Abstract
Background:
Dexmedetomidine has been shown to blunt the stress response to surgery. Hence a study was designed to evaluate the effect of intravenous (IV) Dexmedetomidine infusion during general anesthesia for abdominal surgeries on blood glucose levels and on Sevoflurane requirements during anesthesia.
Materials and Methods:
Forty patients scheduled for abdominal surgery under general anesthesia were divided into Dexmedetomidine (D) group and Placebo (P) group of 20 each. Group D received a loading dose of Inj. Dexmedetomidine at 1 μg/kg/10 min diluted to 20 mL, followed by maintenance with 0.5 μg/kg/h., till the end of surgery. Group P received similar volume of IV normal saline. Anesthesia was maintained with nitrous oxide in oxygen and Sevoflurane keeping entropy between 40 and 60. Data were analyzed using students t test, chi square test and Fisher Exact test as applicable.
Results:
During the first postoperative hour, Dexmedetomidine group showed blood glucose levels of 118.2 ± 16.24 mg/dL, compared to placebo group which was 136.95 ± 19.76 mg/dL and it was statistically significant (P < 0.01). Mean hourly Sevoflurane requirement in Group D was 11.10 ± 2.17 mL, compared to 15.45 ± 3.97 mL in placebo group. In peri-operative period, the heart rate and MAP were significantly lower in Group D, when compared to placebo. Patients in Group D were better sedated and post-operative pain score was better in Group D compared to Group P.
Conclusion:
IV Dexmedetomidine was effective in blunting stress response to surgical trauma as indicated by lower blood glucose levels, and reduces Sevoflurane requirements during entropy guided general anesthesia without affecting time for extubation.
Keywords: Adrenergic alpha-2 receptor agonists, blood glucose, Dexmedetomidine, entropy, metabolic stress response, Sevoflurane
Introduction
Alpha-2 receptors are a subgroup of noradrenergic receptors that mediate the function of the sympathetic nervous system. The alpha-2 receptor activation results in reduction in norepinephrine release, which can be used therapeutically to induce sympatholysis.[1]
Dexmedetomidine was first marketed for Intensive Care Unit (ICU) sedation, to make use of highly selective adrenergic alpha-2 receptor agonist activity. Unlike commonly used sedatives such as Propofol or Midazolam, Dexmedetomidine produces an “interactive” form of sedation, in which patients can be aroused easily with stimulation, and are cooperative once aroused. Because of its sympatholytic properties, Dexmedetomidine was gradually developed as an anesthetic premedication, with the goal of attenuating the sympathetic response to perioperative stresses such as laryngoscopy and intubation.[2]
In addition to sedative effects, Dexmedetomidine has been labeled as “analgesia sparing” by the Food and Drug Administration (FDA). Dexmedetomidine when coadministered with opioids has no depressant effects on respiration, but its analgesic effects offer a significant advantage for patients at risk for respiratory decompensation.[3,4]
The metabolic stress response to surgical trauma is characterized by increased serum levels of catecholamines and other steroid hormones.[5] If this stress response is of prolonged duration, the hyper-metabolic state can lead on to decreased resistance, delayed ambulation and increased morbidity and mortality.[6]
Excitation of the hypothalamus during stress results in the secretion of adrenocorticotrophic hormone (ACTH), which in turn initiates sudden increase in cortisol level. The cortisol mobilizes amino acids and fat from the body stores, and makes them available for synthesis of glucose, thus can cause hyperglycemia.[6] Hence, monitoring blood glucose level can reflect the metabolic stress response to surgery and the effect of Dexmedetomidine in blunting this stress response.
Several studies have indicated that administration of IV Dexmedetomidine during general anesthesia can decrease the minimum alveolar anesthetic concentration (MAC) of Sevoflurane. Thus a clinical study was conducted to assess the effect of Dexmedetomidine on Sevoflurane consumption.
The infusion of Dexmedetomidine will not significantly affect the recovery from anesthesia as indicated by time for tracheal extubation.
The aim of the present study was to observe the effect of intravenous (IV) Dexmedetomidine on levels of blood glucose, which is one among several stress response markers and Sevoflurane requirements under General Anesthesia (GA) for abdominal and abdomino-thoracic surgeries. The secondary goals were to maintain intra- and postoperative hemodynamic stability without affecting the tracheal extubation.
Materials and Methods
After obtaining approval from institutional ethical committee, a randomized, prospective, double blind, controlled, clinical study was formulated, and conducted at our hospital during the month of April 2012 to September 2012. By keeping the confidence limits of 95% to detect 20% change in blood glucose levels as primary criteria for a power of 80%, assuming an equal standard deviation in each group, the required sample size was 12 patients. However, for better validation of results we further increased the sample size to 20 in each group. These 40 patients were randomly allocated into two groups as Group ‘D’ (Dexmedetomidine group) and Group ‘P’ (Placebo group) using computer generated random numbers.
Informed written consent was obtained from all patients. The patients with American Society of Anesthesiologists (ASA) physical status I and II, aged 20-60 years, scheduled for elective abdominal surgery under general anesthesia (GA) were included in the study. Diabetic and hypertensive patients, patients on psychoactive medication, history of alcohol or drug abuse, patients receiving α-2 agonists or antagonist treatment, coronary artery disease and heart block were excluded from the study.
All patients were premedicated with tab. Alprazolam 0.5 mg oral, the night before surgery and a minimum fasting state of 6 h before anesthesia was ensured in all patients. The standard monitoring consisted of electrocardiography (ECG), pulse oximetry (SpO2), noninvasive blood pressure (NIBP), entropy, neuromuscular transmission monitor (NMT) and capnography. Before induction of general anesthesia, an epidural catheter was placed in all patients for the purpose of providing postoperative analgesia. The spectral entropy (SE) was measured with a plug in Datex Ohmeda entropy S/5 module™. All patients were preoxygenated with 100% oxygen for 3 min and inj. Midazolam 0.05 mg/kg IV, inj. Glycopyrrolate 0.01 mg/kg IV, inj. Fentanyl 2 μg/kg IV were administered.
Group D patients were given an initial dose of inj. Dexmedetomidine 1 μg/kg IV made to 20 mL with normal saline, over 10 min. Group C patients received similar volume of normal saline over 10 min. The study drug and placebo infusions were prepared by an anesthesiologist who was not involved in the study and the anesthesiologist recording the details was unaware of the type of infusion patients received. Anesthesia was induced in all the patients with inj. Propofol in successive 30 mg doses every 2 min until Response Entropy (RE) dropped to 50, and confirmed with loss of response to verbal commands. In both the groups, inj. Atracurium 0.5 mg/kg IV was administered, and trachea was intubated after disappearance of train of four twitch response. Additional dose of inj. Propofol 20-30 mg bolus was given if increase in entropy values were observed. A maintenance dose of inj. Dexmedetomidine infusion at 0.5 μg/kg/hour made to 20 mL with normal saline was administered in group D patients and a similar volume of normal saline was administered in Group P, till the end of surgery. Anesthesia was continued with Sevoflurane and 60% nitrous oxide in oxygen and ventilated to maintain end-tidal CO2(EtCO2) between 35 and 40 mmHg. Sevoflurane dial concentration was adjusted to ensure adequate depth of general anesthesia to maintain RE levels between 40 and 60 and difference between RE and SE less than 10, and also to maintain clinical variables like HR, NIBP, mean arterial pressure (MAP) within normal limits. Top up doses of Atracurium were guided by NMT monitor. Infusion of Dexmedetomidine was stopped 30 min prior to expected time of completion of surgery. Sevoflurane administration was turned off at the beginning of skin suturing. At the end of the surgery, residual neuromuscular blockade was reversed with inj. Neostigmine 0.5 mg/kg and inj. Glycopyrrolate 0.005 mg/kg and trachea extubated after satisfactory recovery and presence of response to oral commands. All patients were administered only IV normal saline during study period.
The age, gender, body weight, duration of surgery (minutes), duration of anesthesia (minutes) were recorded. Serial blood glucose levels from fresh capillary whole blood using glucose test strips were estimated at following points – before induction (basal), 30 min after intubation and at one and 6 h after recovery from anesthesia. The total and hourly Sevoflurane requirements were measured at the end of every hour. The usage of Sevoflurane during anesthesia can be calculated as follows.[7]
Dion's Formula:
Usage of volatile anesthetic (mL) = [Dialed concentration × Total fresh gas flow × Duration at that concentration × Molecular weight]/[2412 × Density].
Loke's Formula:
Usage of volatile anesthetic (mL) = [(MAC) (FGF) (60 min) (MW)]/[(Pressure/(R × T))(D)].(FGF - fresh gas flow, MW – molecular weight, R – ideal gas constant and D - density in g/mL). But the Datex Ohmeda Avance S5 system automatically calculates and displays the usage of anesthetic liquid in mL on real-time basis.
The Hemodynamic and Entropy parameters were recorded every 5 min throughout intraoperative and postoperative period. The recovery from anesthesia was measured from the time all anesthetics were turned off. The time required for extubation and later Ramsay sedation score (RSS) after extubation were also observed [Table 1]. The post-operative pain was assessed using visual analogue scale (VAS).
Table 1.
Ramsay sedation score

Statistical analysis was conducted with statistical package for social sciences (SPSS) Version 17 (IL Chicago) for windows statistical package. All the parametric data are presented as mean ± SD and non-parametric data in tables. Parametric data were assessed with independent sample t-test. Bonferroni's correction was applied as appropriate. Non-parametric data with Chi square test and Fischer exact test as appropriate. P value of less than 0.05 was considered as statistically significant.
Results
Demographic parameters such as age, gender, body weight, basal vital parameters and entropy values were uniform in both groups. The duration of surgery and anesthesia were similar in both groups. The types of surgeries are listed in Table 2.
Table 2.
Demographic parameters
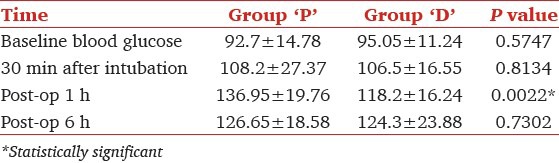
The mean blood glucose levels at 30 min after intubation were comparable in both groups (group P 108.2 ± 27.37 mg/dL, group D 106.5 ± 16.55 mg/dL, P = 0.8134). During 1st h of post-operative period they were elevated in group P (136.95 ± 19.76 mg/dL) while it was stable in group D (118.2 ± 16.24 mg/dL) (P = 0.002). Again the blood glucose levels were similar in both groups at 6 h of postoperative period (group P 126.65 ± 18.58 mg/dL, group D 124.3 ± 23.88 mg/dL, P = 0.7302) [Table 3].
Table 3.
Blood glucose levels
There was a clinically and statistically significant reduction in heart rate (HR) in group D throughout intraoperative period compared to group P (P < 0.05) [Figure 1]. Bradycardia was noticed in 3 out of 20 patients in group D, which was treated with inj. Atropine 0.6 mg IV. There was no incidence of bradycardia in group P. However, there was no statistically significant difference in systolic and diastolic blood pressures and also the MAP were comparable in both groups during intraoperative period.
Figure 1.
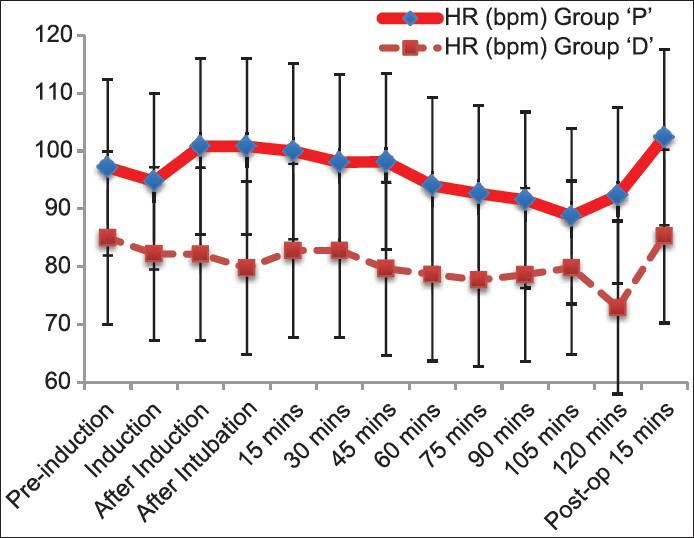
Graph showing comparison of heart rate between the groups. Heart rate was significantly low in group D
End-tidal Sevoflurane concentration was significantly reduced in both the groups during entropy guided anesthesia. It was 1.78 ± 0.90 at induction and 1.13 ± 0.37 at 60th min (P = 0.005) indicating 36% reduction, but in group D it was 1.68 ± 0.54 and 0.74 ± 0.42, respectively (P < 0.001), suggesting a 56% decrease in Et Sevoflurane concentration [Figure 2]. There was statistically significant 28% reduction in the Sevoflurane requirement in the 1st h of anesthesia, as suggested by 11.10 ± 2.17 mL in group D compared to 15.45 ± 3.97 mL in group P (P < 0.001). While it was 10.58 ± 3.4 mL in 2nd h and 7.00 ± 2.33 mL in 3rd h in group P compared to 8.13 ± 2.75 mL and 8.14 ± 1.35 mL group D (P = 0.02 for 2nd h, P = 0.06 for 3rd h), respectively [Table 4]. The time from turning off of Sevoflurane to tracheal extubation was considered as time for extubation and it was 5.4 ± 1.35 min. in group P, compared to 5.5 ± 1.82 min in group D. Ramsay sedation score was significantly higher at 2.6 ± 0.75 in patients treated with Dexmedetomidine, while it was 1.25 ± 0.44 in placebo group (P < 0.001) indicating arousable sedation. Pain assessed by visual analog scale at first hour of postoperative period was 2.05 ± 0.69 in group D, which was significantly lower against 3.65 ± 0.49 in group P (P < 0.001) [Table 5]. In group P, 13 patients and 1 patient in group D required epidural analgesia at end of 1 h postoperatively. Remaining 7 patients in group P and 19 patients in group D received epidural analgesia by the end of 2 h.
Figure 2.
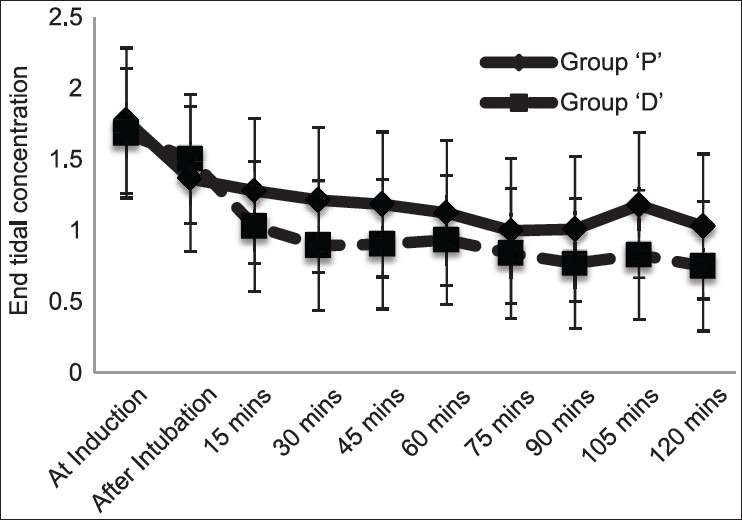
Graph showing comparison of end tidal concentration of Sevoflurane between groups
Table 4.
Hourly Sevoflurane consumption
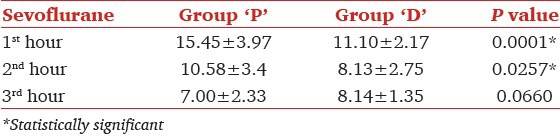
Table 5.
RSS and VAS at 1st hour of postoperative period

Discussion
Dexmedetomidine, a highly selective α2-adrenergic receptor agonist has generated lot of interest for its sedative, analgesic, perioperative sympatholytic, anesthetic-sparing, and hemodynamic-stabilizing properties with a relatively high ratio of α2/α1 activity (1620:1).[8]
The hypnotic and supraspinal analgesic effects of Dexmedetomidine are mediated by suppression of neuronal firing in the locus coeruleus, resulting in inhibition of norepinephrine release and activity in the descending medullo-spinal noradrenergic pathway.[8,9] This suppression of inhibitory control leads to decreased histamine secretion, resulting in hypnosis similar to natural sleep,[4] without ventilatory depression.[10]
The stress response manifests clinically and metabolically with a wide range of endocrinal, immunological and hematological effects. Some of them include sympathetic nervous system activation, insulin resistance and hyperglycemia, cytokine production with a host of immunological and hematological changes.
Blood glucose concentrations increase after surgical incision. The cortisol and catecholamines facilitate glucose production as a result of increased hepatic glycogenolysis and gluconeogenesis. In addition, peripheral use of glucose is decreased due to insulin resistance. The blood glucose concentrations are related to the intensity of the surgical injury; the changes follow closely the increases in catecholamines. Following cardiac surgery, blood glucose concentrations can increase up to 180-216 mg/dL and remain elevated for up to 24 h after surgery. The changes are less significant with minor surgery.[11] In diabetic subjects, poor glycemic control is associated with an increase in diabetic complications, which can be avoided with tight control of blood glucose.[12]
It was demonstrated that mixed venous plasma concentration of Noradrenaline were lower during Sevoflurane anesthesia compared to Desflurane[13] and Dexmedetomidine combined with Sevoflurane anesthesia.[14] Supplementing Sevoflurane anesthesia with drugs such as parecoxib sodium or Fentanyl inhibited stress response to tracheal intubation[15,16] and skin incision[17] as indicated by stable hemodynamic parameters. Ahmed et al. in their study observed that intravenous infusion of inj. Dexmedetomidine reduced stress response to major surgeries as indicated by clinically and statistically significant reduction in blood glucose, interleukin-6, and cortisol levels.[18]
Entropy is a device which helps in analyzing Electroencephalogram (EEG) and yields State entropy (SE) and Response Entropy (RE).[19] SE measures the degree of hypnotic level and RE reflects both EEG and frontal EMG activities. The difference of RE - SE reflects EMG activation, indicating the adequacy of antinociception.[20] Numbers close to 100 indicate that the patient is conscious, and towards zero denote very ‘deep’ anesthesia. For a clinically meaningful anesthesia and a low probability of consciousness following an administration of general anesthetic in a patient, an RE value of 40-60 is considered as adequate, with RE - SE difference of less than 10.[21] Song and others observed that Bispectral Index Monitoring (BIS) during anesthesia helps in reducing Sevoflurane requirements and a faster emergence in patients undergoing tubal ligation procedures.[22] Edno Magalhães and others observed that Dexmedetomidine infusion at a rate of 0.5 μg/kg/h during Sevoflurane anesthesia significantly reduced end-tidal Sevoflurane concentration from 1.6 at 45th min to 1.07 at 105th min, while maintaining hemodynamic stability, although others have found a varying reduction from 17-58% in MAC of Sevoflurane.[23] Dexmedetomidine infusion in a dose of 1 μg/kg over 10 min before the induction of anesthesia and later maintenance dose of 0.2-0.7 μg/kg/h until skin closure can attenuate sympatho-adrenal response to tracheal intubation.[24]v Even a single bolus dose of Dexmedetomidine before induction of anesthesia can cause attenuation of hemodynamic and neuroendocrinal responses to skull-pin insertion in patients undergoing craniotomy.[25] Dexmedetomidine is known to provide hemodynamic stability even during peak of surgical stress.[26] The HR and MAP during the intra and postoperative periods were significantly lower but within hemodynamically stable limits in group D relative to group P. Administration of Dexmedetomidine could blunt the increase in HR and MAP associated with endotracheal intubation in group D.
It was observed that Dexmedetomidine at a plasma concentrations of 0.7 ng/mL decreased the MAC of Sevoflurane by 17%, although lower doses did not reduce the MAC significantly.[27]
The time for extubation was significantly shorter with Sevoflurane when compared to Propofol anesthesia.[28] Dexmedetomidine used as an adjuvant to general anesthesia resulted in better recovery and significant sedation at 2 h.[16] Turan and others observed that Dexmedetomidine 0.5 μg kg-1 stabilizes hemodynamics, and allows easy extubation, without affecting the recovery time.[29,30] Dexmedetomidine induced arousable sedation in the present study as noticed by other authors,[10,22] and pain scores were also lower in patients receiving Dexmedetomidine highlighting its analgesic properties.[24,25] Blood glucose levels are only a tip of iceberg in showcasing the stress response to surgery; assessment of cytokines and interleukins may be better markers for stress response to surgery.
Conclusion
Dexmedetomidine as a preanesthetic medication and intraoperative infusion was effective in blunting metabolic stress response to major surgeries as indicated by stable blood glucose levels. It also decreased intraoperative anesthetic requirement and had significant anesthetic sparing property during entropy guided general anesthesia. In addition, continuous intraoperative administration of Dexmedetomidine does not affect intraoperative cardiovascular stability.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Jorden VS, Avery T. Dexmedetomidine: Clinical update seminars in anesthesia. Perioperotive Med Pain. 2002;21:265–74. [Google Scholar]
- 2.Lee JH, Kim H, Kim HT, Kim MH, Cho K, Lim SH, et al. Comparison of Dexmedetomidine and remifentanil for attenuation of hemodynamic responses to laryngoscopy and tracheal intubation. Korean J Anesthesiol. 2012;63:124–9. doi: 10.4097/kjae.2012.63.2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Günes Y, Gündüz M, Özcengiz D, Özbek H, Isik G. Dexmedetomidine–remifentanil or Propofol-remifentanil anesthesia in patients undergoing intracranial surgery. Neurosurg. 2005;15:122–6. [Google Scholar]
- 4.Hsu YW, Cortinez LI, Robertson KM, Keifer JC, Sum-Ping ST, Moretti EW, et al. Dexmedetomidine pharmacodynamics: Part I: Crossover comparison of the respiratory effects of Dexmedetomidine and remifentanil in healthy volunteers. Anesthesiology. 2004;101:1066–76. doi: 10.1097/00000542-200411000-00005. [DOI] [PubMed] [Google Scholar]
- 5.O’Riain SC, Buggy DJ, Kerin MJ, Watson RW, Moriarty DC. Inhibition of the stress response to breast cancer surgery by regional anesthesia and analgesia does not affect vascular endothelial growth factor and prostaglandin E2. Anesth Analg. 2005;100:244–9. doi: 10.1213/01.ANE.0000143336.37946.7D. [DOI] [PubMed] [Google Scholar]
- 6.Singh M. Stress response and anesthesia altering the peri and post-operative management. Indian J Anaesth. 2003;47:427–34. [Google Scholar]
- 7.Loke J, Shearer WA. Cost of anaesthesia. Can J Anaesth. 1993;40:472–4. doi: 10.1007/BF03009526. [DOI] [PubMed] [Google Scholar]
- 8.Grewal A. Dexmedetomidine: New avenues. J Anaesthesiol Clin Pharmacol. 2011;27:297–302. doi: 10.4103/0970-9185.83670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu SB. Dexmedetomidine sedation in ICU. Korean J Anesthesiol. 2012;62:405–11. doi: 10.4097/kjae.2012.62.5.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Candiotti KA, Bergese SD, Bokesch PM, Feldman MA, Wisemandle W, Bekker AY, et al. Monitored anesthesia care with Dexmedetomidine: A prospective, randomized, double-blind, multicenter trial. Anesth Analg. 2010;110:47–56. doi: 10.1213/ane.0b013e3181ae0856. [DOI] [PubMed] [Google Scholar]
- 11.Desborough JP. The stress response to trauma and surgery. Br J Anaesth. 2000;85:109–17. doi: 10.1093/bja/85.1.109. [DOI] [PubMed] [Google Scholar]
- 12.Khattab M, Khader YS, Al-Khawaldeh A, Ajlouni K. Factors associated with poor glycemic control among patients with type 2 diabetes. J Diabetes Complications. 2010;24:84–9. doi: 10.1016/j.jdiacomp.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 13.Marana E, Russo A, Colicci S, Polidori L, Bevilacqua F, Viviani D, et al. Desflurane versus Sevoflurane: A comparison on stress response. Minerva Anestesiol. 2013;79:7–14. [PubMed] [Google Scholar]
- 14.Scheinin B, Lindgren L, Randell T, Scheinin H, Scheinin M. Dexmedetomidine attenuates sympathoadrenal responses to tracheal intubation and reduces the need for thiopentone and peroperative Fentanyl. Br J Anaesth. 1992;68:126–31. doi: 10.1093/bja/68.2.126. [DOI] [PubMed] [Google Scholar]
- 15.Feng C, Qi SH, Zou YM, Ma XS, Gao DP, Han BQ. [Effects of Dexmedetomidine combined with Fentanyl in patients undergoing anesthesia induction by Sevoflurane.] [Article in Chinese. ]Zhonghua Yi Xue Za Zhi. 2012;92:1889–91. [PubMed] [Google Scholar]
- 16.Patel CR, Engineer SR, Shah BJ, Madhu S. Effect of intravenous infusion of Dexmedetomidine on perioperative haemodynamic changes and postoperative recovery: A study with entropy analysis. Indian J Anaesth. 2012;56:542–6. doi: 10.4103/0019-5049.104571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Z, Lu H, He G, Ma H, Wang J. Non-steroidal anti-inflammatory drugs reduce the stress response during Sevoflurane anesthesia. Acta Anaesthesiol Scand. 2012;56:890–5. doi: 10.1111/j.1399-6576.2012.02730.x. [DOI] [PubMed] [Google Scholar]
- 18.Yacout AG, Osman HA, Abdel-Daem MH, Hammouda SA, Elsawy MM. Effect of intravenous Dexmedetomidine infusion on some proinflammatory cytokines, stress hormones and recovery profile in major abdominal surgery. Alexandria J Med. 2012;48:3–8. [Google Scholar]
- 19.Aho AJ, Lyytikäinen LP, Yli-Hankala A, Kamata K, Jäntti V. Explaining entropy responses after a noxious stimulus, with or without neuromuscular blocking agents, by means of the raw electroencephalographic and electromyographic characteristics. Br J Anaesth. 2011;106:69–76. doi: 10.1093/bja/aeq300. [DOI] [PubMed] [Google Scholar]
- 20.Kawaguchi M, Takamatsu I, Kazama T. Rocuronium dose-dependently suppresses the spectral entropy response to tracheal intubation during Propofol anaesthesia. Br J Anaesth. 2009;102:667–72. doi: 10.1093/bja/aep040. [DOI] [PubMed] [Google Scholar]
- 21.Raid W, Schreiber M, Saeed AB. Monitoring with EEG entropy decreases Propofol requirement and maintains cardiovascular stability during induction of anaesthesia in elderly patients. Eur J Anaesthesiol. 2007;24:684–8. doi: 10.1017/S026502150700018X. [DOI] [PubMed] [Google Scholar]
- 22.Song D, Joshi GP, White PF. Titration of volatile anesthetics using bispectral index facilitates recovery after ambulatory anesthesia. Anesthesiology. 1997;87:842–8. doi: 10.1097/00000542-199710000-00018. [DOI] [PubMed] [Google Scholar]
- 23.Magalhães E, Govêia CS, Ladeira LC, Espíndola BV. Relationship between Dexmedetomidine continuous infusion and end-tidal Sevoflurane concentration, monitored by bispectral analysis. [Article in Portuguese. ] Rev Bras Anestesiol. 2004;54:303–10. doi: 10.1590/s0034-70942004000300003. [DOI] [PubMed] [Google Scholar]
- 24.Keniya VM, Ladi S, Naphade R. Dexmedetomidine attenuates sympathoadrenal response to tracheal intubation and reduces perioperative anaesthetic requirement. Indian J Anaesth. 2011;55:352–7. doi: 10.4103/0019-5049.84846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uyar AS, Yagmurdur H, Fidan Y, Topkaya C, Basar H. Dexmedetomidine attenuates the hemodynamic and neuroendocrinal responses to skull-pin head-holder application during craniotomy. J Neurosurg Anesthesiol. 2008;20:174–9. doi: 10.1097/ANA.0b013e318177e5eb. [DOI] [PubMed] [Google Scholar]
- 26.Dilek O, Yasemin G, Atci M. Preliminary experience with Dexmedetomidine in neonatal anesthesia. J Anaesthesiol Clin Pharmacol. 2011;27:17–22. [PMC free article] [PubMed] [Google Scholar]
- 27.Fragen RJ, Fitzgerald PC. Effect of Dexmedetomidine on the minimum alveolar concentration (MAC) of Sevoflurane in adults age 55 to 70 years. J Clin Anesth. 1999;11:466–70. doi: 10.1016/s0952-8180(99)00081-1. [DOI] [PubMed] [Google Scholar]
- 28.Orhon ZN, Devrim S, Celik M, Dogan Y, Yildirim A, Basok EK. Comparison of recovery profiles of Propofol and Sevoflurane anesthesia with bispectral index monitoring in percutaneous nephrolithotomy. Korean J Anesthesiol. 2013;64:223–8. doi: 10.4097/kjae.2013.64.3.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turan G, Ozgultekin A, Turan C, Dincer E, Yuksel G. Advantageous effects of Dexmedetomidine on haemodynamic and recovery responses during extubation for intracranial surgery. Eur J Anaesthesiol. 2008;25:816–20. doi: 10.1017/S0265021508004201. [DOI] [PubMed] [Google Scholar]
- 30.Aksu R, Akin A, Biçer C, Esmaoğlu A, Tosun Z, Boyaci A. Comparison of the effects of Dexmedetomidine versus Fentanyl on airway reflexes and hemodynamic responses to tracheal extubation during rhinoplasty: A double-blind, randomized, controlled study. Curr Ther Res. 2009;70:209–20. doi: 10.1016/j.curtheres.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]


