Abstract
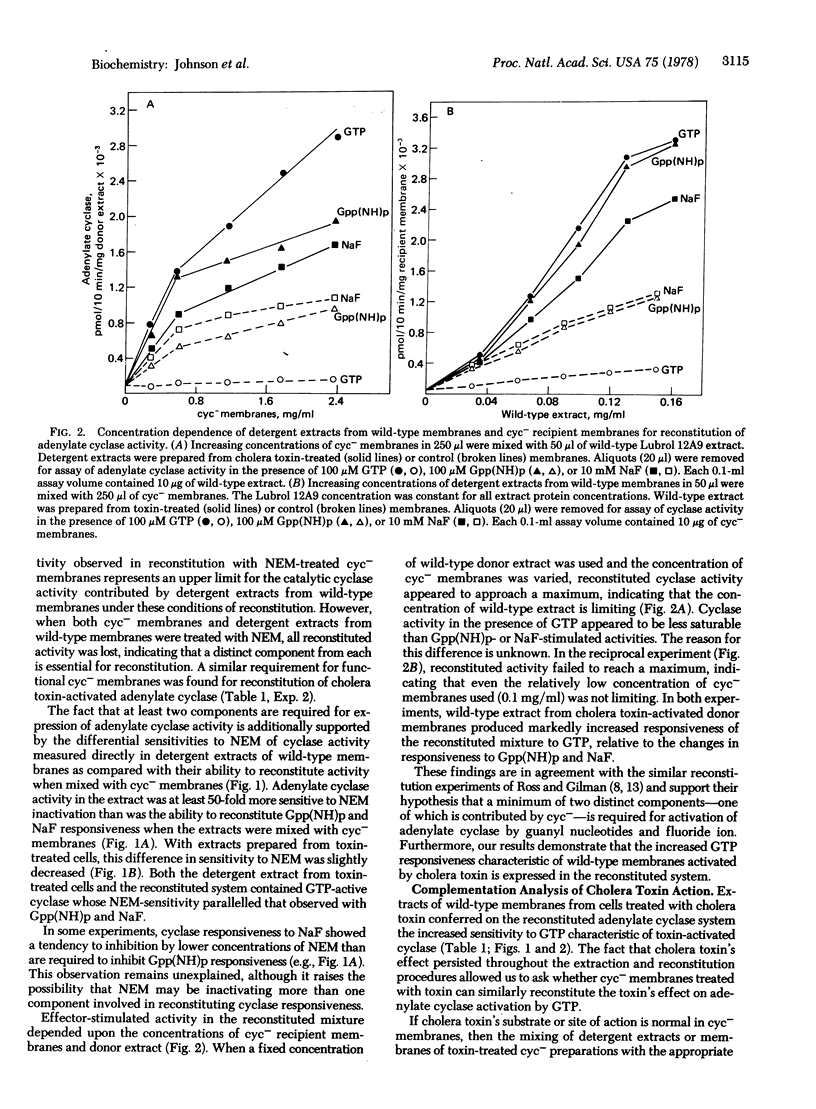
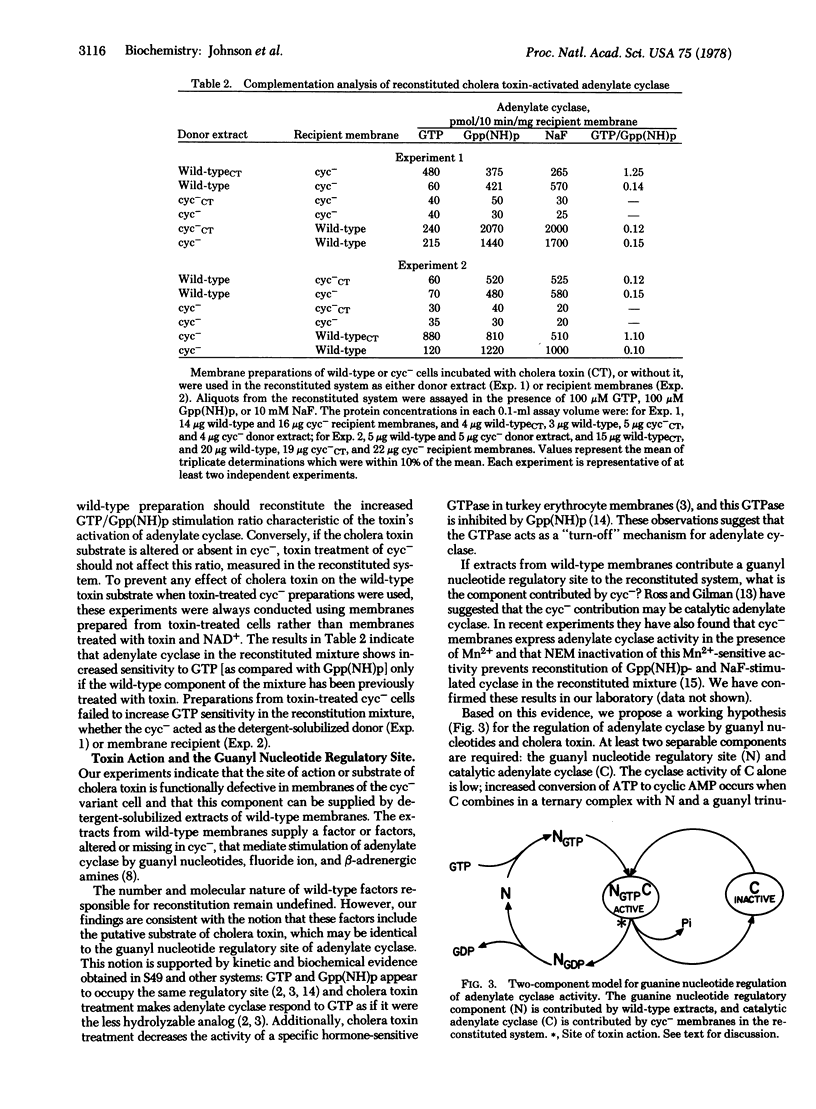
Reconstitution of adenylate cyclase activity responsive to stimulation by guanylyl-5'imidodiphosphate or NaF may be achieved by mixing dilute Lubrol 12A9-solubilized extracts of wild-type S49 membranes with membranes of an adenylate cyclase-deficient variant. Experiments using N-ethylmaleimide to inactivate components of the adenylate cyclase system indicate that distinct components from both wild-type detergent extracts and adenylate cyclase-deficient membranes are essential for reconstitution. These results and conclusions confirm those of E. M. Ross and A. G. Gilman [J. Biol. Chem. (1977) 252, 6966-6969]. Detergent extracts of cholera toxin-treated wild-type membranes yield a reconstituted adenylate cyclase as responsive to GTP as to guanylyl-5'-imidodiphosphate whereas, in the absence of cholera toxin treatment, GTP has little or no effect. Cholera toxin-treated adenylate cyclase-deficient membranes and Lubrol 12A9 extracts from them, however, fail to yield a reconstituted adenylate cyclase that responds to GTP with an increase in cyclase activity. Because treatment of the adenylate cyclase-deficient variants with cholera toxin is without effect on the reconstituted cyclase, we propose that the cholera toxin substrate is absent or altered in the adenylate cyclase-deficient phenotype.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bitensky M. W., Wheeler M. A., Mehta H., Miki N. Cholera toxin activation of adenylate cyclase in cancer cell membrane fragments. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2572–2576. doi: 10.1073/pnas.72.7.2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne H. R., Coffino P., Tomkins G. M. Selection of a variant lymphoma cell deficient in adenylate cyclase. Science. 1975 Feb 28;187(4178):750–752. doi: 10.1126/science.163487. [DOI] [PubMed] [Google Scholar]
- Cassel D., Selinger Z. Activation of turkey erythrocyte adenylate cyclase and blocking of the catecholamine-stimulated GTPase by guanosine 5'-(gamma-thio) triphosphate. Biochem Biophys Res Commun. 1977 Aug 8;77(3):868–873. doi: 10.1016/s0006-291x(77)80058-2. [DOI] [PubMed] [Google Scholar]
- Cassel D., Selinger Z. Mechanism of adenylate cyclase activation by cholera toxin: inhibition of GTP hydrolysis at the regulatory site. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3307–3311. doi: 10.1073/pnas.74.8.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffino P., Bourne H. R., Tomkins G. M. Somatic genetic analysis of cyclic AMP action: selection of unresponsive mutants. J Cell Physiol. 1975 Jun;85(3):603–610. doi: 10.1002/jcp.1040850312. [DOI] [PubMed] [Google Scholar]
- Constantopoulos A., Najjar V. A. The activation of adenylate cyclase. II. The postulated presence of (A) adenylate cyclase in a phospho (inhibited) form (B) a dephospho (activated) form with a cyclic adenylate stimulated membrane protein kinase. Biochem Biophys Res Commun. 1973 Aug 6;53(3):794–799. doi: 10.1016/0006-291x(73)90162-9. [DOI] [PubMed] [Google Scholar]
- Gill D. M. Involvement of nicotinamide adenine dinucleotide in the action of cholera toxin in vitro. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2064–2068. doi: 10.1073/pnas.72.6.2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honjo T., Nishizuka Y., Hayaishi O. Adenosine diphosphoribosylation of aminoacyl transferase II by diphtheria toxin. Cold Spring Harb Symp Quant Biol. 1969;34:603–608. doi: 10.1101/sqb.1969.034.01.069. [DOI] [PubMed] [Google Scholar]
- Johnson G. L., Bourne H. R. Influence of cholera toxin on the regulation of adenylate cyclase by GTP. Biochem Biophys Res Commun. 1977 Sep 23;78(2):792–798. doi: 10.1016/0006-291x(77)90249-2. [DOI] [PubMed] [Google Scholar]
- Moss J., Manganiello V. C., Vaughan M. Hydrolysis of nicotinamide adenine dinucleotide by choleragen and its A protomer: possible role in the activation of adenylate cyclase. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4424–4427. doi: 10.1073/pnas.73.12.4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss J., Vaughan M. Mechanism of action of choleragen. Evidence for ADP-ribosyltransferase activity with arginine as an acceptor. J Biol Chem. 1977 Apr 10;252(7):2455–2457. [PubMed] [Google Scholar]
- Najjar V. A., Constantopoulos A. The activation of adenylate cyclase. I. A postulated mechanism for fluoride and hormone activation of adenylate cyclase. Mol Cell Biochem. 1973 Nov 15;2(1):87–93. doi: 10.1007/BF01738682. [DOI] [PubMed] [Google Scholar]
- Raeburn S., Goor R. S., Schneider J. A., Maxwell E. S. Interaction of aminoacyl transferase II and guanosine triphosphate: inhibition by diphtheria toxin and nicotinamide adenine dinucleotide. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1428–1434. doi: 10.1073/pnas.61.4.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross E. M., Gilman A. G. Reconstitution of catecholamine-sensitive adenylate cyclase activity: interactions of solubilized components with receptor-replete membranes. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3715–3719. doi: 10.1073/pnas.74.9.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross E. M., Gilman A. G. Resolution of some components of adenylate cyclase necessary for catalytic activity. J Biol Chem. 1977 Oct 25;252(20):6966–6969. [PubMed] [Google Scholar]
- Ross E. M., Maguire M. E., Sturgill T. W., Biltonen R. L., Gilman A. G. Relationship between the beta-adrenergic receptor and adenylate cyclase. J Biol Chem. 1977 Aug 25;252(16):5761–5775. [PubMed] [Google Scholar]
- Skogerson L., Moldave K. Characterization of the interaction of aminoacyltransferase II with ribosomes. Binding of transferase II and translocation of peptidyl transfer ribonucleic acid. J Biol Chem. 1968 Oct 25;243(20):5354–5360. [PubMed] [Google Scholar]


