Abstract
Background:
Providing anesthesia for gastrointestinal (GI) endoscopy procedures in morbidly obese patients is a challenge for a variety of reasons. The negative impact of obesity on the respiratory system combined with a need to share the upper airway and necessity to preserve the spontaneous ventilation, together add to difficulties.
Materials and Methods:
This retrospective cohort study included patients with a body mass index (BMI) >40 kg/m2 that underwent out-patient GI endoscopy between September 2010 and February 2011. Patient data was analyzed for procedure, airway management technique as well as hypoxemic and cardiovascular events.
Results:
A total of 119 patients met the inclusion criteria. Our innovative airway management technique resulted in a lower rate of intraoperative hypoxemic events compared with any published data available. Frequency of desaturation episodes showed statistically significant relation to previous history of obstructive sleep apnea (OSA). These desaturation episodes were found to be statistically independent of increasing BMI of patients.
Conclusion:
Pre-operative history of OSA irrespective of associated BMI values can be potentially used as a predictor of intra-procedural desaturation. With suitable modification of anesthesia technique, it is possible to reduce the incidence of adverse respiratory events in morbidly obese patients undergoing GI endoscopy procedures, thereby avoiding the need for endotracheal intubation.
Keywords: Airway, colonoscopy, endoscopic retrograde cholangiopancreatography, endoscopy, morbid obesity
Introduction
“Out of operating room” anesthesia is one of the fastest growing areas of clinical practice. At the Hospital of the University of Pennsylvania, we have had an almost 300% increase [Figure 1] over the last 3 years, much of this attributable to gastrointestinal (GI) endoscopy [Figure 2]. Upper GI endoscopy, in particular, is challenging for the anesthesia provider. The shared airway, increased risk of aspiration, preference for spontaneous ventilation and relative non-availability of rapid help during an emergency due to the remote location, all increase anesthesia risks. The mortality and morbidity is relatively high and is mainly related to airway management.[1] It is to be expected that, morbidly obese patients as a group (individuals with a body mass index [BMI] greater than 40 kg/m2), are likely to have an even higher rate of perioperative complications. Although the American Society of Anesthesia (ASA) recommends that emergency help should be available at all times,[2] it does not specify the quality and nature of emergency support. Again, in the opinion of ASA’ task force on perioperative management of patients with obstructive sleep apnea (OSA), general anesthesia with a secured airway is preferable to moderate or deep sedation for most patients with OSA. A significant number of morbidly obese patients presenting for GI endoscopy have OSA. In spite of this, our approach is one of providing intravenous general anesthesia without endotracheal intubation.
Figure 1.
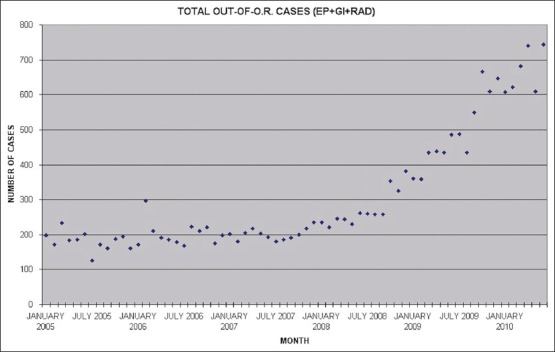
Total out of operating room anesthesia case load. EP: Electrophysiological procedures, GI: Gastrointestinal endoscopy, and RAD: Radiological procedures
Figure 2.
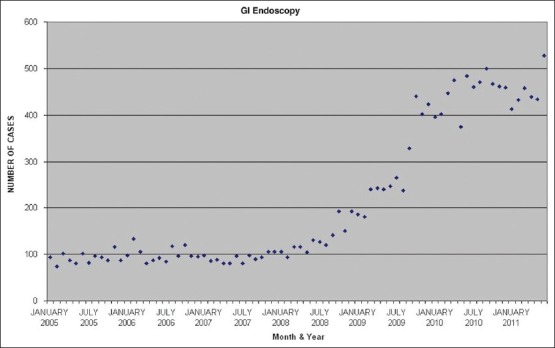
Gastrointestinal endoscopy case load
The goal of this study was to evaluate the optimum airway management for morbidly obese patients undergoing GI endoscopy under total intravenous anesthesia. In order to understand patient safety and risk in this setting, we evaluated the incidence of complications.
Materials and Methods
This was a retrospective cohort study of morbidly obese (BMI >40 kg/m2) adults who presented for outpatient GI endoscopy over a period of 6 months from September 2010 to February 2011. Patients who were electively intubated for the procedure or under the age of 18 years were not included in this retrospective study. The perioperative records were reviewed to obtain the following patient-specific and procedure specific data: Demographics and comorbidities, method of airway management, endoscopic procedure performed, periods of oxygen saturation below 90 and 95% respectively, cardiovascular complications, unexpected endoscope withdrawal to facilitate patients’ oxygenation and any procedure cancellation after the start of the procedure due to anesthesia issues. For statistical analysis, desaturation episode was defined as a fall of pulse oximeter saturation below 95%. Pearson's Chi-square test was used to relate frequency of desaturation to possible predictors (BMI groups and history of OSA). The degree of statistical significance was set at 95% thus taking a P value of less than 0.05 as significant. This retrospective study was approved by the Institutional Review Board of the University of Pennsylvania.
Results
The records of 2035 consecutive patients that underwent outpatient GI endoscopy were reviewed. One hundred nineteen had a BMI greater than 40 and met all inclusion criteria. Characteristics of the 119 individuals included in the cohort are shown in Table 1. The majority of patients were female (61.3%). The mean BMI was 45.4 and ranged from 40 to 79.5. All patients in the cohort had co-morbidities, the most common of which was hypertension (68.1%). Pearson's Chi-square test showed a significant correlation between frequency of desaturation (bellow 95% or a 5% fall) and previous history of OSA. (Chi-square = 11.05, P = 0.004, df = 2) with a likelihood ratio of patients with OSA desaturating 5.11 times the patients without OSA. No statistically significant relation could be established between increasing BMI groups [Table 2] and frequency of desaturation (Chi-square = 3.35, P = 0.76).
Table 1.
Patient characteristics (N=119)
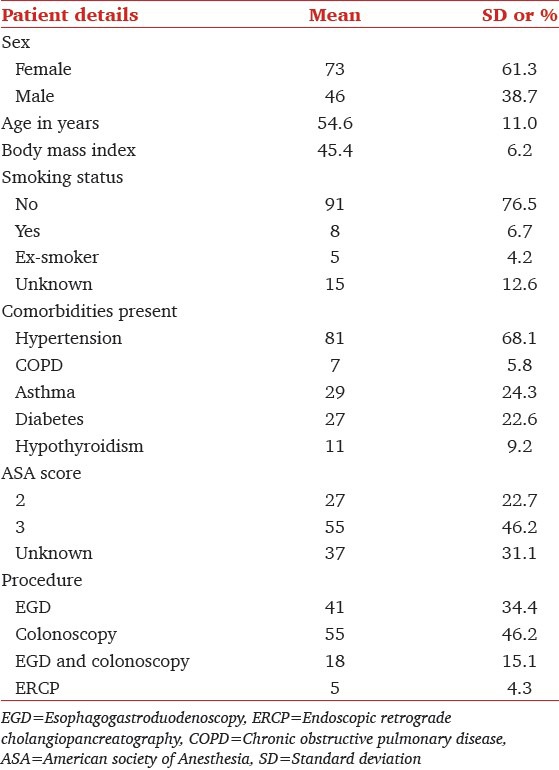
Table 2.
Relation between BMI, history of OSA and procedural complications
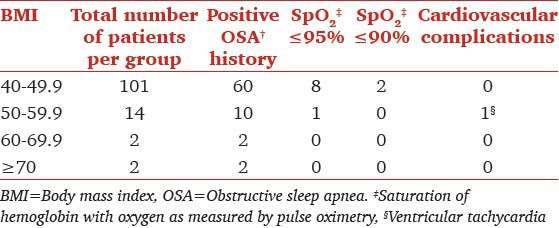
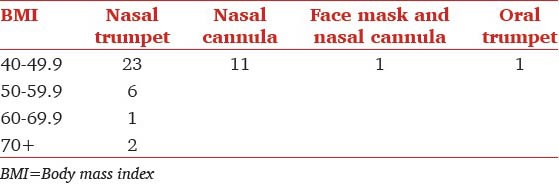
The various airway management techniques used are shown in Table 3. Nasal trumpet was the most commonly used approach among patients across all BMI [Figure 3]. Complications that occurred within the cohort are shown in Table 2. In total, nine individuals experienced periods of oxygen saturation below 95% and two experienced periods of oxygen saturation below 90%. The lowest saturation recorded in one of these two was 89% and another was 82%. The duration of desaturation was very brief. Endoscopy was completed successfully in all patients. One of the patients experienced a very brief ventricular tachycardia that spontaneously reverted to sinus rhythm immediately (no drug administered). There were no airway problems in this patient and oxygenation was 98-99% throughout.
Table 3.
Airway types used by BMI group
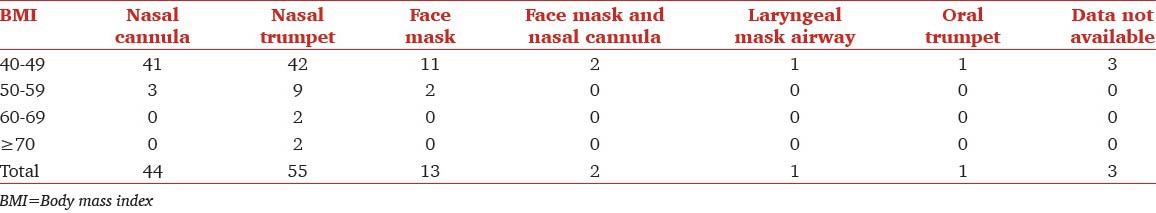
Figure 3.
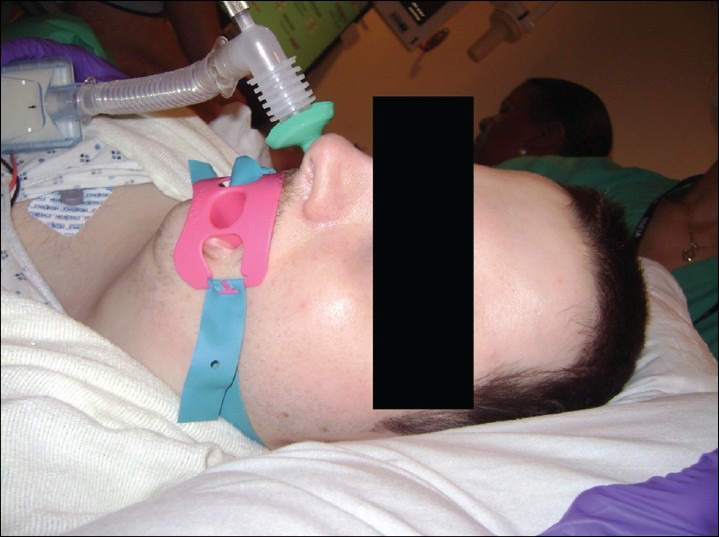
A size 32 nasal trumpet connected to mapleson-C breathing system
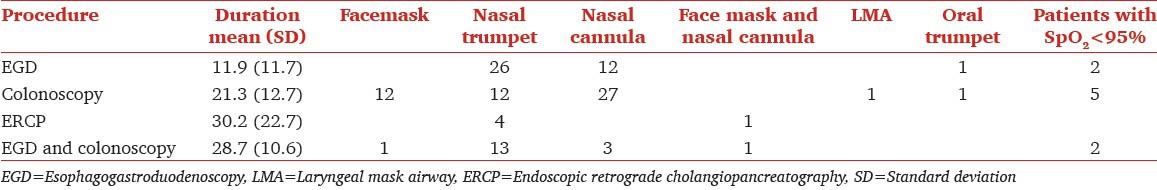
The duration of the procedure is important in trying to understand the significance of our findings. The mean standard deviation of each of the procedures is in Table 4. In general combined esophagogastroduodenoscopy with colonoscopy took much longer. The endoscopic retrograde cholangiopancreatography (ERCP) took longest and our data included all morbidly patients who underwent ERCP during this tile period. Considering the majority of ERCPs were in patients with either pancreatic malignancy or patients who had prior hepatic transplant, the number with morbid obesity was small in this sub group.
Table 4.
Relation between duration of the procedure, desaturation and the airway used
The anesthesia technique was a provider and procedure dependent. The majority of patients undergoing upper endoscopy (with or without colonoscopy) were planned with supplemental oxygen through a nasal trumpet (unless anticoagulated or had gross deviated nasal septum). These patients were asked to breathe 100% oxygen with a face mask. In the vast majority (about two thirds), fentanyl (25-50 mcg) was administered 2-3 min before administering propofol. The dose of propofol (between 0.5 and 2 mg/kg) was based on patient's weight, clinical response and comorbidity. As soon as the patients were unresponsive an attempt was made to insert an appropriate sized nasal airway. In the majority of patients, this was successful on first attempt. In a small minority, further administration of oxygen and propofol was required to facilitate tolerance to the insertion of nasal airway. Soon after insertion of the nasal airway, it was connected to the elbow of Mapleson breathing system (an oxygen flow of 8-10 l/min) as seen in Figure 3. Anesthesia was maintained on propofol infusion at 80-120 mcg/kg/min. In about one-third of the patients undergoing upper endoscopy, after 2-3 min of preoxygenation, propofol was administered until patient was unresponsive and nasal trumpet was inserted. However, the maintenance phase of anesthesia was achieved with a mixture of propofol and remifentanil (5 mcg of remifentanil mixed with each cc of propofol) started at 100-120 mcg/kg/min of propofol (ignoring any calculation for remifentanil). An effort was always made to time the endoscope insertion at peak clinical effect of propofol, which was usually soon after nasal trumpet was inserted. In some instances, oxygen was administered with nasal cannula at 4-6 l/min and nasal trumpet was inserted if saturations were seen to be dropping. An effort was made to keep the saturation close to 100% at all times. One of the patients was administered oxygen with a handheld jet ventilator via nasal trumpet. Although in this patient group (data collected over about 5 months), supraglottic jet ventilation was used on only one occasion, it is a technique used more often with some anesthesia providers for selected cases and procedures including morbidly obese. A colonoscopy was performed with a laryngeal mask airway and an endoscopy was provided supplemental oxygen with an oral trumpet. The “oral trumpet” refers to the nasal trumpet inserted in the mouth, beside the bite-block between the teeth, due to difficulty in inserting a nasal trumpet. The recovery time, from removal of the endoscope to verbal response, was short (3-5 min) with no unexpected prolongation in any of the patients.
The relation between the BMI and the airway is presented in Table 3. As can be appreciated, majority of patients with higher BMI were managed with a nasal trumpet. In additional, Table 5 shows that patients undergoing upper endoscopy got nasal airways significantly more often than patients undergoing colonoscopy alone. Table 6 shows the relationship between airway used and BMI for patients undergoing upper GI endoscopy alone. Clearly higher BMI patients were inserted with a nasal trumpet more often than patients with lower BMI.
Table 5.
Airway device used for the procedure
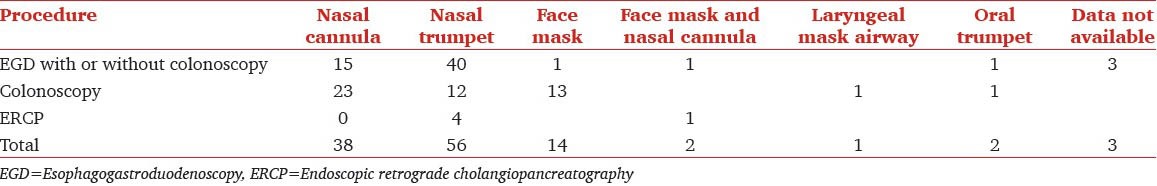
Table 6.
Relation between BMI and airway used for esophagogastroduodenoscopy with or without colonoscopy
For colposcopies (without esophagogastroduodenoscopy), oxygen was administered with a nasal cannula and the depth of anesthesia was adjusted to preserve the spontaneous ventilation. In case of apnea and (or) desaturation, either face mask or nasal trumpet was inserted.
Discussion
The prevalence of morbid obesity is increasing world-wide. Of all patients presenting for outpatient GI endoscopy in our hospital, nearly 6% were morbidly obese, i.e., had a BMI of over 40 kg/m2. We could not find any studies that have specifically reviewed the intraoperative airway management and outcomes in morbidly obese patients presenting for GI endoscopy under anesthesia. However, at least two studies have shown that obese patients run a higher perioperative risk for adverse airway events. In a prospective observational study involving 79 patients undergoing endoscopy under “conscious sedation,” Qadeer et al.[3] found a 51% overall incidence of hypoxemia (defined as saturation below 90% any time during the procedure, irrespective of the duration of hypoxia). They also found a significant difference in the rates of desaturation between non-obese (46%) and obese group (BMI >30 kg/m2 where it was 71%). In patients undergoing upper GI endoscopy with a BMI of over 28,[4] the baseline oxygen saturations were 94.86 ± 0.63% with a fall to 92.57 ± 1.06% after premedication. Endoscope insertion was associated with a further fall to 90.57 ± 1.27%. The saturation decreased below 90% for about 15% of total endoscopy time. In this study, only patients desaturating below 85% were provided supplemental oxygen. Patients in both of these studies were provided conscious sedation, administered by nurses under the supervision of gastroenterologists. One of the key finding in our study was that BMI did not directly relate to the tendency of desaturation. A logical explanation that distinguishes our finding from the previous studies[4,5] is that, anesthesiologist rather than gastroenterologists administered sedation in the present cohort. Our finding supports the ASA directive of propofol use only by practitioners trained in airway management (anesthesia providers in our case). Even the highest BMI subgroups (>60 kg/m2) did not show desaturation episodes in our study. Obesity has previously been reported as an independent risk factor for sedation related complications during propofol-mediated anesthesia for advanced endoscopic procedures.[5] In their prospective study, Wani et al. analyzed data of 1016 patients undergoing advanced endoscopic procedures. In obese subgroup, they found an incidence hypoxemia significantly higher than our practice. However, unlike our practice, wherein use of airway maneuvers is considered routine and part of anesthesia practice, Wani et al. used airway manipulations on an as needed basis.
The incidence of diagnosed OSA in our morbidly obese patient population was 64%. Predictably, this incidence increased with increasing severity of obesity, with incidence of 78% in patients above a BMI of 50 kg/m2. Although the number of patients with a BMI of over 60 was only 4, the incidence of OSA in this group was 100%. This incidence is similar to the one reported by Frey and Pilcher[6] In morbidly obese patients presenting for bariatric surgery, they found a 71% incidence of OSA. OSA rather than BMI values in our study was found to have significant relation to patients desaturation. OSA leads to significant alterations airway (narrowing) and central nervous system. Small doses of sedatives (propofol) can cause exaggerated response leading to airway obstruction. Thus, both dose titration and airway management are challenging in this subgroup. We were able to bypass the airway obstruction by use of nasal trumpet in these patients, but their physiologically altered responses can still lead to desaturation. Majority of our patients were using CPAP or BIPAP at home. Expectedly, treated OSA patients have a lower incidence of perioperative anesthetic complications, which is highlighted by the fact that patients with the highest OSA associated with highest BMI were not the ones who desaturated, as treatment of OSA probably improves physiological responses to sedation and decreases the likelihood of developing apnea.
The notion that the “administration of supplemental oxygen can suppress respiratory drive”, although true, should not, in and of itself, be a reason to avoid administration of supplemental oxygen. Consequently, we suggest that all measures should be taken to prevent hypoxemia especially in morbidly obese with OSA as their functional residual capacity (FRC) is relatively lower and oxygen consumption is relatively higher, on account of higher body weight. In the event of a hypoxemic event, these patients are most challenging to treat as well.
The following is the most likely explanation for incidence of hypoxemia that was significantly less than published study.
Practice of using a nasal airway connected to a Mapleson breathing system [Figure 3] allows administration of high concentration of oxygen at the tracheal inlet. High inflow of oxygen at the supraglottic area (10-15 l) could create sufficient positive pressure, thereby preventing the collapse of soft tissue in the velopharynx. This technique also provides apneic insufflation of oxygen thereby delaying hypoxemia and allowing more time to treat apnea/hypopnea
Use of supraglottic jet ventilation using a hand held jet device to treat apnea or hypopnea (although in this series, only one patient was supplemented with supraglottic jet ventilation). The actual device and the connections are represented in the picture [Figure 4]. The entrained air from the reservoir bag of the attached breathing system (connected to the nasal trumpet) contains a high percentage of oxygen
Ultra morbidly obese are anesthetized and endoscopy performed in supine-head up position. This position can help in preserving FRC by limiting basal lung atelectasis
By using a combined opioid (fentanyl-remifentanil)- propofol technique, propofol requirements are reduced and coughing is suppressed. In the majority of the cases, spontaneous ventilation can be maintained.
Figure 4.
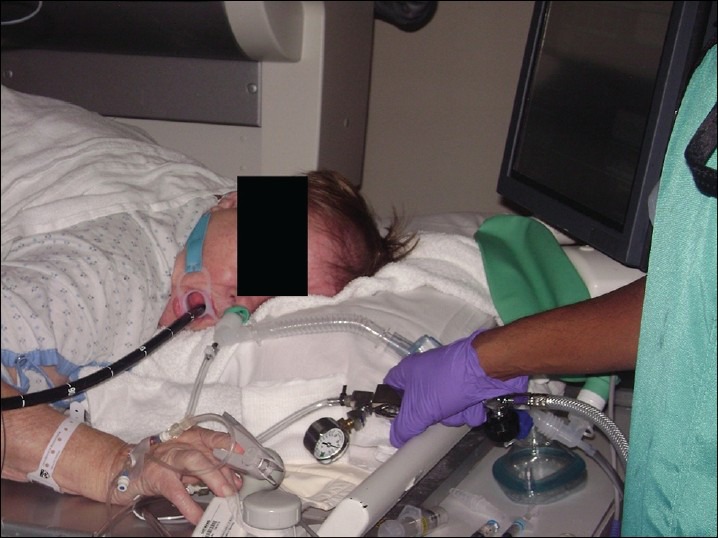
CO2 out port of an elbow adapter used to deliver supraglottic jet ventilation via nasal trumpet during an endoscopic retrograde cholangiopancreatography
The dose of propofol is controversial in morbidly obese patients.[7,8] All dosing methods have one aim, to predict the relationship between infusion rates and plasma concentrations (or more accurately, brain concentrations). The various theoretical pharmacokinetic compartments will not increase at the same rate as bodyweight and are likely a function of the percentage of muscle and fat mass, among other factors. The induction dose is likely to be a function of cardiac output with a tendency to use lean body weight for this part of the calculation. However, clearance and volumes of distribution are more likely related to total body weight than lean body weight and the infusions should be based on this. In our study, dosing was largely based on patient's clinical response with the aim of preserving spontaneous respiration. Preserving spontaneous ventilation is important since apnea signifies deeper anesthesia and positive pressure ventilation requires withdrawal of the endoscope in patients undergoing upper GI endoscopies.
Use of remifentanil-propofol mixtures is another controversial area. The mixture is likely to remain stable for at least 30 min.[9] Although the context sensitive halftimes are different for both, given that most GI procedures are under 60 min, it is unlikely to be of any clinical significance. The need to vary the dose of individual components (hypnotic and opioid) is not relevant as GI procedures are not painful like surgeries that involve a skin incision. Based on our findings, propofol along with a short acting opioid, based anesthesia seems to work best in the setting of morbidly obese patients presenting for endoscopy.
Several studies have looked at the mode of delivery of supplementary oxygen for patients undergoing upper GI endoscopy.[10,11,12,13,14,15] No data is available regarding the best mode of delivery of supplementary oxygen for patients undergoing GI endoscopy let alone morbidly obese patients. Based on our findings, we recommend a sequence of “preoxygenation-induction-insertion of nasal trumpet (connected to a Mapleson-C breathing system)-gastroduodenoscope insertion” for all upper GI endoscope procedures in morbidly obese patients.
Our study could be criticized for its retrospective nature. However, to do a study of this nature prospectively will be extremely difficult. Moreover, the results may be confounded by the presence of an observer. It might be ethically challenging to do a prospective study in this high risk area of anesthesia.
Conclusions
Increasing BMI does not bear direct correlation to the likelihood of patient's desaturating under sedation for GI procedures. Preoperative history of OSA irrespective of associated BMI values can be potentially used as a predictor of intra-procedural desaturation. With suitable modification of anesthesia technique, it is possible to reduce the incidence of adverse respiratory events in morbidly obese patients undergoing GI endoscopy procedures. Contrary to popular teaching, it is possible to anesthetize these patients without endotracheal intubation. The need to abort the procedure or withdraw the endoscope to facilitate treatment of apnea or hypopnea can be minimized.
Acknowledgement
Mr. Divakara Gouda, Cherry East High School, NJ, USA.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Metzner J, Posner KL, Domino KB. The risk and safety of anesthesia at remote locations: The US closed claims analysis. Curr Opin Anaesthesiol. 2009;22:502–8. doi: 10.1097/ACO.0b013e32832dba50. [DOI] [PubMed] [Google Scholar]
- 2.Gross JB, Bachenberg KL, Benumof JL, Caplan RA, Connis RT, Coté CJ, et al. Practice guidelines for the perioperative management of patients with obstructive sleep apnea: A report by the American Society of Anesthesiologists Task Force on Perioperative Management of patients with obstructive sleep apnea. Anesthesiology. 2006;104:1081–93. doi: 10.1097/00000542-200605000-00026. [DOI] [PubMed] [Google Scholar]
- 3.Qadeer MA, Rocio Lopez A, Dumot JA, Vargo JJ. Risk factors for hypoxemia during ambulatory gastrointestinal endoscopy in ASA I-II patients. Dig Dis Sci. 2009;54:1035–40. doi: 10.1007/s10620-008-0452-2. [DOI] [PubMed] [Google Scholar]
- 4.Dhariwal A, Plevris JN, Lo NT, Finlayson ND, Heading RC, Hayes PC. Age, anemia, and obesity-associated oxygen desaturation during upper gastrointestinal endoscopy. Gastrointest Endosc. 1992;38:684–8. doi: 10.1016/s0016-5107(92)70564-1. [DOI] [PubMed] [Google Scholar]
- 5.Wani S, Azar R, Hovis CE, Hovis RM, Cote GA, Hall M, et al. Obesity as a risk factor for sedation-related complications during propofol-mediated sedation for advanced endoscopic procedures. Gastrointest Endosc. 2011;74:1238–47. doi: 10.1016/j.gie.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frey WC, Pilcher J. Obstructive sleep-related breathing disorders in patients evaluated for bariatric surgery. Obes Surg. 2003;13:676–83. doi: 10.1381/096089203322509228. [DOI] [PubMed] [Google Scholar]
- 7.Lemmens HJ. Perioperative pharmacology in morbid obesity. Curr Opin Anaesthesiol. 2010;23:485–91. doi: 10.1097/ACO.0b013e32833b0a8c. [DOI] [PubMed] [Google Scholar]
- 8.Ingrande J, Brodsky JB, Lemmens HJ. Lean body weight scalar for the anesthetic induction dose of propofol in morbidly obese subjects. Anesth Analg. 2011;113:57–62. doi: 10.1213/ANE.0b013e3181f6d9c0. [DOI] [PubMed] [Google Scholar]
- 9.Stewart JT, Warren FW, Maddox FC, Viswanathan K, Fox JL. The stability of remifentanil hydrochloride and propofol mixtures in polypropylene syringes and polyvinylchloride bags at 22 degrees-24 degrees C. Anesth Analg. 2000;90:1450–1. doi: 10.1097/00000539-200006000-00037. [DOI] [PubMed] [Google Scholar]
- 10.Bell GD, Bown S, Morden A, Coady T, Logan RF. Prevention of hypoxaemia during upper-gastrointestinal endoscopy by means of oxygen via nasal cannulae. Lancet. 1987;1:1022–4. doi: 10.1016/s0140-6736(87)92282-3. [DOI] [PubMed] [Google Scholar]
- 11.Brandl S, Borody TJ, Andrews P, Morgan A, Hyland L, Devine M. Oxygenating mouthguard alleviates hypoxia during gastroscopy. Gastrointest Endosc. 1992;38:415–7. doi: 10.1016/s0016-5107(92)70467-2. [DOI] [PubMed] [Google Scholar]
- 12.Basavana G. Goudra, Preet Mohinder Singh, Ashish C. Sinha. “Anesthesia for ERCP: Impact of Anesthesiologist's Experience on Outcome and Cost,”. Anesthesiology Research and Practice 2013. 2013:5. doi: 10.1155/2013/570518. Article ID 570518. doi: 10.1155/2013/570518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goudra BG, Singh PM, Sinha AC. Outpatient endoscopic retrograde cholangiopancreatography: Safety and efficacy of anesthetic management with a natural airway in 653 consecutive procedures. Saudi J Anaesth. 2013;7:259–65. doi: 10.4103/1658-354X.115334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bell GD, Quine A, Antrobus JH, Morden A, Burridge SM, Coady TJ, et al. Upper gastrointestinal endoscopy: A prospective randomized study comparing continuous supplemental oxygen via the nasal or oral route. Gastrointest Endosc. 1992;38:319–25. doi: 10.1016/s0016-5107(92)70424-6. [DOI] [PubMed] [Google Scholar]
- 15.Wang CY, Ling LC, Cardosa MS, Wong AK, Wong NW. Hypoxia during upper gastrointestinal endoscopy with and without sedation and the effect of pre-oxygenation on oxygen saturation. Anaesthesia. 2000;55:654–8. doi: 10.1046/j.1365-2044.2000.01520.x. [DOI] [PubMed] [Google Scholar]


