Abstract
Heparin is a critically important anticoagulant drug that was contaminated with a persulfonated polysaccharide in 2008, resulting in a number of severe adverse reactions, some leading to death. Controversy remains as to the precise composition of the 2008 contaminant and new information suggests that heparin may now be subject to adulteration with a new, difficult to detect, contaminant, N-sulfo oversulfated chondroitin sulfate. This study synthesizes this new potential contaminant and describes the use of radical depolymerization followed by liquid chromatography-mass spectrometry to detect N-sulfo oversulfated chondroitin sulfate and to confirm the structure of the 2008 contaminant as oversulfated chondroitin sulfate and not oversulfated heparan sulfate.
Keywords: heparin, oversulfated chondroitin sulfate, oversulfated heparan sulfate, N-sulfated oversulfated chondroitin sulfate, contaminant, radical depolymerization, mass spectrometry
Heparin is an important and widely used anticoagulant that is an essential component of modern medicine.1 This polysaccharide natural product is prepared in ton quantities principally from pig intestinal tissues.2 In 2008 there was a contamination crisis involving much of the world’s heparin supply coming from China and associated with severe anaphlyactoid reactions leading to patient deaths.3 The contamination of heparin was believed to be deliberate and the adulterant causing these reactions was identified as an oversulfated chondroitin sulfate (OSCS)4 that was prepared by the chemical sulfonation5 of an inexpensive nutraceutical polysaccharide, chondroitin sulfate (CS).6 In the aftermath of the 2008 contamination crisis, there was some disagreement as to whether or not OSCS was the sole contaminant in the adulterated heparin and it was speculated that heparan sulfate (HS), a side-stream in heparin manufacturing, might have been chemically sulfonated to prepare oversulfated heparan sulfate (OSHS) and also used as an adulterant.7 A rigorous discussion of this possibility ensued,8,9 but a consensus arose that the principal contaminant present was OSCS and that no conclusive evidence supported the presence of OSHS in contaminated lots of heparin.10,11
Since the contamination crisis there has been extensive efforts aimed at ensuring the safety of the heparin supply.6 Better control has been placed on the collection and processing of animal tissues used to prepare raw heparin, new analytical methods have been develop to detect OSCS in heparin,12 the monographs controlling heparin active pharmaceutical ingredient (API) have been revised,13 and new efforts are underway to chemically synthesize or bioengineer heparins to reduce dependence on animal-sourced heparin.14 The updated United States Pharmacopeia (USP) monograph now contains two assays useful for determining the absolute concentration of OSCS in heparin API, relying on proton nuclear magnetic resonance spectrometry (1H-NMR) and ion-exchange high performance liquid chromatography (HPLC).15 One additional assay examines the relative amounts of glucosamine (present in heparin and HS) and galactosamine (present in chondroitin sulfates), to test for both OSCS contaminant and chondroitin sulfate impurities, both containing galactosamine.13 Because of the polyanionic nature of glycosaminoglycans, capillary electrophoresis (CE) has also applied to analyze both contaminants and impurities in heparin, such as OSCS and dermatan sulfate.16 Despite these efforts there was a disturbing report that a new contaminant, N-deacetylated/N-sulfonated OSCS (NSOSCS), which is difficult to detect using current analytical methods, was making its way into heparin API in Asian countries.15 The current USP 1H-NMR assay, focused on the absence of the N-acetyl signal associated with OSCS at 2.08 ppm, fails to detect the N-deacetylated polysaccharide, NSOSCS, and the current HPLC assay is often not performed at a sufficiently high salt elution to detect the presence of the very highly sulfated NSOSCS (OSCS has four negatively charged sulfo groups/ disaccharide repeating unit while NSOSCS has five sulfo groups/ disaccharide repeating unit). Moreover, the relative ratio of glucosamine to galactosamine can be readily manipulated through the addition of small amounts of the inexpensive monosaccharide, glucosamine. The similar capillary electrophoresis (CE) migration rates of glycosaminoglycan-based contaminants also make it difficult to distinguish these polysaccharides by CE. Furthermore, enzymatic depolymerization reactions using polysaccharide lyases, pioneered in our laboratory for the selective depolymerization of natural glycosaminoglycans for oligosaccharide analysis,17,18 does not work on the unnatural structures present in chemically sulfonated glycosaminoglycans. Based on these concerns, our laboratory undertook to devise a sensitive assay that could be used to detect all chemically sulfonated glycosaminoglycan-based contaminants.
Reactive oxygen species (ROS), generated through the use of chemical reagents and radiation have been previously used to depolymerize sulfated polysaccharides.19–22 Unlike enzymatic or base catalyzed depolymerization, ROS-based depolymerization is relatively non-selective fragmenting a wide variety of polysaccharides, even ones resistant to enzymatic depolymerization.23 ROS-based depolymerization has been applied to glycosaminoglycans, such as heparin,19 chondroitin/dermatan sulfate,20 hyaluronan,21 and marine glycosaminoglycans.22 These studies demonstrate that ROS-based depolymerization is highly reproducible route to degrade glycosaminoglycans. Moreover, unlike acid hydrolysis, there is no preferential cleavage of side chains or sulfo groups and the primary structure of the molecule is retained after ROS-based depolymerization. As a consequence, we selected ROS-based depolymerization to investigate the glycosaminoglycans resistant to enzymatic depolymerization, including OSCS, OSHS and NSOSCS, using copper ion and hydrogen peroxide followed by liquid chromatography-mass spectrometry (LC-MS).
The syntheses of oversulfated polysaccharides were adapted from published methods (see Supporting Information for detailed Methods and Figure S1). OSCS was prepared through the treatment of the tetrabutylammonium salt of CS with sulfur trioxide-pyridine complex in dimethylformamide.5 OSHS was synthesized from porcine intestinal HS and relied on the same chemistry but required a subsequent N-sulfonation in aqueous sodium bicarbonate to replace N-sulfo groups lost on O-sulfonation24 The synthesis of NSOSCS began with de-N-acetylation of CS using hydrazine/hydrazine sulfate, O-sulfonation in dimethylformamide, and aqueous N-sulfonation. 24 1H-NMR characterization of each derivative confirmed their structures (Figures S2–S4).
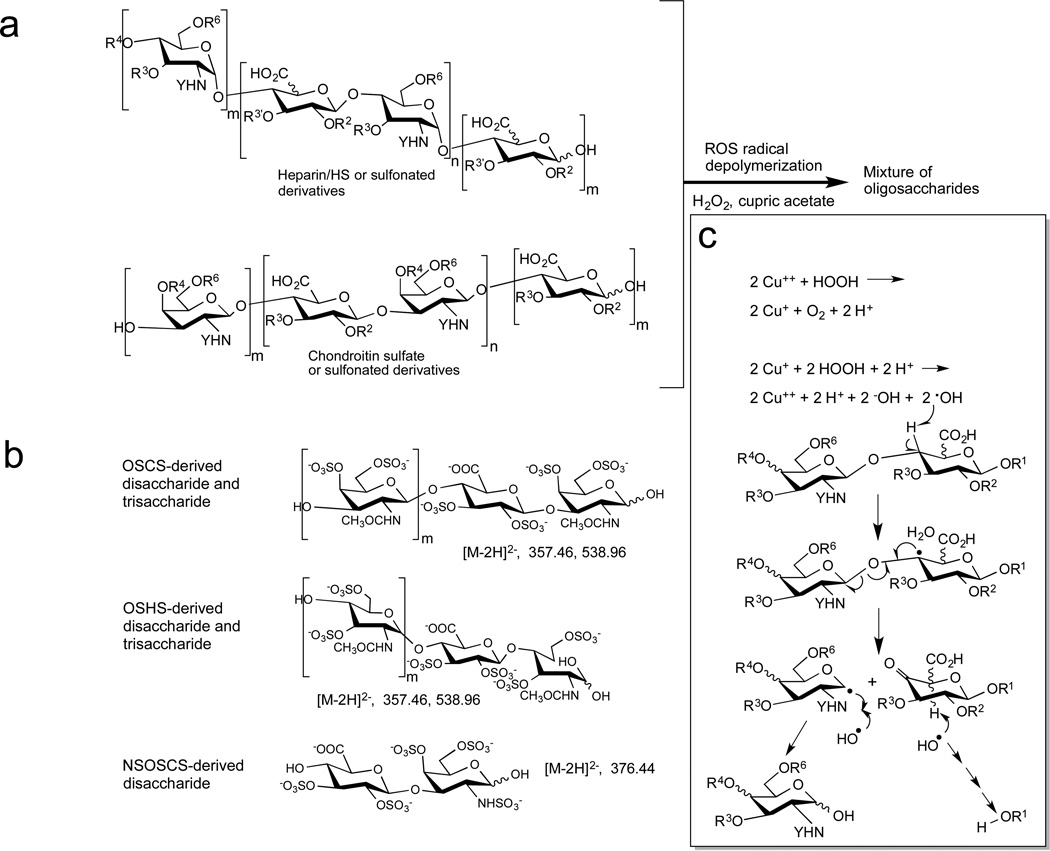
The oxidative depolymerization of OSCS, OSHS, NSOSCS and contaminated heparin relied on treatment with hydrogen peroxide and cupric acetate at 45 °C for 3 h, after which the reaction was quenched with sodium bisulfite (Figure 1).22 The level, of polysaccharide degradation, was controlled by altering the amount of hydrogen peroxide (Figure S5), and the conditions for nearly completely degradation (0.6% H2O2 in water v/v) were applied. Hydrophilic interaction liquid chromatography (HILIC)-MS analysis, which had been recently demonstrated on low molecular weight heparins,25 was utilized for the direct analysis of ROS-treated chemically sulfonated glycosaminoglycans.
Figure 1.
Oxidative depolymerization and HILIC-MS of heparin, OSHS, OSCS and NSOSCS. a. Structure of polysaccharides and their persulfonated derivatives, where R is H or SO3− with the superscript referring to its ring-position, Y is COCH3, or SO3−, m = 0 or 1, n = 8–60 (depending on chain molecular weight), and their oxidative depolymerization to a mixture of oligosaccharides. b. HILIC-MS the mixture of oligosaccharides afforded through oxidative depolymerization affords the following structures with diagnostic [M-2H]2− ions. c. Proposed mechanism for radical depolymerization of heparin, heparan sulfate, chondroitin sulfate and their sulfonated derivatives.19,26 Cupric acetate and hydrogen peroxide for hydroxyl radicals that can abstract the hydrogen, resulting in a radical species that can fragment the chain at the glycosidic linkage. Further radical reactions can result in the recovery of the reducing end sugar breakdown of the non-reducing end sugar. R3 and R4 are H or SO3− or the anomeric carbon of the next sugar, Y is COCH3, or SO3−.
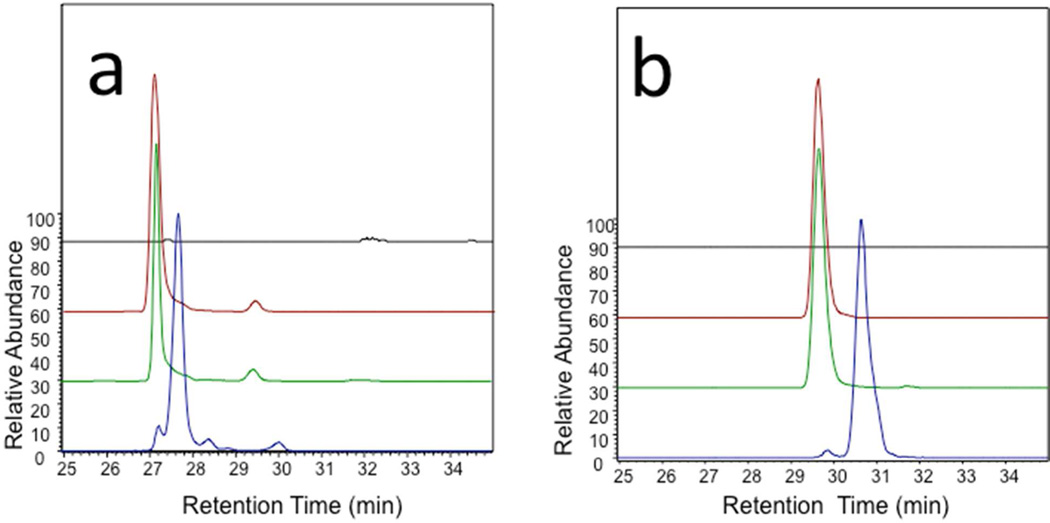
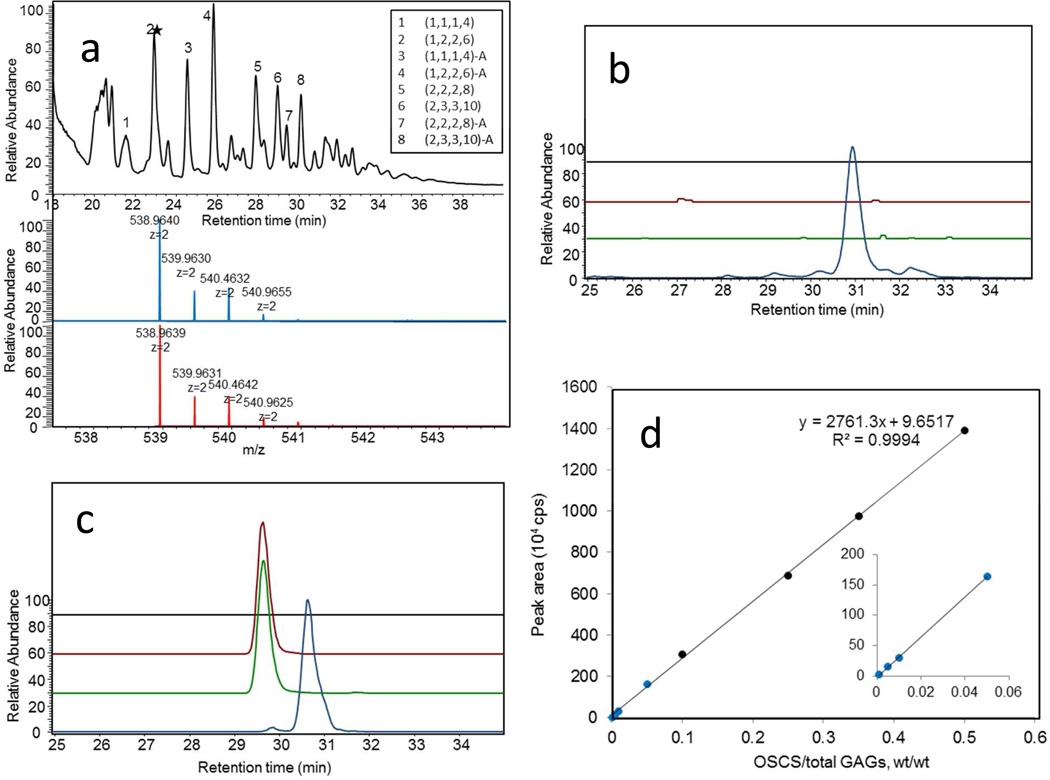
Direct analysis of oxidatively depolymerized OSCS and OSHS by HILIC-MS showed m/z 357.4674 and m/z 538.9639 peaks corresponding to [M-2H]2− ion for tetrasulfated disaccharide and hexasulfated trisaccharide, respectively (Figure 2). The OSCS- derived and OSHS-derived hexasulfated trisaccharides showed baseline separation on HILIC so they were used for quantitative analysis (Figures 2 and 3). Using extracted ion chromatography, as little as 0.1 wt% OSCS could be detected in heparin (Figure 3) with a 97–105% recovery (Table 1). Examination of a formulated sample of contaminated heparin showed 19.45 ± 0.37 wt% OSCS and no OSHS present (Figure 3b) consistent with its previous analysis by a number of other methods.11 Next, we analyzed oxidatively depolymerized NSOSCS by HILIC-MS and it showed an m/z 376.4405 peak corresponding to [M-2H]2− ion for a unique pentasulfated disaccharide (Figure 3c).
Figure 2.
Extracted ion chromatograms of peaks a. at m/z 357.4674 (± 5 ppm) and at b. m/z 538.9639 (± 5 ppm). Samples are heparin (black), contaminated heparin sample (red), oversulfated chondroitin sulfate (green), and oversulfated heparan sulfate (blue).
Figure 3.
HILIC-MS analysis of the mixture of oligosaccharides afforded through oxidative depolymerization of heparin, OSHS, OSCS and NSOSCS. a. High resolution mass analysis of depolymerized oligomers. The total ion chromatogram of ROS radical depolymerized OSCS analyzed by HILIC-MS. Peak 2, indicated by the star was selected as a characteristic marker for the presence of OSCS. The mass spectrum for peak 2 ((1,2,2,6), where the oligosaccharide compositions are given as (HexA, HexN, Ac, SO3)) is shown (blue). The theoretical mass data for 2 (1,2,2,6) is also presented (red). b. HILIC-MS EIC of mass 538.9639 (± 5 ppm) of oxidatively depolymerized: heparin (black), contaminated heparin sample (red), OSCS (green), and OSHS (blue). c. HILIC-MS EIC of mass 376.4405 (± 5 ppm) of oxidatively depolymerized: heparin (black), contaminated heparin sample (red), OSCS (green), and NSOSCS (yellow). d. Detection of different amounts of OSCS added to heparin following oxidative depolymerization by HILIC-MS EIC of mass 538.963.
Table 1.
Recovery of OSCS from contaminated heparin sample.
| Contaminated heparin, µg |
Amount of OSCS in contaminated heparin, µg |
Amount of OSCS added, µg |
Determined amount of OSCS, µg |
OSCS recovery, % |
RSD % n = 3 |
|
|---|---|---|---|---|---|---|
| 1 | 95.0 | 18.5 | 5.0 | 23.4 | 99.5 | 2.72 |
| 2 | 90.0 | 17.5 | 10.0 | 28.9 | 105 | 1.76 |
| 3 | 80.0 | 15.6 | 20.0 | 36.3 | 102 | 1.82 |
| 4 | 70.0 | 13.6 | 30.0 | 42.5 | 97.3 | 2.93 |
Following oxidative depolymerization the absence of a characteristic HILIC-MS peaks for OSHS tetrasulfated disaccharide and hexasulfated trisaccharide, corresponding to [M-2H]2− ions of m/z 357.4674 and m/z 538.9639, again confirm the identity of the contaminant as OSCS in a 2008 contaminated heparin sample.
A rapid and sensitive method for the analysis of unnatural, chemically sulfonated polysaccharide contaminants in heparin has been developed that relies on oxidative depolymerization followed by LC-MS. This method confirms that OSCS was the sole contaminant in Chinese heparin contaminated in 2008 and that OSHS was not present. This method is also capable of detecting a new potential contaminant, NSOSCS, which cannot be detected by current USP assays, and has been recently reported present in heparin API produced in Asia. NSOSCS contaminated heparin samples are not currently available to test our method, so it was only tested on heparin spiked with NSOCS.
METHODS
Chemical synthesis of oversulfated glycosaminoglycans
OSCS was synthesized by the chemical O-sulfonation of the tetrabutylammonium salt of chondroitin sulfate with sulfur trioxide-pyridine in anhydrous N, N’-dimethylformamide. OSHS and NSOSCS were synthesized from heparan sulfate and chondroitin sulfate, respectively, by hydrazine/hydrazine sulfatecatalyzed de-N-acetylation and oxidation with iodine followed by chemical O-sulfonation of their tetrabutylammonium salts with sulfur trioxide-pyridine in anhydrous N, N’-dimethylformamide and subsequent N-sulfonation in aqueous sodium carbonate using sulfur trioxide-trimethylamine complex.
Controlled oxidative depolymerization and HILIC LC-FTMS analysis
Glycosaminoglycan samples were degraded by controlled oxidative depolymerization using hydrogen peroxide and cupric acetate. Sodium bisulfite was added to terminate the reaction by removing excess unreacted hydrogen peroxide and the reaction mixture was then lyophilized. The mixture of oligosaccharides, generated from oxidative depolymerization of heparin, OSHS, OSCS and NSOSCS, were fractionated by HILIC-HPLC column using an aqueous ammonium acetate-acetonitrile gradient connected online to the standard ESI source of LTQ-Orbitrap XL FT MS.
Supplementary Material
ACKNOWLEDGMENTS
The authors are grateful for the support of the National Institutes of Health in the form of grants GM38060, HL096972, ES020903 to RJL and by grants from the China Scholarship Council and Program for Changjiang Scholars and Innovative Research Team in University (IRT1188) and National Marine Public Welfare Scientific Research Project of China (No.201105029).
Footnotes
ASSOCIATED CONTENT
Supporting Information
Complete experimental methods, compound characterization and spectral data. This material is available free of charge via the internet at http://pubs .acs.org.
Notes
The authors declare no competing financial interest.
REFERENCES
- 1.Linhardt RJ. J Med Chem. 2003;46:2551–2554. doi: 10.1021/jm030176m. [DOI] [PubMed] [Google Scholar]
- 2.Bhaskar U, Sterner E, Hickey AM, Onishi A, Zhang F, Sordick JS, Linhardt RJ. Appl. Microbiol. Biotechnol. 2012;93:1–16. doi: 10.1007/s00253-011-3641-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kishimoto TK, Viswanathan K, Ganguly T, Elankumaran S, Smith S, Pelzer K, Lansing JC, Sriranganathan N, Zhao G, Gargova ZG, HaKim AA, Bailey GS, Fraser B, Roy S, Cotrone TR, Buhse L, Whary M, Fox J, Nasr M, Pan GJD, Shriver Z, Langer RS, Venkataraman G, Austen KF, Woodcock J, Sasisekharan R. New Engl. J. Med. 2008;358:2457–2467. doi: 10.1056/NEJMoa0803200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guerrini M, Beccati D, Shriver Z, Naggi A, Viswanathan K, Bisio A, Capila I, Lansing JC, Guglieri S, Fraser B, Hakim AA, Gunay NS, Zhang Z, Robinson L, Buhse L, Nasr M, Woodcock J, Langer R, Venkataraman G, Linhardt RJ, Casu B, Torri G, Sasisekharan R. Nat. Biotechnol. 2008;26:669–775. doi: 10.1038/nbt1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maruyama T, Toida T, Yu G, Linhardt RJ. Carbohydr. Res. 1998;306:35–43. doi: 10.1016/s0008-6215(97)10060-x. [DOI] [PubMed] [Google Scholar]
- 6.Liu H, Zhang Z, Linhardt RJ. Nat. Prod. Rep. 2009;26:313–321. doi: 10.1039/b819896a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pan J, Qian Y, Zhou X, Pazandak A, Frazier SB, Weiser P, Lu H, Zhang L. Glycobiol. Insight. 2010:1–12. [PMC free article] [PubMed] [Google Scholar]
- 8.Pan J, Qian Y, Zhou X, Pazandak A, Frazier SB, Weiser P, Lu H, Zhang L. Nat. Biotechnol. 2010;28:203–207. doi: 10.1038/nbt0310-203. [DOI] [PubMed] [Google Scholar]
- 9.Guerrini M, Shriver Z, Naggi A, Casu B, Linhardt RJ, Torri G, Sasisekharan R. Nat. Biotechnol. 2010;28:207–211. [Google Scholar]
- 10.Guerrini M, Zhang Z, Shriver Z, Naggi A, Masuko S, Langer R, Casu B, Linhardt RJ, Sasisekharan R. Proc. Nat. Acad. Sci. USA. 2009;106:16956–16961. doi: 10.1073/pnas.0906861106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Z, Xiao Z, Masuko S, Zhao W, Sterner E, Bansal V, Fareed J, Dordick J, Zhang F, Linhardt RJ. Anal. Biochem. 2011;408:147–156. doi: 10.1016/j.ab.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beni S, Limtiaco JF, Larive CK. Anal. Bioanal. Chem. 2011;399:527–539. doi: 10.1007/s00216-010-4121-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.United States Pharmacopeia, Monograph on heparin sodium.
- 14.Linhardt RJ, Liu J. Curr. Opin. Pharmacol. 2012;12:217–219. doi: 10.1016/j.coph.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toida T. Abstracts of Papers, 245th ACS National Meeting New Orleans, Louisiana. Washington, DC. April 7–11, 2013; American Chemical Society; 2013. CARB 49. [Google Scholar]
- 16.Somsen G, Mol R, Jong GJ. J. Chromatogr. A. 2010;1217:3978–3991. doi: 10.1016/j.chroma.2010.01.026. [DOI] [PubMed] [Google Scholar]
- 17.Linhardt RJ. In: Analysis of Glycoconjugates. Varki A, editor. V 2. Boston: Wiley Interscience; 1994. pp. 17.13.17–17.13.32. [Google Scholar]
- 18.Xiao Z, Tappen BR, Ly M, Zhao W, Canova LP, Guan H, Linhardt RJ. J. Med. Chem. 2011;54:603–610. doi: 10.1021/jm101381k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vismara E, Pierini M, Mascellani G, Liverani L, Lima M, Guerrini M, Torri G. Thrombos. Haemostas. 2010;103:613–622. doi: 10.1160/TH09-02-0084. [DOI] [PubMed] [Google Scholar]
- 20.Ofman D, Slim GC, Watt DK, Yorke S. Carbohydr. Polym. 1997;33:47–56. [Google Scholar]
- 21.Zhao X, Yang B, Li L, Zhang F, Linhardt RJ. Carbohydr. Polym. 2013;96:503–509. doi: 10.1016/j.carbpol.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen S, Li G, Wu N, Guo X, Liao N, Ye X, Liu D, Xue C, Chai W. BBA-Gen. Subjects. 2013;1830:3054–3066. doi: 10.1016/j.bbagen.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 23.Linhardt RJ. Curr. Protoc. Mol. Biol. 2001:17–17.13B. doi: 10.1002/0471142727.mb1713bs48. [DOI] [PubMed] [Google Scholar]
- 24.Nadkarni VD, Toida TT, Gorp CV, Schubert RL. Carbohydr. Res. 1996;290:87–96. doi: 10.1016/0008-6215(96)00129-2. [DOI] [PubMed] [Google Scholar]
- 25.Li L, Zhang F, Zaia J, Linhardt RJ. Anal. Chem. 2012;84:8822–8829. doi: 10.1021/ac302232c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kennett EC, Davies MJ. Free Radic. Biol. Med. 2009;47:389–400. doi: 10.1016/j.freeradbiomed.2009.05.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.