Abstract
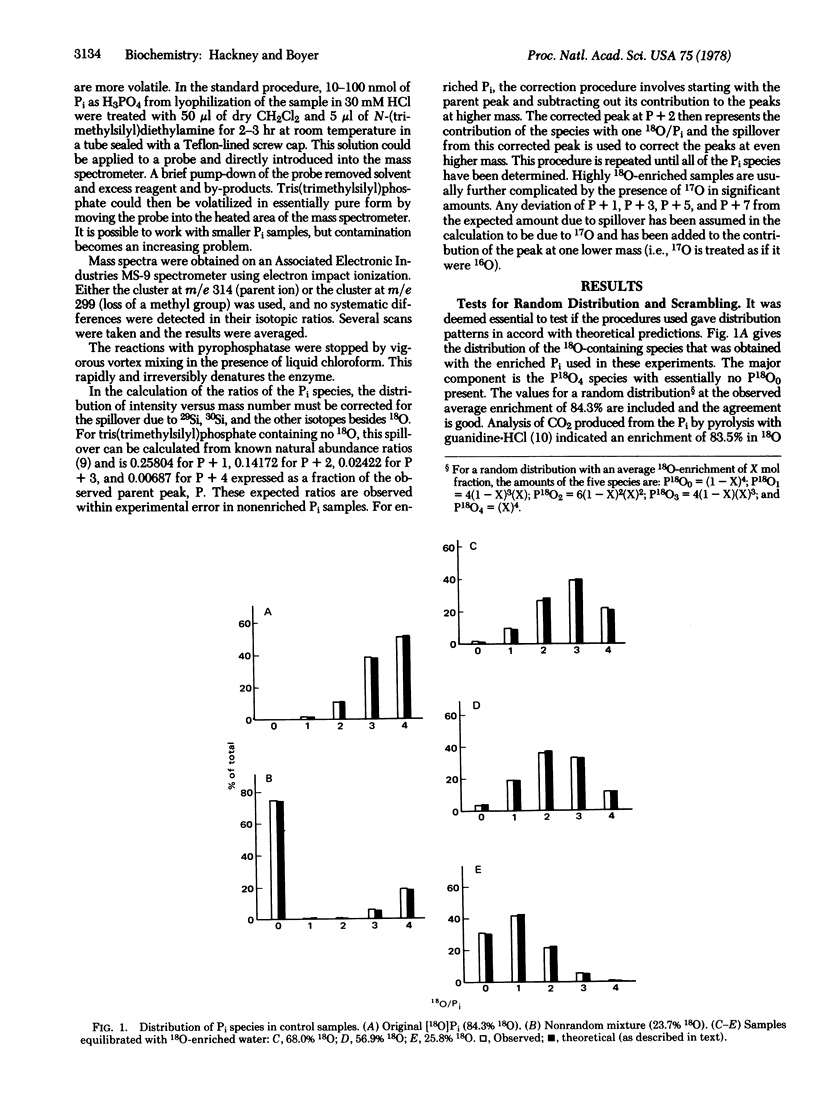
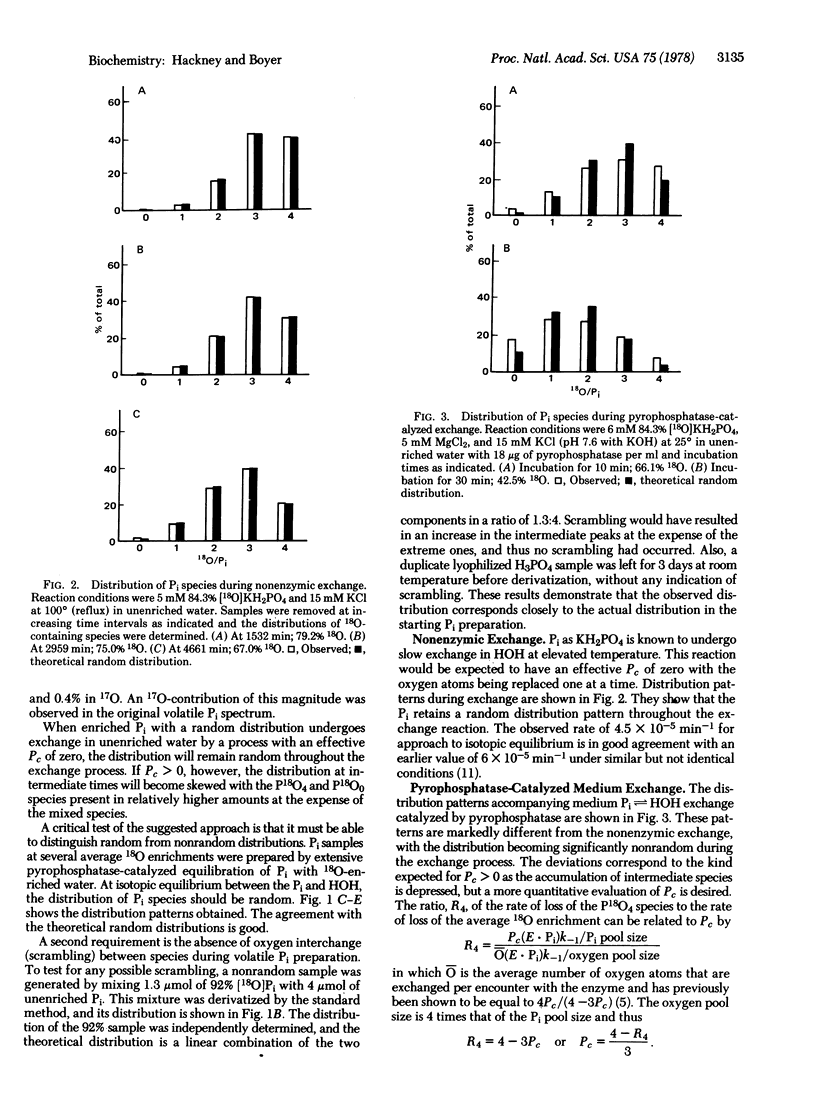
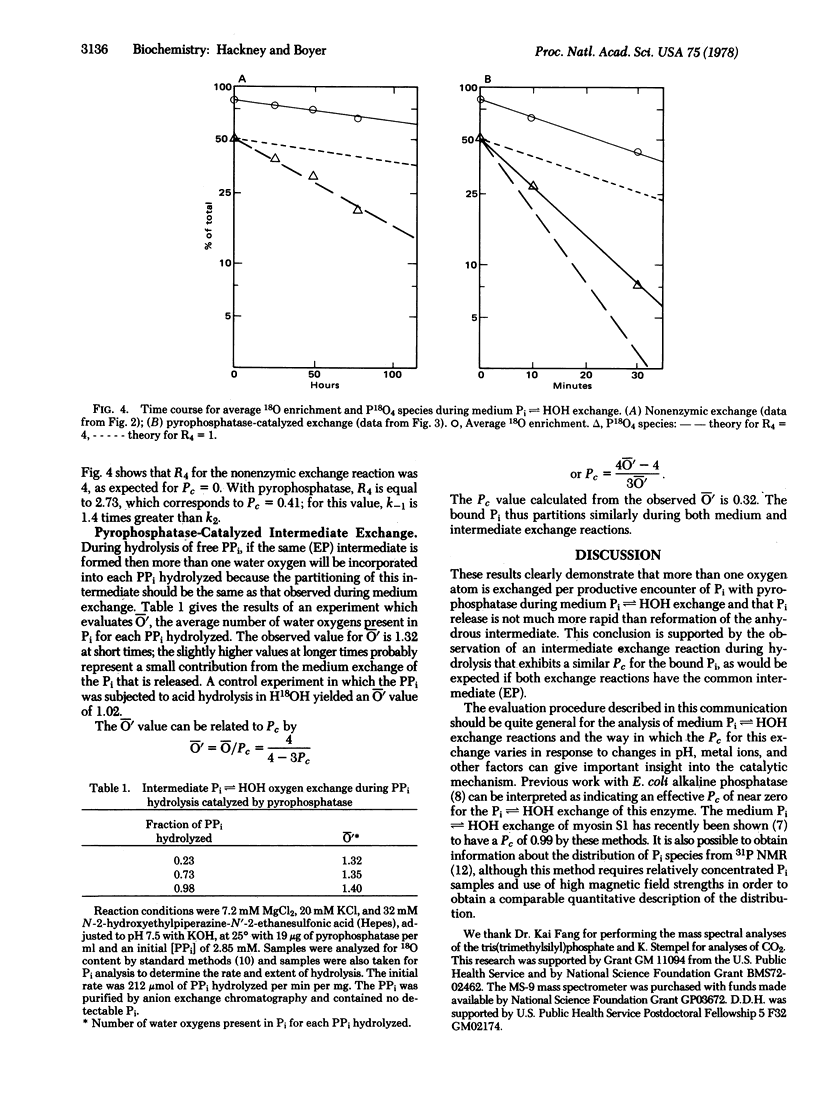
During the rapid exchange of oxygens of Pi with water catalyzed by yeast inorganic pyrophosphatase, Pi at the catalytic site may either dissociate or undergo reversible loss of an oxygen to water. The effective partitioning of bound Pi during exchange starting with medium Pi containing 18O in all four oxygens has been evaluated by mass spectral analysis of the change in the distribution of Pi species containing zero to four 18O oxygens per Pi. This analysis indicates that the rate of Pi release from the enzyme is only 1.4 times faster than the rate of reformation of the anhydrous intermediate. A similar partitioning of bound Pi is observed during PPi hydrolysis, indicating that hydrolysis and medium exchange have common intermediates. The approach should be applicable to study of related phosphate oxygen exchanges.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyer P. D., de Meis L., da Gloria Costa Carvalho M., Hackney D. D. Dynamic reversal of enzyme carboxyl group phosphorylation as the basis of the oxygen exchange catalyzed by sarcoplasmic reticulum adenosine triphosphatase. Biochemistry. 1977 Jan 11;16(1):136–140. doi: 10.1021/bi00620a023. [DOI] [PubMed] [Google Scholar]
- COHN M. Phosphate-water exchange reaction catalyzed by inorganic pyrophosphatase of yeast. J Biol Chem. 1958 Jan;230(1):369–379. [PubMed] [Google Scholar]
- Cohn M., Hu A. Isotopic (18O) shift in 31P nuclear magnetic resonance applied to a study of enzyme-catalyzed phosphate--phosphate exchange and phosphate (oxygen)--water exchange reactions. Proc Natl Acad Sci U S A. 1978 Jan;75(1):200–203. doi: 10.1073/pnas.75.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eargle D. H., Jr, Licko V., Kenyon G. L. Kinetic studies of 18O exchange of inorganic phosphate using mass spectral measurements on the tris-(trimethylsilyl) derivative. Anal Biochem. 1977 Jul;81(1):186–195. doi: 10.1016/0003-2697(77)90612-1. [DOI] [PubMed] [Google Scholar]
- Kanazawa T., Boyer P. D. Occurrence and characteristics of a rapid exchange of phosphate oxygens catalyzed by sarcoplasmic reticulum vesicles. J Biol Chem. 1973 May 10;248(9):3163–3172. doi: 10.2172/4473783. [DOI] [PubMed] [Google Scholar]
- Levine D., Reid T. W., Wilson I. B. The free energy of hydrolysis of the phosphoryl-enzyme intermediate in alkaline phosphatase catalyzed reactions. Biochemistry. 1969 Jun;8(6):2374–2380. doi: 10.1021/bi00834a018. [DOI] [PubMed] [Google Scholar]
- SCHWARTZ J. H., LIPMANN F. Phosphate incorporation into alkaline phosphatase of E. coli. Proc Natl Acad Sci U S A. 1961 Dec 15;47:1996–2005. doi: 10.1073/pnas.47.12.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEIN S. S., KOSHLAND D. E. Solubilization of certain L- and D-Peptidases of hog kidney particulates. Arch Biochem Biophys. 1952 Jul;39(1):230–231. [PubMed] [Google Scholar]


