Abstract
The molecular architecture of membrane vesicles prepared from Escherichia coli ML 308-225 has been studied by using crossed immunoelectrophoresis, and a reference pattern of 52 discrete immunoprecipitates has been established. Progressive immunoadsorption experiments conducted with untreated control vesicles and with physically disrupted vesicles demonstrate that the membrane-associated immunogens fall into two categories: (i) those immunogens typified by ATPase (ATP phosphohydrolase, EC 3.6.1.3) and NADH dehydrogenase [NADH: (acceptor) oxidoreductase, EC 1.6.99.3] whose expression is minimal unless the vesicles are disrupted; and (ii) immunogens such as Braun's lipoprotein that are expressed to similar extents in untreated and in disrupted vesicles. A mathematical relationship between the peak area subtended by an immunoprecipitate in the crossed immuno-electrophoresis system and the quantity of vesicles used in the adsorption process has been derived. This relationship allows quantitation of the degree to which specific membrane immunogens partition between exposed and unexposed surfaces of the vesicle membrane. The results demonstrate conclusively that >95% of the membrane in the vesicle preparations is in the form of sealed sacculi with the same polarity as the intact cell. Moreover, the findings provide a strong indication that dislocation of immunogens from the inner to the outer surface of the membrane during vesicle preparation does not occur to an extent exceeding 11%.
Keywords: crossed immunoelectrophoresis, zymograms, antibody adsorption, membrane topology
Full text
PDF

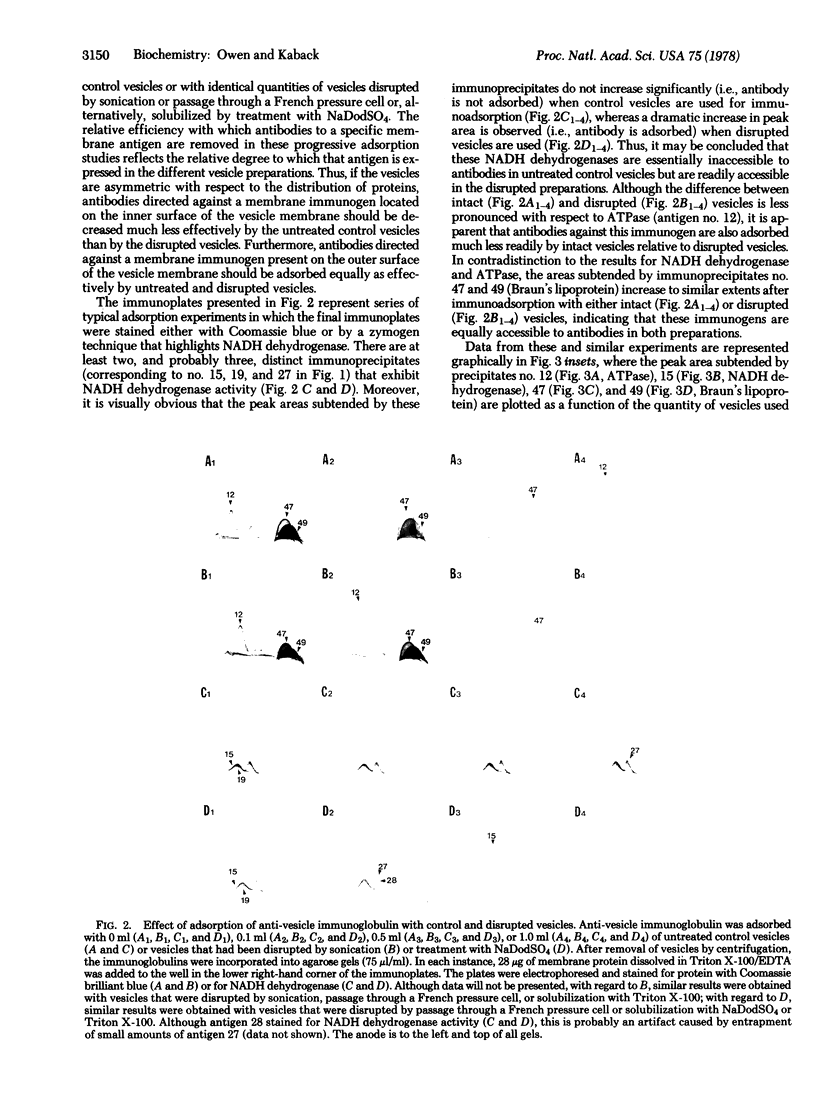
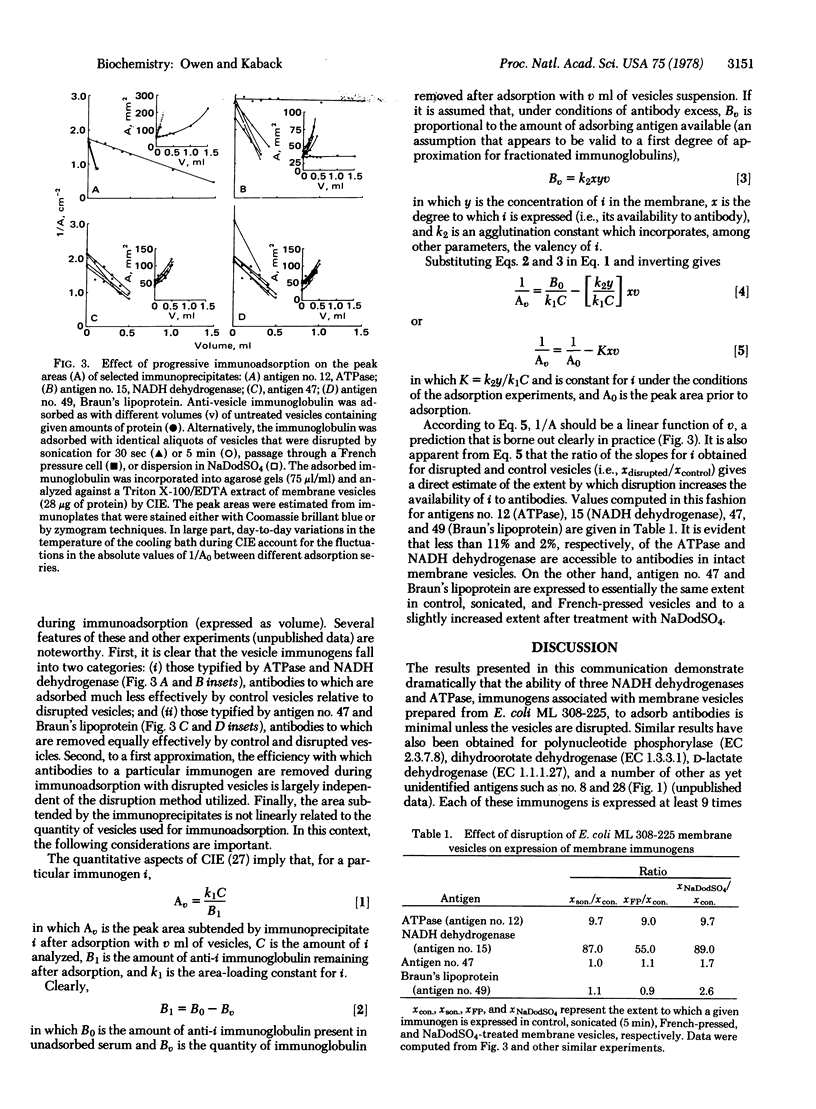

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler L. W., Rosen B. P. Functional mosaicism of membrane proteins in vesicles of Escherichia coli. J Bacteriol. 1977 Feb;129(2):959–966. doi: 10.1128/jb.129.2.959-966.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer M. E. Ultrastructure and organization of the bacterial envelope. Ann N Y Acad Sci. 1974 May 10;235(0):6–28. doi: 10.1111/j.1749-6632.1974.tb43254.x. [DOI] [PubMed] [Google Scholar]
- Braun V., Bosch V., Klumpp E. R., Neff I., Mayer H., Schlecht S. Antigenic determinants of murein lipoprotein and its exposure at the surface of Enterobacteriaceae. Eur J Biochem. 1976 Mar 1;62(3):555–566. doi: 10.1111/j.1432-1033.1976.tb10190.x. [DOI] [PubMed] [Google Scholar]
- Braun V. Covalent lipoprotein from the outer membrane of Escherichia coli. Biochim Biophys Acta. 1975 Oct 31;415(3):335–377. doi: 10.1016/0304-4157(75)90013-1. [DOI] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulley J. R., Grieve P. A. A simple technique for eliminating interference by detergents in the Lowry method of protein determination. Anal Biochem. 1975 Mar;64(1):136–141. doi: 10.1016/0003-2697(75)90415-7. [DOI] [PubMed] [Google Scholar]
- Futai M. Orientation of membrane vesicles from Escherichia coli prepared by different procedures. J Membr Biol. 1974;15(1):15–28. doi: 10.1007/BF01870079. [DOI] [PubMed] [Google Scholar]
- Futai M., Tanaka Y. Localization of D-lactate dehydrogenase in membrane vesicles prepared by using a french press or ethylenediaminetetraacetate-lysozyme from Escherichia coli. J Bacteriol. 1975 Oct;124(1):470–475. doi: 10.1128/jb.124.1.470-475.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare J. F., Olden K., Kennedy E. P. Heterogeneity of membrane vesicles from Escherichia coli and their subfractionation with antibody to ATPase. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4843–4846. doi: 10.1073/pnas.71.12.4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson K. E., Hjertén S. Localization of the Tween 20-soluble membrane proteins of Acholeplasma laidlawii by crossed immunoelectrophoresis. J Mol Biol. 1974 Jun 25;86(2):341–348. doi: 10.1016/0022-2836(74)90023-0. [DOI] [PubMed] [Google Scholar]
- Kaback H. R. Molecular biology and energetics of membrane transport. J Cell Physiol. 1976 Dec;89(4):575–593. doi: 10.1002/jcp.1040890414. [DOI] [PubMed] [Google Scholar]
- Kaback H. R. Transport across isolated bacterial cytoplasmic membranes. Biochim Biophys Acta. 1972 Aug 4;265(3):367–416. doi: 10.1016/0304-4157(72)90014-7. [DOI] [PubMed] [Google Scholar]
- Kaback H. R. Transport studies in bacterial membrane vesicles. Science. 1974 Dec 6;186(4167):882–892. doi: 10.1126/science.186.4167.882. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MITCHELL P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961 Jul 8;191:144–148. doi: 10.1038/191144a0. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc. 1966 Aug;41(3):445–502. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Performance and conservation of osmotic work by proton-coupled solute porter systems. J Bioenerg. 1973 Jan;4(1):63–91. doi: 10.1007/BF01516051. [DOI] [PubMed] [Google Scholar]
- Owen P., Salton M. R. Antigenic and enzymatic architecture of Micrococcus lysodeikticus membranes established by crossed immunoelectrophoresis. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3711–3715. doi: 10.1073/pnas.72.9.3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen P., Salton M. R. Membrane asymmetry and expression of cell surface antigens of Micrococcus lysodeikticus established by crossed immunoelectrophoresis. J Bacteriol. 1977 Dec;132(3):974–978. doi: 10.1128/jb.132.3.974-985.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos S., Kaback H. R. The electrochemical proton gradient in Escherichia coli membrane vesicles. Biochemistry. 1977 Mar 8;16(5):848–854. doi: 10.1021/bi00624a006. [DOI] [PubMed] [Google Scholar]
- Ramos S., Kaback H. R. The relationship between the electrochemical proton gradient and active transport in Escherichia coli membrane vesicles. Biochemistry. 1977 Mar 8;16(5):854–859. doi: 10.1021/bi00624a007. [DOI] [PubMed] [Google Scholar]
- Ramos S., Kaback H. R. pH-dependent changes in proton:substrate stoichiometries during active transport in Escherichia coli membrane vesicles. Biochemistry. 1977 Sep 20;16(19):4270–4275. doi: 10.1021/bi00638a022. [DOI] [PubMed] [Google Scholar]
- Ramos S., Schuldiner S., Kaback H. R. The electrochemical gradient of protons and its relationship to active transport in Escherichia coli membrane vesicles. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1892–1896. doi: 10.1073/pnas.73.6.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salton M. R., Netschey A. Physical chemistry of isolated bacterial membranes. Biochim Biophys Acta. 1965 Oct 18;107(3):539–545. doi: 10.1016/0304-4165(65)90198-4. [DOI] [PubMed] [Google Scholar]
- Short S. A., Kaback H. R., Kohn L. D. Localization of D-lactate dehydrogenase in native and reconstituted Escherichia coli membrane vesicles. J Biol Chem. 1975 Jun 10;250(11):4291–4296. [PubMed] [Google Scholar]
- Smyth C. J., Friedman-Kien A. E., Salton M. R. Antigenic analysis of Neisseria gonorrhoeae by crossed immunoelectrophoresis. Infect Immun. 1976 Apr;13(4):1273–1288. doi: 10.1128/iai.13.4.1273-1288.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth C. J., Siegel J., Salton M. R., Owen P. Immunochemical analysis of inner and outer membranes of Escherichia coli by crossed immunoelectrophoresis. J Bacteriol. 1978 Jan;133(1):306–319. doi: 10.1128/jb.133.1.306-319.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroobant P., Kaback H. R. Ubiquinone-mediated coupling of NADH dehydrogenase to active transport in membrane vesicles from Escherichia coli. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3970–3974. doi: 10.1073/pnas.72.10.3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuda H., Kaback H. R. Sodium-dependent methyl 1-thio-beta-D-galactopyranoside transport in membrane vesicles isolated from Salmonella typhimurium. Biochemistry. 1977 May 17;16(10):2130–2136. doi: 10.1021/bi00629a013. [DOI] [PubMed] [Google Scholar]
- Weiner J. H. The localization of glycerol-3-phosphate dehydrogenase in Escherichia coli. J Membr Biol. 1974;15(1):1–14. doi: 10.1007/BF01870078. [DOI] [PubMed] [Google Scholar]
- Wickner W. Fractionation of membrane vesicles from coliphage M13-infected Escherichia coli. J Bacteriol. 1976 Jul;127(1):162–167. doi: 10.1128/jb.127.1.162-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Thienen G., Postma P. W. Coupling between energy conservation and active transport of serine in Escherichia coli. Biochim Biophys Acta. 1973 Oct 25;323(3):429–440. doi: 10.1016/0005-2736(73)90188-0. [DOI] [PubMed] [Google Scholar]



