Abstract
Hypothesis:
Absence of normal posterior alpha rhythm is an indirect indicator of seizure disorder.
Materials and Methods:
Study group consists of 116 child and adolescent patients in the age range of 5-17 years, with established history of seizure disorder. Follow-up cases of seizure disorder formed first comparison group, patients with a history of pseudo-seizures formed second comparison group and patients with a history of headache formed the third comparison group.
Results:
In significant 48.3% (56) patients within the study group there was no visible alpha rhythm. Whereas, this absent alpha rhythm criteria was seen in only 11.2% (4) patients in first and 15% (8) patients in second and in only 6.1% (2) patients in third comparison groups.
Discussion:
Absent alpha rhythm- a criterion seems to have a certain amount of specificity for electroencephalograms (EEGs) with seizure disorder patients. Presence of seizure activity and absence of alpha activity in EEG significantly correlated to each other (Significant at < 0.01 level). Absent alpha rhythm appears to be a state marker rather than a trait marker of seizure disorder.
Keywords: Absent alpha, adolescent seizure disorder, alpha rhythm
INTRODUCTION
Alpha rhythm has been defined as “a rhythm at 8-13 Hz occurring during wakefulness over the posterior regions of the head, generally with higher voltage over the occipital areas. Amplitude is variable but is mostly below 50 μV in adults. It is best seen with eyes closed and under conditions of physical relaxation and relative mental inactivity. It is blocked or attenuated by attention, especially visual and mental effort.”[1] One should here note the difference between the terms alpha rhythm and alpha activity: Alpha activity refers to activity in the range of 8-13 Hz and alpha rhythm is the activity of 8-13 Hz with specific characteristics as defined above.[2]
It was a preliminary observation by the author that in addition to standard expression of seizure pattern, some electroencephalograms (EEGs) showed complete absence of “normal posterior alpha rhythm”, in patients with a history of seizure disorder. In addition, absence of “normal posterior alpha rhythm” was also seen in few reportedly “normal EEGs” of established epileptic patients. This observation has led to the formulation of a hypothesis that “absence of normal posterior alpha rhythm is an indirect indicator of seizure disorder.” Thus, present study aims to validate/nullify above hypothesis through visual EEG analysis report of child and adolescent patients with a history of seizure disorder.
MATERIALS AND METHODS
The study was conducted at the EEG lab of Universal College of Medical Sciences-Teaching Hospital, Bhairahawa, Nepal. Study duration was 1½ years (January 2008-July 2009). The study sample consists of child and adolescent patients in the age range of 5-17 years, with established history of seizure disorder (including computerized tomography [CT] proved NCC), whose EEG's were reported by the author in the specified time period. Exclusion criteria were patients with a history of mental retardation, co-morbid major neuro-psychiatric illnesses, post-traumatic seizure, alcohol withdrawal seizure, uncooperative patients, EEGs done after inducing sleep and the EEGs that were ordered by the author himself.
Procedure
We have 21-channel computerized EEG machine to record the EEG data of our patients. We follow the standard “International 10-20 system” of electrode placement. EEGs are normally recorded for a minimum of 25-30 min for each patient. Recording is routinely done by our technician, who has been properly trained regarding electrode placements, recording of data, noting down artifacts (like eye-blink, eye-movement, movement of body parts, etc.) and other ongoing important activities like presence of seizure during recording. He has also been trained to carry out reactivity procedures such as eye-opening, photic stimulation and hyperventilation.
For analyzing the data, thus obtained, we used both “bipolar” as well as “referential” montages. Computerized signal analysis gave us the advantage of re-examining the same EEG signal using different montages or filter setting. Report was being given in a typed printed page, in a semi-structured format used in most EEG centers. Following comments were reported in the beginning: Technical quality of recording, patient's level of cooperation and alertness, artifacts noted. Pattern of background EEG activity was noted then. Abnormal Transient events were noted in the end; e.g., presence of focal seizure, focal slowing, generalized seizure, generalized slowing, etc., Detail notes were taken specifically on the pattern of alpha activity seen: Its frequency, distribution, location, symmetry, synchrony, progress, reactivity, rhythmicity, etc.
Author (who rated these EEGs) used to keep himself blind to the diagnosis, provided by the clinician, until the completion of EEG report. A total number of EEGs reported by the author during the specified time period was 513. Amongst these 513 reports, 293 patients were in the age range of 5-17 years and 171 of them had a diagnosis of seizure disorder.
A total of 55 reports were set aside for various exclusion criteria [Table 1]. EEGs of rest 116 patients were considered for the analysis of the present study. Thus, our study group comprised of these 116 patients with definite history of seizure disorder (Group A). A total of 36 patients with a history of “follow-up cases of seizure disorder” formed first comparison group (Group B). This group comprised of patients with all established cases of epilepsy, who were already on anti-epileptic drugs for a variable period of time. All 53 patients with a history suggestive of pseudoseizure, fainting spells, hyperventilation and patients with doubtful history of seizure formed our second comparison group (Group C). Thirty-three patients with history of various type headaches formed the third comparison group (Group D). Age criterion was relaxed for patients with a history of headache, to enable to get adequate number of sample for effective comparison.
Table 1.
List of cases excluded from the study (Total=55)

Descriptive statistics, Chi-square test and t-test were used to analyze the data thus available for the study.
RESULTS
Age and sex distribution chart was shown in Table 2. Study group was equally represented by either sex patients. As commonly reported, Group C and D was over-represented by females. Mean age in Groups A, B and C was similar. As we did not get enough samples in group D (headache), we had to take samples from higher age group, which has been reflected by higher mean average age group in group D.
Table 2.
Few demographic & clinical characteristics
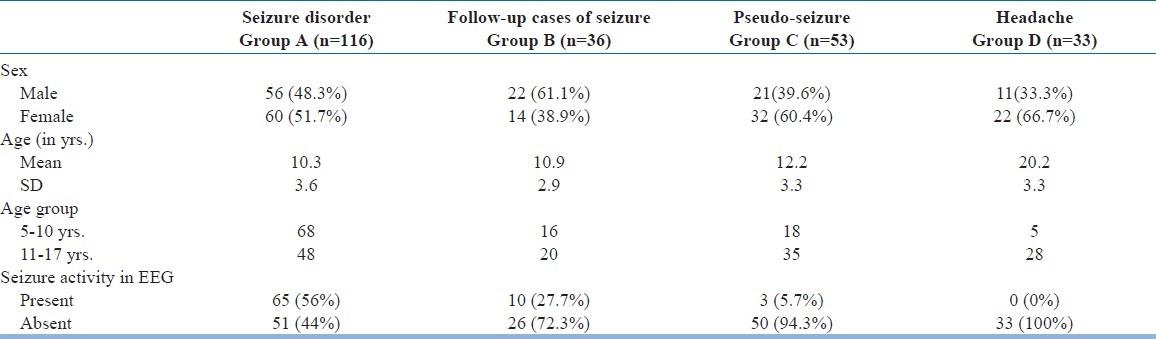
Fifty-six percent (65) of the patients in the Group A (study group) showed seizure activity in their EEG [Table 2]. Whereas, only 27.7% (10) patients in Group B and 5.7% (3) patients in Group C had seizure discharge in their EEGs. Seizure discharge was not seen in any of patients with a diagnosis of headache (Group D).
Table 3 reveals that in the study group (Group A), only 51.7% (60) patients had normal posterior alpha rhythm. In comparison, 88.8% (32) patients in Group B, 85% (45) patients in Group C and 93.9% (31) patients in Group D had shown normal posterior alpha rhythm. In significant 48.3% (56) patients within the study group (Group A) there was no visible alpha rhythm. Therefore, this “absent alpha rhythm” criteria was seen in only 11.2% (4) patients in Group B and 15% (8) patients in Group C and in only 6.1% (2) patients in Group D. “Absent alpha rhythm”, a criterion was conceptualized by the combination of total absence of alpha activity and/or presence of asynchronous poor occasional alpha activity. Absent alpha rhythm is a reflection of combination of these two patterns of alpha wave presentation in EEG.
Table 3.
Distribution of alpha rhythm
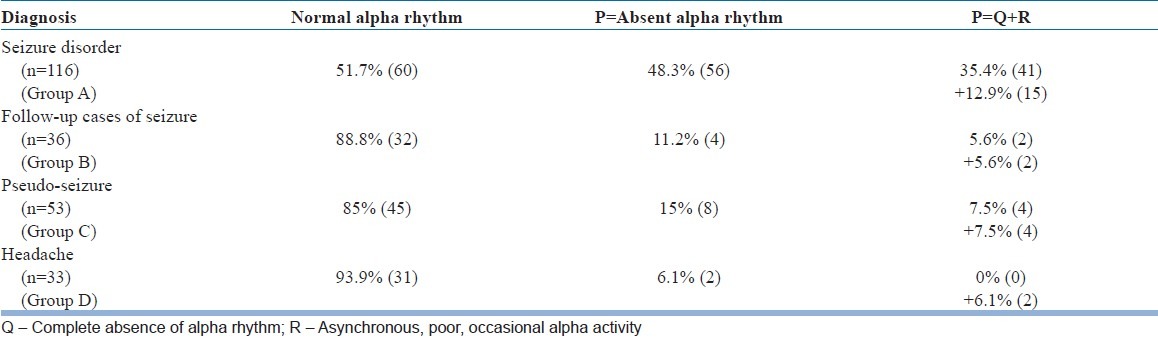
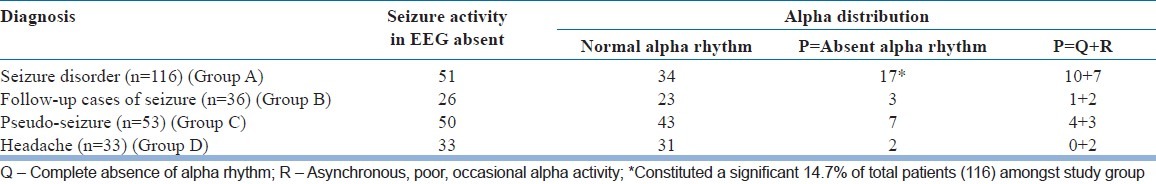
Table 4 shows that amongst seizure disorder patients, where seizure activity was seen in EEG (65), only 40% (26) of them had normal alpha rhythm in their EEG. Whereas, in Group B amongst follow-up cases of seizure disorder, 9 out of 10 patients (90%) with seizure activity showed normalizing effect of Alpha rhythm. In the next table [Table 5] we see that amongst 51 patients in the study group (Group A), where there was no seizure activity in EEG, overall 33.3% (17) of these patients did not show alpha rhythm in their EEG. They constituted a significant 14.7% of total patients (116) amongst study group.
Table 4.
Presence of seizure activity in EEG & Alpha distribution pattern compared

Table 5.
Absent seizure activity in EEG & Alpha distribution pattern compared
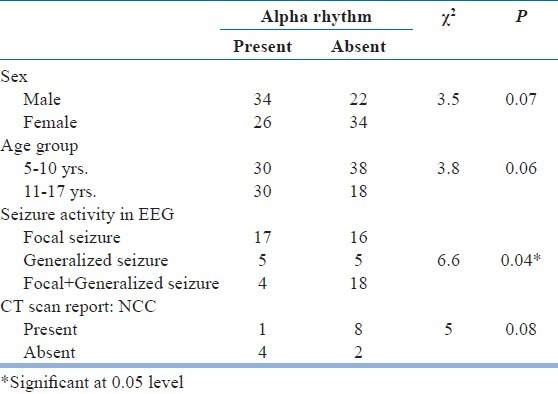
Relationship between epilepsy, seizure activity and alpha activity in EEG was compared in Table 6. It was revealed that the presence of seizure activity and absent of alpha activity in EEG significantly correlated to each other (Significant at <0.01 level). Pattern of the background activity in EEGs with absent alpha rhythm was analyzed in Table 7. Almost 59% patients within the study group, where there was the absence of normal posterior alpha rhythm, a slower background was noted. This effectively ruled out any drug related side-effect as a possible cause of “absent alpha rhythm”. Finally, alpha distribution pattern was compared within the study group as shown in Table 8. It was found that there was non-significant increase in “absent alpha rhythm” pattern amongst younger girls. EEGs showing both focal as well as generalized seizure discharge had more often “absent alpha” pattern. Similarly, CT scan showing organic brain lesion (neurocysticercosis) had shown characteristic “absent alpha rhythm” pattern more frequently.
Table 6.
Epilepsy, seizure activity and alpha activity in EEG (n=116)
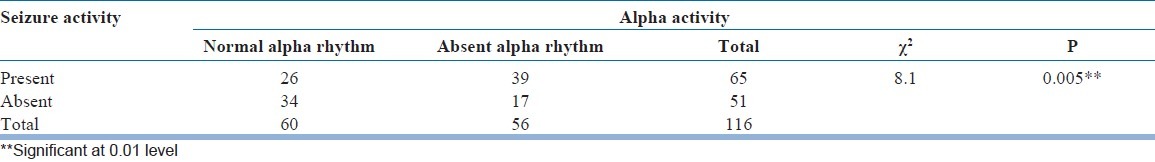
Table 7.
Pattern of background activity in EEGs with absent alpha rhythm

Table 8.
Alpha distribution pattern compared in study group
DISCUSSION
At the age of 36 months, 82% of normal children born at full term show a mean occipital alpha rhythm frequency of 8 Hz (range: 7.5-9.5 Hz). The occipital region is the maximal alpha rhythm voltage in 65% of adults and 95% of children.[3,4] Theta waves are more prominent than alpha waves up to the age of 5 years. Between 5 and 6 years, the amounts of alpha and theta activity are about equal. After this, age alpha waves are normally more prominent.[5] This is the reason we have chosen 5 years as the lower cut of age for the present study.
All electroencephalographers accept that the occipital alpha rhythm should be the starting point for visual EEG analysis. The initial questions usually asked are the following: Is an occipital alpha rhythm present; are its characteristics appropriate for the age? If there is little or no occipital alpha rhythm, is it because of the patient's eyes are open (reactivity) or because the patient is drowsy or asleep (state)? Is it an idiosyncrasy of a normal adult (genetic) or is it an abnormal finding?[3]
It has been accepted that some persons (adults; rarely children) who are apparently normal show no alpha activity, at least under the conditions of a routine clinical recording.[3] The amount of alpha activity reported to be varying considerably from one person to another and 11% of the population may have little or no alpha rhythm.[6] These individual differences in alpha rhythm have been known for long and that there are persons with little or no regular alpha waves.[7] The alpha rhythm is the dominant activity when the eyes are closed in most relaxed but awake adults. In the EEG of an occasional normal adult, alpha rhythm is not seen and pathological significance should not be attached to this finding.[8]
The presence of some low-voltage, 5-6 Hz rhythmic frontocentral activity in an adult may, in the transient absence of an occipital alpha rhythm, merely signify the patient's drowsiness; in the total absence of an occipital alpha rhythm, however, such activity may have pathological significance. Similarly, the presence of frontocentral theta activity would be more ominous, if the occipital alpha rhythm itself were slow (e.g., 7 Hz).[3] Children rarely show low amplitude (<20 μV in T5-O1) alpha rhythm and in contrast to adults, either absent or very low amplitude alpha rhythms (<15 μV in T5-O1) are abnormal between 3 and 12 years of age.[5]
This is what literature commonly says about alpha rhythm. However, existing literature is characteristically silent about the pattern of alpha rhythm in relation to seizure disorder patients. The findings of this paper will probably force us to think on the relationship between seizure activity and alpha rhythm in EEG, especially, in relation to seizure disorder in child and adolescent patients.
We found in significant 48.3% (56) patients within the study group (Group A) there was no visible alpha rhythm. Whereas, this “absent alpha rhythm” criteria was seen in only 11.2% (4) patients in Group B and 15% (8) patients in Group C and in only 6.1% (2) patients in Group D. Thus, “absent alpha rhythm”- a criterion seems to have a certain amount of “specificity” for EEGs with seizure disorder patients (Study group). We found that 56% (65) of the patients in the Group A (study group) showed seizure activity in their EEG. This presence of seizure activity and absent of alpha activity in EEG was significantly correlated to each other (Significant at <0.01 level).
Alpha rhythm was normal in only 40% of patients with seizure activity in their EEG in the study group. Whereas, in Group B amongst “follow-up cases of seizure disorder”, 90% (9 out of 10) patients with seizure activity in EEG showed the presence of normal alpha rhythm [Table 4]. This probably indicates that following treatment, abnormalities in alpha rhythm returns to normalcy faster than the disappearance of seizure discharge. Normalizing alpha rhythm in Group B, may indicate that “absent alpha rhythm” is a “state marker” rather than a “trait marker” of seizure disorder.
An interictal seizure discharge rate of 56% that was seen in the present study is at par with that has been reported in the literature.[9,10] EEG as a diagnostic instrument for epilepsy suffers because of such low level of interictal seizure discharge rate. If we accept our hypothesis that “absent alpha rhythm” is an indirect/additional indicator of seizure discharge (excluding other known possible causes of absent alpha rhythm), then the sensitivity of interictal EEG is increased to more than 70% (56% seizure discharge + 14.7% absent alpha rhythm) as a diagnostic instrument of epilepsy, at least in child and adolescent epilepsy patient. Specially, we can think of prioritizing these 14.7% patients to order for second EEG with special instructions like going for a sleep EEG. It has been reported earlier that repeating the out-patient EEG ultimately demonstrates interictal epileptic discharges in 80-90% of patients.[11,12]
Limitations
Author envisaged few limitations of the present study. Inherent bias of a single man study, as has already been defined and discussed in literature is applicable to present study as well.
Comparison Group-B (follow-up cases of seizure disorder) was not a clearly defined group. Duration of follow-up was not defined, as adequate data was not available in the history provided by the respective clinicians. Comparison Group C (pseudoseizure/fainting spells) was also a loosely defined group. Definite history of pseudoseizure, doubtful history of seizure, vertigo and fainting spells, hyperventilation syndrome, etc., formed this group. But even though the group was a loosely cohesive one, findings were definitely significant. Comparison Group D (headache) was not age matched one, as we did not get an adequate sample of headache in the required age range.
Every experienced electroencephalographer has his or her personal approach to EEG interpretation. This is also true for the manner in which the EEG report is being written.[4] Thus, EEG analysis is a subjective report given by electroencephalographer and there lies the possibility of some inherent bias in reporting. But, now-a-days computerized signal analysis gave us the advantage of re-examining the same EEG signal using different montages or filter setting - thereby increasing the objectivity of the report in visual analysis. By keeping himself blind to diagnosis prior to reporting, author tried to decrease possible bias in reporting.
SUMMARY
“Absent alpha rhythm”- a criterion seems to have a certain amount of “specificity” for EEGs with seizure disorder patients. Presence of seizure activity and absence of alpha rhythm in EEG significantly correlated to each other (significant at <0.01 level). “Absent alpha rhythm” also appears to be a “state marker” rather than a “trait marker” of seizure disorder.
Finally, if the hypothesis that “absent alpha rhythm is an indirect/additional indicator of seizure discharge” is accepted, then the sensitivity of EEG is increased to more than 70% as a diagnostic instrument of epilepsy, at least in child and adolescent epilepsy patients.
The findings of this paper will probably force us to think on the relationship between seizure activity and alpha rhythm in EEG, specially, in relation to seizure disorder in child and adolescent patients.
ACKNOWLEDGMENT
The author would like to thank all the staffs of EEG lab for their technical and other assistance and UCMS authority for their kind permission to carry out the study. I confirm that I have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.IFSECN. A glossary of terms most commonly used by clinical electroencephalographers. Electroencephalogr Clin Neurophysiol. 1974;37:538–48. doi: 10.1016/0013-4694(74)90099-6. [DOI] [PubMed] [Google Scholar]
- 2.Westmoreland BF. Normal and benign EEG patterns. Am J EEG Technol. 1982;22:3–31. [Google Scholar]
- 3.Kellaway P. Orderly approach to visual analysis: Elements of normal EEG and their characteristics in children and adults. In: Ebersole JS, Padley TA, editors. Current Practice of Clinical Electroencephalography. New York: Lippincott Williams and Wilkins; 2003. pp. 100–59. [Google Scholar]
- 4.Niedermeyer E. The normal EEG of the waking adult. In: Niedermeyer E, DaSilva L, editors. Electroencephalography: Basic Principles, Clinical Applications and Related Fields. New York: Lippincott Williams and Wilkins; 1999. pp. 149–67. [Google Scholar]
- 5.Fisch BJ. The normal EEG from premature age to the age of 19 years. In: Fisch BJ, editor. Speelmann's EEG Primer. 2nd ed. Amsterdam: Elsevier; 1991. pp. 175–211. [Google Scholar]
- 6.Gibbs FA, Gibbs EL, Lennox WG. Electroencephalographic classification of epileptic patients and control subjects. Arch Neurol Psychiatry. 1943;50:111–28. [Google Scholar]
- 7.Adrian ED. The discovery of Berger. In: Remond A, editor. Handbook of Electroencephalography and Clinical Neurophysiology. Vol. 1. Amsterdam: Elsevier; 1971. pp. 5–10. [Google Scholar]
- 8.Katz L. A review of cerebral rhythms in the waking EEG. Am J EEG Technol. 1971;11:77–100. [Google Scholar]
- 9.Marsan CA, Zivin LS. Factors related to the occurrence of typical paroxysmal abnormalities in the EEG records of epileptic patients. Epilepsia. 1970;11:361–81. doi: 10.1111/j.1528-1157.1970.tb03903.x. [DOI] [PubMed] [Google Scholar]
- 10.Pillai J, Sperling MR. Interictal EEG and the diagnosis of epilepsy. Epilepsia. 2006;47(Suppl 1):14–22. doi: 10.1111/j.1528-1167.2006.00654.x. [DOI] [PubMed] [Google Scholar]
- 11.Binnie CD. Epilepsy in adults: Diagnostic EEG investigation. In: Kimura H, editor. Recent Advances in Clinical Neurophysiology. Amsterdam: Elsevier; 1996. pp. 217–22. [Google Scholar]
- 12.Salinsky M, Kanter R, Dasheiff RM. Effectiveness of multiple EEGs in supporting the diagnosis of epilepsy: An operational curve. Epilepsia. 1987;28:331–4. doi: 10.1111/j.1528-1157.1987.tb03652.x. [DOI] [PubMed] [Google Scholar]


