | 0.0008 | 0.0010 |
| RPL41A, RPL41B | At2g40205, At3g08520 | 25 | 100 | 95 | 0.3252 | 0.0003 | 0.0010 |
| RPS13A, RPS13B | At4g00100, At3g60770 | 151 | 99 | 85 | 1.5649 | 0.0029 | 0.0018 |
| RPS21A, RPS21B | At3g53890, At5g27700 | 82 | 94 | 83 | 3.9235 | 0.0297 | 0.0076 |
| RPL11A, RPL11C | At2g37190, At5g60670 | 166 | 93 | 81 | 1.0114 | 0.0572 | 0.0566 |
| RPL28A, RPL28B | At2g19730, At4g29410 | 143 | 90 | 85 | 0.7896 | 0.0597 | 0.0756 |
*All paralog pairs shown in this table have identical protein length.
API2 and RPL36aA are expressed in overlapping patterns
As mentioned above, the non-allelic non-complementation between API2 and RPL36aA is best understood when there is at least some overlap of expression in the same cells. To experimentally demonstrate this, we fused the promoter regions of both genes to the GUS reporter gene. GUS staining of 5 API2pro:GUS and 5 RPL36aApro:GUS independent transgenic lines in the Col-0 background uncovered an identical expression pattern for both genes. Both constructs were ubiquitously expressed, with particularly intense GUS staining in young and actively proliferating tissues, such as those of developing leaves, floral buds and root apices (Figure 5). Interestingly, the relative intensities of the GUS staining matched our qRT-PCR results (Figure 3), with the RPL36aA promoter conferring higher expression levels in wild-type vegetative leaves than the API2 promoter.
Figure 5. Visualization of API2pro:GUS and RPL36aApro:GUS transgene activity in the Col-0 background.
(a), (m) Seedlings and (b), (n) cotyledons. (c–f), (o–r) Vegetative leaves from the (c), (o) first, (d), (p) third (e), (q) fifth and (f), (r) seventh nodes. (g), (s) Inflorescences. (h), (t) Immature and (i), (u) mature flowers. (j), (v) Immature siliques, (k), (w) root tips and (l), (x) mature siliques. Pictures were taken (a), (m) 6, (b–f), (k), (n–r), (w) 14 and (g–j), (l), (s–v), (x) 35 das. Scale bars indicate (a), (c–e), (g), (j), (l), (m), (o–q), (s), (v), (x) 1 mm, (b), (f), (i), (n), (r), (u) 500 μm, (h), (t) 200 μm and (k), (w) 100 μm.
api2 and rpl36aa genetically interact with asymmetric leaves mutants
Some ribosomal proteins are known to be involved in leaf developmental programs, as their mutations enhance the adaxial-abaxial polarity defects of the asymmetric leaves1 (as1) and as2 mutants24,25. Moreover, Horiguchi et al.26 showed that the extent of this enhancement varies among mutants affected in different ribosomal proteins. To determine whether api2 and rpl36aa can enhance the polarity defects of as mutants, we isolated the api2 as1-1, api2 as2-1, rpl36aa as1-1 and rpl36aa as2-1 double mutants. Because both paralogous proteins share an identical sequence and expression pattern, any phenotypic differences observed in these mutants are likely to reflect the contribution of individual paralogs to the pool of RPL36a subunits. Both api2 and rpl36aa enhanced the phenotype of the as2-1 mutant, although to a different extent (Figure 6e–h). Both api2 as2-1 and rpl36aa as2-1 double mutants exhibited trumpet-shaped (peltate) leaves (Figure 6f, g), which we did not observe in the as2-1 single mutant (Figure 6d, e). In addition to this, the rpl36aa as2-1 double mutant exhibited radial leaves (Figure 6h), which can be interpreted as a consequence of a more severe abaxialization. These phenotypes were not observed in the double mutant combinations involving the as1-1 mutation (Figure 6b, c).
Figure 6. Double mutant combinations of api2 and rpl36aa with as1-1 and as2-1.
(a–d) Rosettes of the (a) as1-1, (b) api2 as1-1, (c) rpl36aa as1-1 and (d) as2-1 mutants. (e–f) Details of vegetative leaves from (e) as2-1, (f) api2 as2-1 and (g), (h) rpl36aa as2-1 mutant plants. All these mutants were homozygous for the mutations indicated. Pictures were taken (a–c) 14 and (d–f) 18 das. Scale bars indicate 1 mm.
Discussion
Following a positional cloning approach, we have determined that the api2 mutant carries a lesion at a gene encoding the RPL36a ribosomal protein. This ribosomal protein is encoded by two paralogous genes, API2 (RPL36aB) and At3g23390 (RPL36aA), which have not previously been characterized in Arabidopsis. Our results indicate that both paralogs are functionally equivalent (redundant), as shown by the non-allelic non-complementation observed in the F1 progeny of crosses involving mutant alleles of both genes. This functional equivalence is also supported by the identical protein sequences and expression patterns conferred by the promoter regions of API2 and its paralog. This non-allelic non-complementation indicates that a critical level of protein function is not reached in doubly heterozygous plants, a quantitative effect that is expected only when the two genes are expressed in the same cells and perform identical function. Although this type of non-complementation is a relatively rare phenomenon, numerous additional instances have been documented in Arabidopsis, often involving ribosomal proteins of the large and small subunits of the cytosolic ribosome25,27,30,31. The observation that loss-of-function mutations damaging ribosomal proteins are usually recessive in Arabidopsis26, with only a few examples of semi-dominance32,33, is in striking contrast with the dominance or haplolethality of loss-of-function (haploinsufficient) Minute mutations of Drosophila melanogaster34. The Minute mutations represent the most abundant phenotypic class in Drosophila and affect genes coding for ribosomal proteins. However, unlike in Arabidopsis, each ribosomal protein is typically encoded by a single gene in the genome of Drosophila34. This fundamental difference reflects how the need to keep a balance between the different ribosomal proteins after a whole-genome duplication has shaped the genome during plant evolution.
Loss of API2 or RPL36aA functions led to a deleterious recessive syndrome that was very similar, not to say undistinguishable, from the phenotypes of many other mutants carrying lesions in genes encoding ribosomal proteins22,24,25,26,30,31,35. Our reciprocal crosses indicate that the simultaneous inactivation of both paralogs causes lethality and prevents transmission of the mutant alleles through the male and female gametophytes, as has also been reported for other pairs of paralogous ribosomal proteins in Arabidopsis36. Indeed, we were not able to isolate plants with fewer than two active (out of the four) copies of the genes encoding the RPL36a ribosomal protein. The observation that only synonymous nucleotide substitutions have accumulated in the coding region of these paralogous genes (none of which changes the protein sequence) suggests that a matching number of non-synonymous changes have also occurred but must have been selected against by strong purifying selection. A similar situation is likely to hold for other ribosomal proteins for which lethality, non-allelic non-complementation and/or dosage effects have been described. The presence of identical sequences in both paralogs (at the amino acid level) is not a peculiarity of the RPL36a paralogy group, as several instances of gene pairs encoding identical ribosomal proteins have been reported in the literature (e.g. RPS18, RPS29, RPS30, RPL11, RPL21, RPL23, RPL38, RPL40 and RPL41)37. We have found that these proteins have also been subjected to strong purifying selection, as inferred from the low dN/dS values for pairs of paralogous genes, highlighting the critical roles played by such proteins in the ribosome. Purifying selection has also been shown to account for the sequence conservation of other proteins that play essential, highly conserved functions in eukaryotes, such as members of the histone H4 protein family29. We propose that combined haploinsufficiency and strong purifying selection have driven the biased retention of genes coding for paralogous ribosomal proteins after the duplication of plant genomes, as has been documented for the Arabidopsis genome7,8. The stoichiometry and structural constrains of a multisubunit complex, such as the ribosome, are likely the ultimate causes behind this retention16.
Because API2 and RPL36aA are identical at the amino acid level, any phenotypic difference between the api2 and rpl36aa loss-of-function mutant alleles should be attributed to differences in the spatio-temporal expression pattern or expression levels of the two genes. In our particular case, we observed that rpl36aa enhanced the polarity defects of the as2-1 mutant (as inferred from the fully radialized lateral organs seen in the rpl36aa as2-1 double mutant) to a greater extent than api2 (as the api2 as2-1 double mutant showed trumpet-shaped, but not fully radialized leaves). Interestingly, we found RPL36aA to be expressed at higher levels than API2 in wild-type plants, suggesting that the stronger effect caused by the rpl36aa mutation is due to the fact that RPL36aA is the main contributor of RPL36a subunits. Our results favor the hypothesis that, at least in plants, the phenotypic differences observed between mutant alleles of paralogous genes, which have been reported in some cases36,38, might result from differences in the expression patterns or the expression levels of individual paralogous proteins, rather than from the differential specificity for certain mRNAs of an heterogeneous population of ribosomes17,19,39,40,41.
Methods
Plant material and growth conditions
Arabidopsis thaliana (L.) Heynh. Columbia-0 (Col-0) and Landsberg erecta (Ler) wild-type accessions and the SALK_148438 line (named here rpl36aa; accession number N648438), were obtained from the Nottingham Arabidopsis Stock Centre (NASC). The api2/api2 mutant was isolated after EMS mutagenesis of Landsberg erecta (Ler) seeds20. To standardize genetic backgrounds, we outcrossed the api2/api2 mutant three times to Col-0 [api2/api2 (Col-0)]. Growth conditions and crosses were performed as described previously20,42.
Map-based cloning
The api2 mutation was low-resolution mapped as described in Ponce et al.43. For fine-mapping, we used the cer466314, cer459267 and cer453616 InDel polymorphisms (Table S1) from Monsanto (http://www.arabidopsis.org/browse/Cereon). To identify the api2 mutation, the entire transcriptional unit of At4g14320 was amplified using the API2_F1 and API2_R1 primers (Table S1). The resulting 1660-bp PCR product was sequenced on an ABI PRISM 3130xl Genetic Analyser (Applied Biosystems). The presence of the T-DNA insertion in rpl36aa was confirmed by PCR using the rpl36aa_F/R primers designed with the T-DNA Primer Design tool (http://signal.salk.edu/tdnaprimers.2.html) (Table S1).
RNA isolation, cDNA synthesis and qRT-PCR
Total RNA from 20–30 mg of rosette leaves, collected 14 das, was isolated using TRI Reagent (Sigma) and first-strand cDNA synthesis and relative expression quantification were performed as described in Jover-Gil et al.44, using the primers listed on Table S1.
Genetic constructs and plant transformation
To make the 35Spro:RPL36aB and 35Spro:RPL36aA transgenes, we amplified the full-length coding sequence of At4g14320 and At3g23390 genes from Col-0 cDNA using Phusion polymerase (Thermo Scientific) and the primers shown in Table S1. The resulting products were cloned into the pENTR/D-TOPO vector (Invitrogen) and transferred into the pMDC32 destination vector45 in a reaction with LR clonase II (Invitrogen). To make the RPL36aBpro:GUS and RPL36aApro:GUS transgenes, 389-bp and 716-bp fragments from the upstream region of the starting codon of At4g14320 and At3g23390 genes, respectively, were amplified from Col-0 genomic DNA using Phusion polymerase and the API2pro_F/R and RPL36aApro_F/R primers, which include the attB1 and attB2 sequences at their 5′ ends (Table S1). The amplification products were cloned into the pGEM-T Easy221 entry vector (kindly provided by B. Scheres), by recombination with BP clonase II (Invitrogen), and then transferred into pMDC163 destination vector45 in an LR clonase II (Invitrogen) reaction. Competent Agrobacterium tumefaciens LBA4404 cells were transformed with these constructs. Col-0, api2 and rpl36aa plants were transformed by the floral dip method46.
GUS assay and microscopy
GUS staining was performed as described in Robles et al.47, with minor modifications. In brief, plant tissue was incubated in ice-cold 90% acetone for 15 min and then in X-Gluc buffer solution (2 mM 5-bromo-4-chloro-3-indolyl-β-glucuronic acid, 50 mM sodium phosphate, pH 7.2, 5 mM potassium ferrocyanide, 50 mM potassium ferricyanide, and 0.2% Triton X-100) for 14 h at room temperature. After GUS detection, the tissue was cleared with 96% ethanol and mounted in an 8:2:1 (chloral hydrate:glycerol:water) solution. Samples were examined with a Nikon D-Eclipse C1 microscope.
Bioinformatic analysis of selection
To determine the impact of selection, we calculated synonymous (dS) and non-synonymous (dN) substitution rates for pairs of aligned sequences using the codeml program of the PAML (Phylogenetic Analysis by Maximum Likelihood) package48, as implemented in the PAL2NAL program (http://www.bork.embl.de/pal2nal/)49.
Author Contributions
H.C. and J.L.M. conceived and designed the research. R.C.S. and H.C. performed the research. R.C.S, H.C. and J.L.M. wrote the article.
Supplementary Material
Supplementary information
Acknowledgments
We wish to thank J.M. Serrano, F.M. Lozano, T. Trujillo, L. Serna and J.M. Sánchez-Larrosa for their excellent technical assistance. Research in the laboratory of J.L.M. is supported by grants from the Ministerio de Economía y Competitividad of Spain [BFU2011-22825 and CSD2007-00057 (TRANSPLANTA)], the Generalitat Valenciana (PROMETEO/2009/112) and the European Commission [LSHG-CT-2006-037704 (AGRON-OMICS)]. H.C. is a recipient of a Marie Curie International Reintegration Grant (PIRG03-GA-2008-231073). R.C.S. holds a fellowship from the Ministerio de Economía y Competitividad of Spain (BES-2009-014106).
References
- Soltis D. E. et al. Polyploidy and angiosperm diversification. Am. J. Bot. 96, 336–348 (2009). [DOI] [PubMed] [Google Scholar]
- Force A. et al. Preservation of duplicate genes by complementary, degenerative mutations. Genetics 151, 1531–1545 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birchler J. A. & Veitia R. A. The gene balance hypothesis: from classical genetics to modern genomics. Plant Cell 19, 395–402 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birchler J. A. & Veitia R. A. Gene balance hypothesis: connecting issues of dosage sensitivity across biological disciplines. Proc. Natl. Acad. Sci. USA 109, 14746–14753 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simillion C., Vandepoele K., Van Montagu M. C., Zabeau M. & Van de Peer Y. The hidden duplication past of Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 99, 13627–13632 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langham R. J. et al. Genomic duplication, fractionation and the origin of regulatory novelty. Genetics 166, 935–945 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas B. C., Pedersen B. & Freeling M. Following tetraploidy in an Arabidopsis ancestor, genes were removed preferentially from one homeolog leaving clusters enriched in dose-sensitive genes. Proc. Natl. Acad. Sci. USA 16, 934–946 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeling M. Bias in plant gene content following different sorts of duplication: tandem, whole-genome, segmental, or by transposition. Annu. Rev. Plant Biol. 60, 433–453 (2009). [DOI] [PubMed] [Google Scholar]
- Wilson D. N. & Doudna Cate J. H. The structure and function of the eukaryotic ribosome. Cold Spring Harb. Perspect. Biol. 4, a011536 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimberly B. T. et al. Structure of the 30S ribosomal subunit. Nature 407, 327–339 (2000). [DOI] [PubMed] [Google Scholar]
- Ban N., Nissen P., Hansen J., Moore P. B. & Steitz T. A. The complete atomic structure of the large ribosomal subunit at 2.4 Å resolution. Science 289, 905–920 (2000). [DOI] [PubMed] [Google Scholar]
- Yusupov M. M. et al. Crystal structure of the ribosome at 5.5 Å resolution. Science 292, 883–896 (2001). [DOI] [PubMed] [Google Scholar]
- Ben-Shem A. et al. The structure of the eukaryotic ribosome at 3.0 Å resolution. Science 334, 1524–1529 (2011). [DOI] [PubMed] [Google Scholar]
- Rabl J., Leibundgut M., Ataide S. F., Haag A. & Ban N. Crystal structure of the eukaryotic 40S ribosomal subunit in complex with initiation factor 1. Science 331, 730–736 (2011). [DOI] [PubMed] [Google Scholar]
- Klinge S., Voigts-Hoffmann F., Leibundgut M., Arpagaus S. & Ban N. Crystal structure of the eukaryotic 60S ribosomal subunit in complex with initiation factor 6. Science 334, 941–948 (2011). [DOI] [PubMed] [Google Scholar]
- Barakat A. et al. The organization of cytoplasmic ribosomal protein genes in the Arabidopsis genome. Plant Physiol. 127, 398–415 (2001). [PMC free article] [PubMed] [Google Scholar]
- Carroll A. J., Heazlewood J. L., Ito J. & Millar A. H. Analysis of the Arabidopsis cytosolic ribosome proteome provides detailed insights into its components and their post-translational modification. Mol. Cell. Proteomics 7, 347–369 (2008). [DOI] [PubMed] [Google Scholar]
- Chang I. F., Szick-Miranda K., Pan S. & Bailey-Serres J. Proteomic characterization of evolutionarily conserved and variable proteins of Arabidopsis cytosolic ribosomes. Plant Physiol. 137, 848–862 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giavalisco P. et al. High heterogeneity within the ribosomal proteins of the Arabidopsis thaliana 80S ribosome. Plant Mol. Biol. 57, 577–591 (2005). [DOI] [PubMed] [Google Scholar]
- Berná G., Robles P. & Micol J. L. A mutational analysis of leaf morphogenesis in Arabidopsis thaliana. Genetics 152, 729–742 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano-Cartagena J., Robles P., Ponce M. R. & Micol J. L. Genetic analysis of leaf form mutants from the Arabidopsis Information Service collection. Mol. Gen. Genet. 261, 725–739 (1999). [DOI] [PubMed] [Google Scholar]
- Van Lijsebettens M. et al. An S18 ribosomal protein gene copy at the Arabidopsis PFL locus affects plant development by its specific expression in meristems. EMBO J 13, 3378–3388 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T., Kim G. T. & Shinozaki K. Disruption of an Arabidopsis cytoplasmic ribosomal protein S13-homologous gene by transposon-mediated mutagenesis causes aberrant growth and development. Plant J. 22, 257–264 (2000). [DOI] [PubMed] [Google Scholar]
- Pinon V. et al. Three PIGGYBACK genes that specifically influence leaf patterning encode ribosomal proteins. Development 135, 1315–1324 (2008). [DOI] [PubMed] [Google Scholar]
- Yao Y., Ling Q., Wang H. & Huang H. Ribosomal proteins promote leaf adaxial identity. Development 135, 1325–1334 (2008). [DOI] [PubMed] [Google Scholar]
- Horiguchi G. et al. Differential contributions of ribosomal protein genes to Arabidopsis thaliana leaf development. Plant J. 65, 724–736 (2011). [DOI] [PubMed] [Google Scholar]
- Creff A., Sormani R. & Desnos T. The two Arabidopsis RPS6 genes, encoding for cytoplasmic ribosomal proteins S6, are functionally equivalent. Plant Mol. Biol. 73, 533–546 (2010). [DOI] [PubMed] [Google Scholar]
- Muller H. J. Further studies on the nature and causes of gene mutations. Proceedings of the Sixth International Congress of Genetics, Ithaca, New York. 1, 213–255 (1932). [Google Scholar]
- Piontkivska H., Rooney A. P. & Nei M. Purifying selection and birth-and-death evolution in the histone H4 gene family. Mol. Biol. Evol. 19, 689–697 (2002). [DOI] [PubMed] [Google Scholar]
- Rosado A. et al. Auxin-mediated ribosomal biogenesis regulates vacuolar trafficking in Arabidopsis. Plant Cell 22, 143–158 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujikura U., Horiguchi G., Ponce M. R., Micol J. L. & Tsukaya H. Coordination of cell proliferation and cell expansion mediated by ribosome-related processes in the leaves of Arabidopsis thaliana. Plant J. 59, 499–508 (2009). [DOI] [PubMed] [Google Scholar]
- Imai A., Komura M., Kawano E., Kuwashiro Y. & Takahashi T. A semi-dominant mutation in the ribosomal protein L10 gene suppresses the dwarf phenotype of the acl5 mutant in Arabidopsis thaliana. Plant J. 56, 881–890 (2008). [DOI] [PubMed] [Google Scholar]
- Weijers D. et al. An Arabidopsis Minute-like phenotype caused by a semi-dominant mutation in a RIBOSOMAL PROTEIN S5 gene. Development 128, 4289–4299 (2001). [DOI] [PubMed] [Google Scholar]
- Marygold S. J. et al. The ribosomal protein genes and Minute loci of Drosophila melanogaster. Genome Biol. 8, R216 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szakonyi D. & Byrne M. E. Ribosomal protein L27a is required for growth and patterning in Arabidopsis thaliana. Plant J. 65, 269–281 (2011). [DOI] [PubMed] [Google Scholar]
- Degenhardt R. F. & Bonham-Smith P. C. Arabidopsis ribosomal proteins RPL23aA and RPL23aB are differentially targeted to the nucleolus and are disparately required for normal development. Plant Physiol. 147, 128–142 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll A. J. The Arabidopsis cytosolic tibosomal proteome: from form to function. Front. Plant Sci. 4, 32 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcone Ferreyra M. L., Casadevall R., Luciani M. D., Pezza A. & Casati P. New evidence for differential roles of l10 ribosomal proteins from Arabidopsis. Plant Physiol. 163, 378–391 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komili S., Farny N. G., Roth F. P. & Silver P. A. Functional specificity among ribosomal proteins regulates gene expression. Cell 131, 557–571 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauro V. P. & Edelman G. M. The ribosome filter hypothesis. Proc. Natl. Acad. Sci. USA 99, 12031–12036 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiguchi G., Van Lijsebettens M., Candela H., Micol J. L. & Tsukaya H. Ribosomes and translation in plant developmental control. Plant Sci. 191–192, 24–34 (2012). [DOI] [PubMed] [Google Scholar]
- Ponce M. R., Quesada V. & Micol J. L. Rapid discrimination of sequences flanking and within T-DNA insertions in the Arabidopsis genome. Plant J. 14, 497–501 (1998). [DOI] [PubMed] [Google Scholar]
- Ponce M. R., Robles P., Lozano F. M., Brotons M. A. & Micol J. L. in Arabidopsis protocols, 2nd edition edited by Salinas J., & Sánchez-Serrano J. J., eds. (Humana Press, 2006), pp. 105–113. [Google Scholar]
- Jover-Gil S. et al. The microRNA pathway genes AGO1, HEN1 and HYL1 participate in leaf proximal-distal, venation and stomatal patterning in Arabidopsis. Plant Cell Physiol. 53, 1322–1333 (2012). [DOI] [PubMed] [Google Scholar]
- Curtis M. D. & Grossniklaus U. A gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiol. 133, 462–469 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough S. J. & Bent A. F. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743 (1998). [DOI] [PubMed] [Google Scholar]
- Robles P. et al. The RON1/FRY1/SAL1 gene is required for leaf morphogenesis and venation patterning in Arabidopsis. Plant Physiol. 152, 1357–1372 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman N. & Yang Z. A codon-based model of nucleotide substitution for protein-coding DNA sequences. Mol. Biol. Evol. 11, 725–736 (1994). [DOI] [PubMed] [Google Scholar]
- Suyama M., Torrents D. & Bork P. PAL2NAL: robust conversion of protein sequence alignments into the corresponding codon alignments. Nucleic Acids Res. 34, W609–612 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information