Abstract
Retargeting of lentiviral vector entry to cell types of interest is a key factor in improving the safety and efficacy of gene transfer. In this study we show that the retargetable envelope glycoproteins of measles virus (MV), namely, the hemagglutinin (H) responsible for receptor recognition and the fusion protein (F), can pseudotype human immunodeficiency virus 1 (HIV-1) vectors when their cytoplasmic tails are truncated. We then pseudotyped HIV-1 vectors with MV glycoproteins displaying on H either the epidermal growth factor or a single-chain antibody directed against CD20, but without the ability to recognize their native receptors. Gene transfer into cells that expressed the targeted receptor was several orders of magnitude more efficient than into cells that did not. High-target versus nontarget cell discrimination was demonstrated in mixed cell populations, where the targeting vector selectively eliminated CD20-positive cells after suicide gene transfer. Remarkably, primary human CD20-positive B lymphocytes were transduced more efficiently by the CD20-targeted vector than by a vector pseudo-typed with the vesicular stomatitis virus G (VSV-G) protein. In addition, the CD20-targeted vector was able to transduce even unstimulated primary B cells, whereas VSV-G pseudotyped vectors were unable to do so. Because MV enters cells through direct fusion at the cell membrane, this novel targeting system should be widely applicable.
INTRODUCTION
Lentiviral vectors allow stable long-term transgene expression in nondividing cells and in tissues. These properties have made them ideal gene delivery vehicles for research and therapeutic applications, 1 including in clinical trials.2 However, further refinements in vector design are required for improving the safety and efficacy of lentiviral vector–mediated gene transfer. Special attention has to be given to measures that restrict gene transfer to the cell type relevant for a particular therapeutic application. These include the use of tissue-specific promoters (promoter targeting), and detargeting of irrelevant cell types from gene expression by inserting target sequences for tissue-specific miRNAs.1,3
Ideally, however, gene transfer should be restricted at the step of cell entry itself; however, an effective and universally applicable system for cell entry targeting is still not available. Lentiviral vectors are usually pseudotyped with the glycoprotein (G) of vesicular stomatitis virus (VSV) or the envelope (Env) proteins of γ-retroviruses such as murine leukemia virus (MLV). These proteins confer a broad tropism that mediates gene transfer into all human cell types. There are only a few exceptions that allow for selective transduction of a specific cell population.4,5 Although the rules governing the incorporation of foreign glycoproteins into lentiviral particles are not yet fully understood, high cell-surface expression levels in the packaging cell are always of critical importance. 6,7 Moreover, modifications in the cytoplasmic tail of the glycoprotein may be essential.8,9
Attempts to engineer receptor usage were initiated >15 years ago, but this protein engineering task proved to be difficult, especially in respect of envelope proteins that combine the receptor attachment and membrane fusion functions, such as the retroviral Env or VSV-G. In many cases, although vector particles did specifically bind the retargeted receptor, gene transfer was absent or very inefficient. 10,11 Alternative targeting strategies have been developed based on specific requirements such as the surface expression of special proteases on the target cell.12,13 Recently, lentiviral vectors have been pseudotyped with engineered Sindbis virus glycoproteins without the ability to recognize their natural receptor and have been modified to either noncovalently bind a monoclonal antibody directed against a surface antigen, or to become co-incorporated into vector particles together with a complete antibody molecule.14,15 Although such vector particles have shown promising targeting capabilities, this strategy has limitations because of the noncovalent linkage to the antibody, and the requirement for the targeted receptor to become endocytosed after vector binding so as to activate the membrane fusion function of the Sindbis virus glycoprotein by low pH.
In contrast, the measles virus (MV) glycoproteins, namely, hemagglutinin (H) and fusion protein (F), mediate cell entry directly at the cell membrane in a pH-independent manner.16 H protein attaches the virus particle to the MV receptors, which are CD46 and SLAM for the MV vaccine strains.16 CD46, a complement regulatory protein, is expressed on all human cell types except erythrocytes, while SLAM is confined to leukocyte subsets, especially the activated T and B cells. Moreover, H forms a complex with F and fulfils fusion helper functions, for which residues in the membrane proximal part of the H cytoplasmic tail are essential.17 For MV, a particularly efficient and compliant cell entry retargeting system has recently been established: the receptor contact residues in H protein are mutated18 and a single-chain antibody (scAb) is fused to its ectodomain.19 Such retargeted MVs infect MV receptor–negative cell lines when these cells express the cognate cell-surface antigen and selectively spread through antigen-positive tumor cells both in vitro and in vivo.19–22
We hypothesized that the H and F proteins of MV can confer efficient cell entry targeting to lentiviral vectors. In this study we show that cytoplasmic tail variants of H and F mediate efficient pseudotyping of human immunodeficiency virus 1 (HIV-1) vector particles. We also demonstrate retargeting of such particles to epidermal growth factor receptor (EGFR)-expressing cells and to CD20-positive lymphocytes.
RESULTS
Pseudotyping of lentiviral vector particles with MV glycoproteins
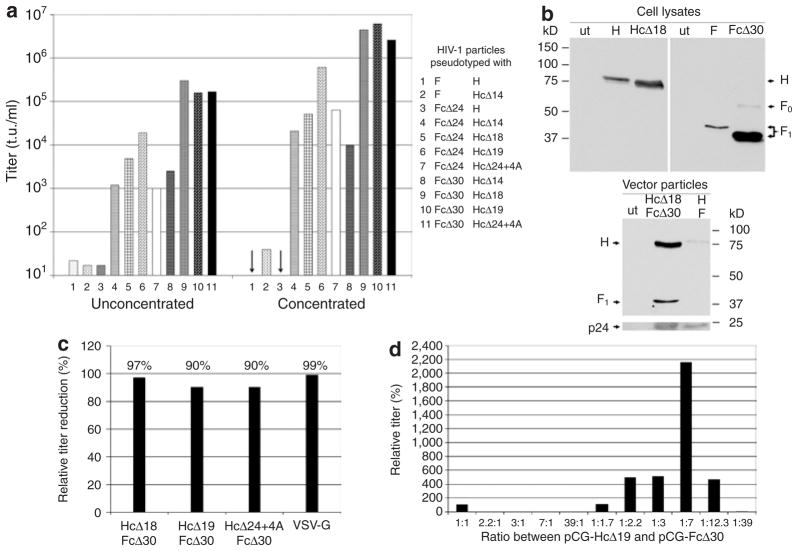
Preliminary experiments showed that HIV-1 vector particles produced in the presence of the unmodified MV glycoproteins were unable to mediate gene transfer. Assuming that sequences in the cytoplasmic tails of the F and H proteins of MV prevented pseudotyping, we took advantage of cytoplasmic tail variants characterized earlier in terms of fusion function.17 We screened 15 H protein variants carrying stepwise truncations and amino acid exchanges in their cytoplasmic tails, and two F protein variants for their ability to mediate transfer of the gfp gene into HT1080 cells (Figure 1). Truncation of both cytoplasmic tails allowed efficient pseudotyping (Figure 2a and Supplementary Figure S1). The highest titers were obtained when the F protein cytoplasmic tail was truncated by 30 residues, leaving only three amino acids, two of them being positively charged. Among the H protein variants, there was a clear peak of optimal truncation when 18 or 19 residues were deleted (variants HcΔ18 and HcΔ19). Further truncation reduced the titers, although replacing the deleted residues by alanine could restore optimal titers in the case of the variant HcΔ24+4A. All three H protein variants that allowed most efficient pseudotyping have been shown earlier to be active in fusion helper function.17 The screening identified three combinations, namely HcΔ18/FcΔ30, HcΔ19/FcΔ30, and HcΔ24+4A/FcΔ30, that allowed the most efficient pseudotyping of HIV-1 vector particles with titers of ~105 transducing units (t.u.)/ml on HT1080 cells (Figure 2a). After concentration titers >106 t.u./ml were obtained (Figure 2a). All further experiments were performed with the HcΔ18/FcΔ30 variants.
Figure 1. Overview of the fusion (F) and hemagglutinin (H) protein variants of measles virus used for pseudotyping.
Amino acid sequences of the cytoplasmic tails of (a) the F protein and (b) the H protein variants are shown. Note that H is a type II transmembrane protein. The best titer of pseudotyped human immunodeficiency virus 1 vectors titrated on HT1080 cells for each possible F/H combination that was tested is shown [screening titer (t.u./ml)]. Presence (+) or absence (−) of the fusion helper function of each H variant as determined by ref. 17 is indicated. Variants used for further studies are depicted in bold lettering.
Figure 2. T ransduction of HT1080 cells with human immunodeficiency virus 1 (HIV-1) vector particles pseudotyped with fusion (F) and hemagglutinin (H) protein variants of measles virus (MV).
HEK-293T cells were cotransfected with the HIV-1 packaging plasmid pCMVΔR8.9, the green fluorescent protein (GFP) transfer plasmid pHR′-CMV-GFP, and plasmids encoding the indicated F and H protein variants. After 48 hours, cell supernatants were used for the transduction of HT1080 cells, either directly or after concentration. (a) Screening titers of selected pseudotype vectors are shown. The arrows indicate titers <10 t.u./ml. (b) Western blot analysis of HIV-1 vector particles pseudotyped with the indicated proteins (bottom panel) and cell lysates of HEK-293T packaging cells producing the respective vectors (top panel). The blotted proteins were analyzed utilizing anti-F, anti-H, or anti-p24 antibodies, as appropriate. Either cell lysate or concentrated cell supernatant from untransfected HEK-293T cells was loaded as negative control (ut). (c) HT1080 cells were transduced in the presence or absence of 10 μmol/l azido-thymidine (AZT) by concentrated HIV-1 vector particles pseudotyped with the indicated glycoproteins. The relative titer reduction in the presence of AZT is shown. (d) In order to optimize titers, the ratio of the plasmids encoding the HcΔ19 or FcΔ30 protein variants used for vector production in HEK-293T cells was varied. Each pseudotype vector produced was titrated on HT1080 cells, and its relative titer, normalized to that obtained after transfection of a 1:1 ratio of pCG-HcΔ19 and pCG-FcΔ30 (100%), is shown.
Biochemical analysis confirmed the formation of pseudotypes: in cell lysates of vector particle–producing cells, the truncated proteins HcΔ18 and FcΔ30 were detectable according to their expected molecular weights (Figure 2b). Although western blot analysis suggested increased protein expression levels after cytoplasmic tail truncation, especially for FcΔ30, there was no difference in cell-surface expression levels between H and HcΔ18, and between F and FcΔ30, as determined by fluorescence-activated cell sorting (FACS) analysis (data not shown). In the released HIV-1 vector particles, only low amounts of unmodified H protein and no unmodified F protein were detectable. The HcΔ18 and FcΔ30 proteins, in contrast, were present in significant amounts, thereby demonstrating that cytoplasmic tail truncation had resulted in enhancing particle incorporation (Figure 2b).
The transduction of various cell lines being positive or negative for the MV receptors revealed that receptor usage of the pseudo-typed particles is identical to that of MV particles (Supplementary Table S1). In order to assess whether the observed titers were attributable to pseudotransduction, which means green fluorescent protein (GFP) protein transfer from the producer to the target cells, we determined the sensitivity of gene transfer toward a reverse transcriptase inhibitor.23 In the presence of azido-thymidine, titers of HIV-1 vector particles pseudotyped with the MV glycoproteins decreased by 90–97%, comparable to the 99% titer reduction observed for VSV-G-pseudotyped vector particles (Figure 2c). It is therefore evident that pseudotransduction makes only a negligible contribution to the titers.
During MV infection, less H than F mRNA is produced.24 We therefore expected that altering the ratio of the amounts of H and F plasmids in the packaging cells might enhance the titers. Increasing the relative level of pCG-HcΔ19 had the effect of reducing the titers in comparison with those obtained by transfecting equivalent amounts of both plasmids. In contrast, higher levels of pCG-FcΔ30 considerably increased the titers. The optimal ratio was determined to be sevenfold more F than H plasmid, and this ratio resulted in a more than tenfold increase in titers (Figure 2d).
Retargeting of HIV-1 vector particles pseudotyped with modified MV glycoproteins
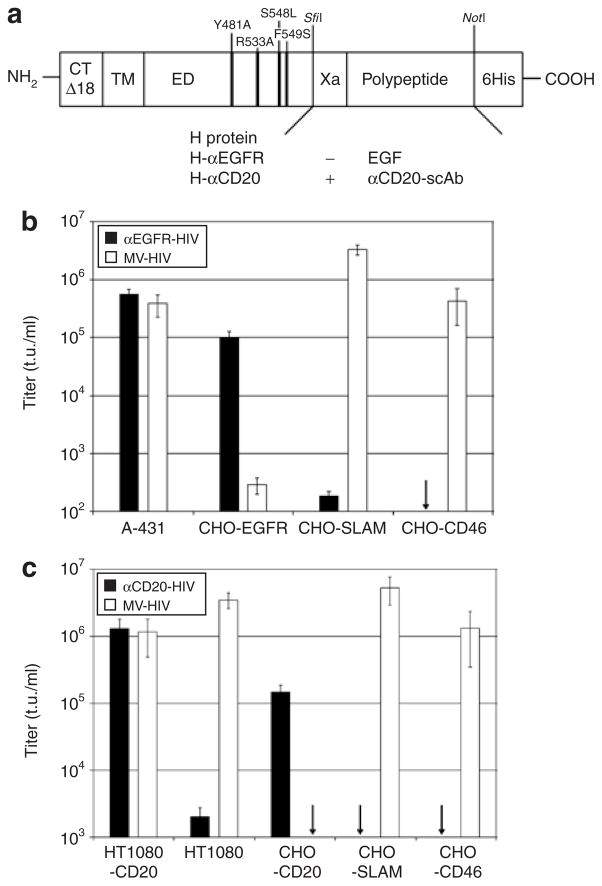
Next, cell-targeting vectors specific for the EGF receptor (EGFR) or the CD20 B-cell surface marker were generated. For this purpose the cytoplasmic tail of an H protein variant with inability to recognize the native receptors CD46 and SLAM (mutations Y481A, R533A, S548L, F549S)19 was truncated by 18 amino acids. At its ectodomain, either EGF or a scAb directed against CD20 (αCD20-scAb) was displayed, resulting in the constructs H-αEGFR and H-αCD20, respectively (Figure 3a). These were used together with FcΔ30 to generate the respective targeting vectors, αEGFR-HIV and αCD20-HIV. The untargeted pseudotype vector MV-HIV, based on the HcΔ18 and FcΔ30 variants, was used as a control.
Figure 3. Targeting of epidermal growth factor receptor (EGFR) and CD20-positive cells.
(a) Schematic drawing of the modified hemagglutinin (H) protein variants used for retargeting. The following modifications are indicated: four point mutations in the ectodomain (ED) ablating the natural receptor usage of H, truncation of the cytoplasmic tail by 18 amino acids (CTΔ18), the displayed polypeptide, and a factor Xa cleavage site (Xa). The position of the transmembrane domain (TM) is also indicated. (b) Concentrated vector particles pseudotyped with FcΔ30 and H-αEGFR were used for the transduction of EGFR-, CD46-, or SLAM-expressing cell lines. Vector particles pseudotyped with FcΔ30 and HcΔ18 (MV-HIV) were used as controls. The titers are mean values and SD from four independent experiments. The arrow indicates a titer <102 t.u./ml. (c) Concentrated particles pseudotyped with FcΔ30 and H-αCD20 were used for the transduction of the CD20-positive and CD20-negative HT1080 and CHO cell lines. MV-HIV particles were used as controls. The titers are based on mean values and SD from four independent experiments. The arrows indicate titers <103 t.u./ml. CHO, Chinese hamster ovary; HIV, human immunodeficiency virus; MV, measles virus; scAb, single-chain antibody.
In order to evaluate the targeting potential of the αEGFR-HIV vector we transduced a panel of Chinese hamster ovary (CHO) cell lines stably expressing EGFR, CD46, or SLAM, as well as the human cell line A-431 naturally overexpressing EGFR.25,26 On the basis of titrations of the αEGFR-HIV and MV-HIV vector stocks on A-431 cells, which showed susceptibility toward both vector types, serial dilutions containing equivalent amounts of A-431 transducing units were applied to the CHO cell lines. The αEGFRHIV vector–transduced CHO-EGFR cells, while transduction of CHO-CD46 and CHO-SLAM cells remained at background levels even when the highest possible amount of vector particles was applied (Figure 3b). In contrast, MV-HIV vector particles efficiently transduced all cell lines that expressed MV receptors, while CHO-EGFR cells were transduced only at background levels (Figure 3b). The background level of transduction (5 × 102 t.u./ml) was defined by applying the same amounts of HIV-1 vectors pseudotyped with the Env protein of the ecotropic Moloney MLV (MoMLV), which lacks a receptor on human and CHO cells (data not shown).
Stocks of the αCD20-HIV vector were standardized against MV-HIV vector particles on HT1080-CD20 cells. Next, serial dilutions containing equivalent amounts of transducing units were analyzed on CHO cell lines expressing CD20, CD46, or SLAM, as well as on HT1080 and HT1080-CD20 cells. The titer of the αCD20-HIV vector on HT1080-CD20 cells was >106 t.u./ml, which was almost three-fold higher than the titer on the parent CD20-negative HT1080 cells (Figure 3c). Similar observations were made in the CHO cell lines, which were transduced only when CD20 was expressed. The MV-HIV vector, in contrast, infected the different MV receptor–positive cell types with similar efficiency, but did not transduce CHO-CD20 cells (Figure 3c). HIV vectors pseudotyped with the mutant receptor–blind H protein without a ligand did not show transduction above background levels on any of the cell lines to which they were applied (data not shown). These data clearly demonstrate that cell entry of the αEGFR-HIV and αCD20-HIV vectors occurred through the retargeted receptor. Target receptor–positive cell lines were transduced at least 102-fold more efficiently by the targeting vectors than target receptor–negative cell lines, independent of the vector dose.
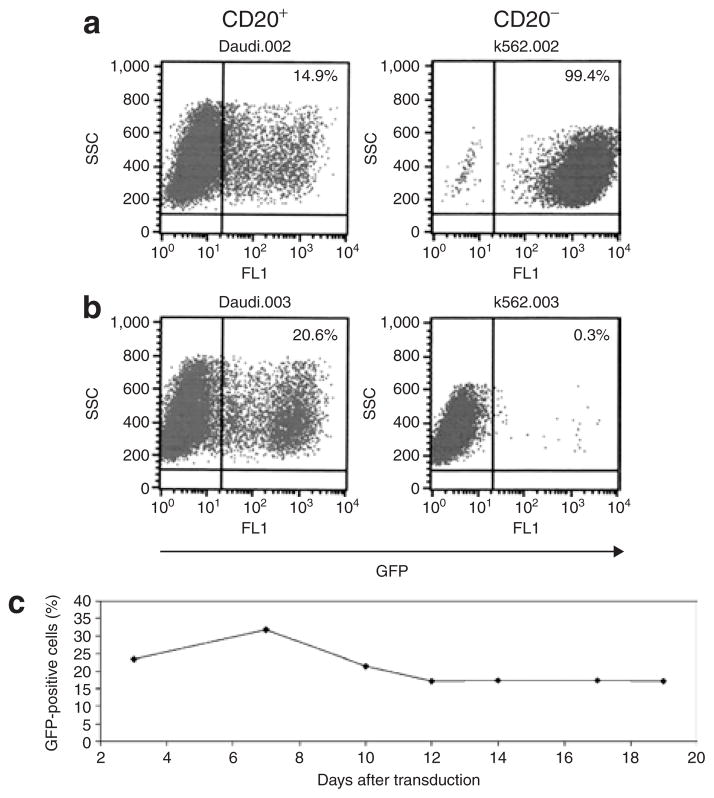
Lymphocytes naturally expressing CD20 were also selectively transduced by the αCD20-HIV vector. The CD20-positive B cell line, Daudi, and the CD20-negative myeloid cell line, K-562, were separately incubated with αCD20-HIV vector particles or VSV-G-pseudotyped HIV vector particles. While the latter transduced both cell lines efficiently (Figure 4a), the αCD20-HIV vector selectively transduced Daudi cells, whereas K-562 cells remained largely GFP-negative (Figure 4b). Remarkably, the transduction efficiency in Daudi cells was slightly higher with the use of the αCD20-HIV vector than with the VSV-G-HIV vector. It is clear from these findings that cells naturally expressing CD20 are efficiently transduced by the αCD20-HIV vector.
Figure 4. Selective transduction of CD20-positive B cell lines.
Concentrated (a) VSV-G-HIV vector particles [multiplicity of infection 5 (MOI 5)] or (b) αCD20-HIV vector particles (MOI 0.5) were used for the transduction of CD20-positive Daudi cells and CD20-negative K-562 cells, respectively. The percentage of green fluorescent protein (GFP)-positive cells was determined by fluorescence-activated cell sorting (FACS) analysis. (c) In order to ascertain the stability of cell transduction, Raji cells were cultivated for 19 days after transduction with the αCD20-HIV vector. The percentage of GFP-positive cells was determined by FACS analysis at the indicated time-points. HIV, human immunodeficiency virus; VSV-G, vesicular stomatitis virus G.
In order to verify gene expression stability, the CD20-positive B cell line, Raji, was incubated with αCD20-HIV vector particles, and the number of GFP-positive cells was determined over a period of 19 days. The data demonstrate that a constant level of 17–30% GFP-positive cells was detectable over the whole observation period, thereby confirming the stability of gene transfer and expression mediated by the αCD20-HIV vector (Figure 4c).
Selective killing of CD20-positive cells in a mixed cell population
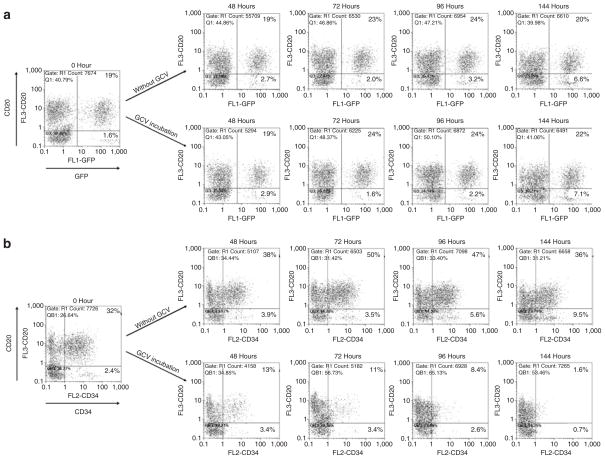
Next we investigated whether the targeting capability of the αCD20-HIV vector could be applied to selectively kill CD20-positive cells in a mixture of CD20-positive and -negative cells. In order to allow cell killing, a suicide gene coding for an F protein (CD34TK39), composed of a hypersensitive mutant of the herpes simplex virus thymidine kinase (TK39) and a truncated version of the cell surface antigen CD34, was used.27 Transduced cells express CD34 on their surfaces and can thereby be distinguished from untransduced cells. As control, αCD20-HIV vector particles containing the packaged gfp gene were used. A 1:1 mixture of CD20-positive Raji and CD20-negative K-562 cells was transduced with each of the two vector types, respectively. At different time-points after gancyclovir treatment, the relative numbers of GFP/CD20 and CD34/CD20 double-positive cells were determined. Both CD20-targeted vector particles selectively transduced the CD20-positive cell fraction. There were ~20% CD20/GFP and ~30% CD20/CD34 double-positive cells, whereas only ~1–2.5% of the CD20-negative cells were transduced (Figure 5). Normalized to the total cell number, this means that 32 and 55% of the CD20-positive cells, respectively, and 4 and 6% of the CD20-negative cells, respectively, had become GFP- and CD34-positive.
Figure 5. S elective killing of CD20-positive cells in a mixed cell population.
2 × 103 CD20+ Raji cells and 2 × 103 CD20− K-562 cells were mixed and then transduced with the αCD20-HIV vector packaged with either (a) the gfp gene or (b) the cd34tk39 gene. Five days after transduction (time-point 0 hours), half of the transduced cells were incubated in 10 μmol/l gancyclovir (GCV)-containing medium while the other half was left as control in GCV-free medium. At time-point 0 hours and at the indicated time-points, the percentages of CD20+/GFP+ and CD20+/CD34+ cells were determined by fluorescence-activated cell sorting analysis. GFP, green fluorescent protein; HIV, human immunodeficiency virus.
In contrast, when the cell mixture was incubated with VSV-G-HIV vector particles, no differences in the transduction rates of CD20-positive and -negative cells were observed (data not shown). Over time, the transduction patterns remained similar, demonstrating the stable integration and expression of the transferred genes and the viability of the transduced cells. However, there was a slight increase in the number of reporter gene-positive CD20-negative cells in the absence of cell killing (Figure 5), suggesting that transduction by the αCD20-HIV vector may have resulted in loss of the CD20 surface marker in a small fraction of the cells.
As expected, incubation with gancyclovir had no influence on the fraction of CD20/GFP double-positive cells (Figure 5a). In contrast, the CD20-positive cell fraction transduced with the cd34tk39 gene was efficiently and selectively killed by gancyclovir (Figure 5b); starting with 32% double-positive cells, almost all the double-positive cells had disappeared after 6 days. It is clear, therefore, that the CD20 targeting vector can selectively kill CD20-positive cells in mixed cell populations.
Transduction of primary human B cells
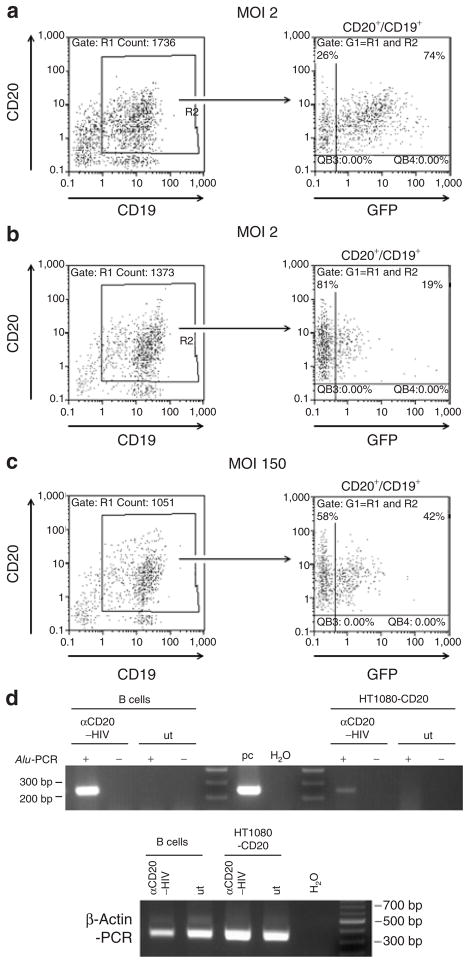
Primary human B cells were isolated from peripheral blood mononuclear cells, activated for 48 hours with a cytokine cocktail, and incubated with the αCD20-HIV vector or the VSV-G-HIV vector. Most of the isolated cells were CD20/CD19 double-positive B cells (Figure 6). Remarkably, >70% of these cells were GFP-positive after transduction with the αCD20-HIV vector (Figure 6a), whereas only ~20% had become GFP-positive with the VSV-G-HIV vector (Figure 6b). These results were confirmed with B cells from three further donors (Supplementary Table S2). Increasing the multiplicity of infection (MOI) of the VSV-G-HIV vector to 150 resulted in raising the fraction of GFP-positive B cells approximately twofold (Figure 6c). However, at such high MOIs, pseudotransduction may significantly contribute to the GFP fluorescence of VSV-G-HIV vector–transduced cells.28 The considerably lower mean fluorescence intensity of VSV-G-HIV vector–transduced B cells supports this possibility.
Figure 6. Transduction of primary human B lymphocytes.
Primary human B cells were isolated from human peripheral blood mononuclear cells, activated for 48 hours, and then (a) transduced with the αCD20-HIV vector at a multiplicity of infection (MOI) of 2. In parallel, a second aliquot of cells was transduced with the VSV-G-HIV vector at (b) an MOI of 2 or (c) an MOI of 150. Seventy-two hours after transduction, the percentage of CD20+/CD19+/GFP+ triple-positive cells was determined by fluorescence-activated cell sorting analysis. (d) The genomic DNA of cells presented in a and HT1080-CD20 cells transduced at an MOI of 0.7 by αCD20-HIV was isolated 6 days after transduction and analyzed using a two-step Alu-PCR protocol for genomic integration of vector sequences. β-Actin sequences were amplified to demonstrate the integrity of isolated genomic DNA. +, two-step Alu-PCR protocol; −, vector-specific PCR (second round) without previous Alu-PCR; GFP, green fluorescent protein; HIV, human immunodeficiency virus; pc, positive control (50 ng pSEW plasmid); ut, untransduced cells; VSV-G, vesicular stomatitis virus G.
In order to demonstrate chromosomal integration of vector DNA transferred by the αCD20-HIV vector, a two-step PCR amplification assay (Alu-PCR) was performed using primers that bind to cellular genomic Alu and proviral gag sequences in the first cycle, and vector-specific primers binding in the long-terminal repeat in the second cycle.29 Integration of vector DNA is indicated if a significant increase in the vector-specific signal is obtained by two steps of amplification, as compared to that attained without a preceding Alu-PCR. Integration of vector DNA was detected in both transduced HT1080-CD20 and B cells, whereas no signal was detected in untransduced control cells and in PCR controls (Figure 6d). In contrast, B cells transduced by the VSV-G-HIV vector (high MOI) were negative in the Alu-PCR, further suggesting that pseudotransduction makes a significant contribution to the GFP fluorescence obtained with this vector (data not shown).
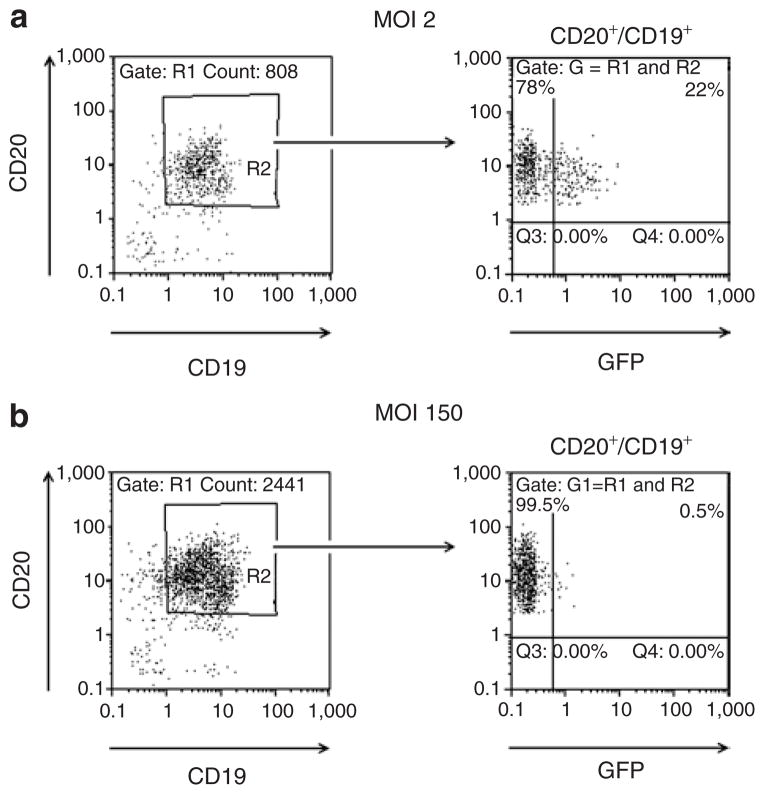
The unexpectedly high efficiency of transduction of primary B cells with the αCD20-HIV vector prompted us to test the ability of the αCD20-HIV vector to transduce unstimulated B cells. For this purpose, primary human B cells were transduced immediately after isolation, without ever coming in contact with activating cytokines, either before or after transduction. As expected, these cells were completely resistant to transduction with the VSV-G-HIV vector, even at MOIs of 150 or higher (Figure 7; Supplementary Table S2). Most surprisingly, the αCD20-HIV vector was able to transduce unstimulated B cells derived from three different donors at efficiencies of ~20% (Figure 7; Supplementary Table S2).
Figure 7. Transduction of quiescent primary human B lymphocytes.
Primary human B cells were isolated from human peripheral blood mononuclear cells, shown to be negative for CD69, and were then transduced with (a) the αCD20-HIV vector at a multiplicity of infection (MOI) of 2 or (b) the VSV-G-HIV vector at an MOI of 150. Forty-eight hours after transduction, the percentage of CD20+/CD19+/GFP+ triple-positive cells was determined by fluorescence-activated cell sorting analysis. GFP, green fluorescent protein; HIV, human immunodeficiency virus; VSV-G, vesicular stomatitis virus G.
Selective transduction of B cells in primary human lymphocytes
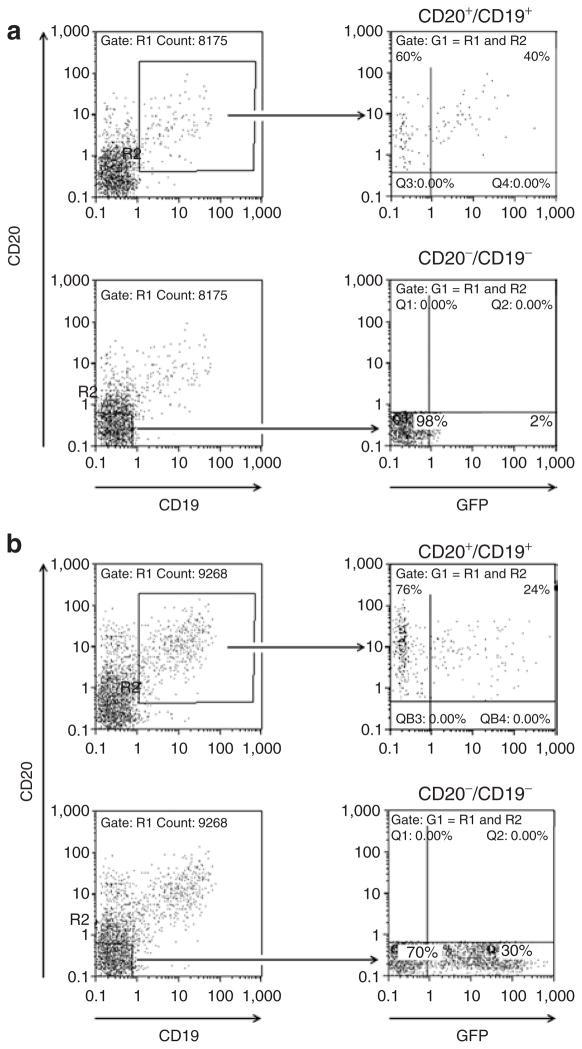
Human CD20+/CD19+ B cells and CD3+ T cells were isolated from human blood by CD19 and CD3 antibody–mediated immunomagnetic selection, and activated with a cytokine cocktail. Then the cell mixture containing ~70% T cells and 5% B cells was transduced with the αCD20-HIV vector or the VSV-G-HIV vector. With the αCD20-HIV vector, ~40% of the B cells had become GFP-positive, while the CD20−/CD19− cell fraction, consisting mainly of T cells, remained GFP-negative (Figure 8a). In contrast, the VSV-G-HIV vector was able to transduce both cell fractions, at approximately equivalent efficiencies (Figure 8b), without discriminating between the two cell populations. These data have been confirmed with a B- and T-cell mixture from a second donor (data not shown). These findings show that the αCD20-HIV vector effectively targets primary human CD20-positive cells, even when the target cell population is highly underrepresented.
Figure 8. S elective transduction of primary human B cells in mixed lymphocytes.
Primary human B and T cells were isolated from human blood and incubated with cytokines that activate B and T cells. The cell mixture was then transduced with (a) the αCD20-HIV vector or (b) the VSV-G-HIV vector at an MOI of 2. Forty-eight hours after transduction, the percentages of CD20+/CD19+/GFP+ cells (upper panels) and CD20−/CD19−/GFP+ cells (bottom panels) were determined by fluorescence-activated cell sorting analysis. One representative example out of two independent experiments is shown. GFP, green fluorescent protein; HIV, human immunodeficiency virus; VSV-G, vesicular stomatitis virus G.
DISCUSSION
In this study, we report a novel lentiviral cell entry targeting system based on the MV glycoproteins. We demonstrated its high selectivity by carrying out experiments targeting two different types of cell-surface molecules, namely the EGFR (a typical type I transmembrane protein that becomes rapidly endocytosed after ligand binding), and CD20, a membrane tetraspan calcium channel that is usually not internalized after antibody binding.25,30,31 For retargeting, either a natural ligand (EGF) or a scAb can be displayed as C-terminal extension of H.
The use of the retargeted receptor for cell entry was demonstrated by the highly increased titers of the targeting vectors (>100-fold for the EGFR and 1,000-fold for CD20) when CHO cells expressed the target receptors instead of the natural MV receptors. The almost complete absence of background transduction of nontargeted cells with this system was demonstrated by the selective killing of CD20-positive human lymphocytes while cocultured CD20-negative cells proliferated unaffected. Membrane fusion through CD20 was pH-independent, as confirmed by the formation of syncytia under neutral pH conditions in HT1080-CD20, but not in HT1080 cells after cotransfection of plasmids encoding H-αCD20 and FcΔ30 (Supplementary Figure S2). This shows that the pH-independent MV membrane fusion machinery can use totally different types of cell-surface proteins as receptors, and can thereby be exploited for the targeting of lentiviral vectors.
The key issues in establishing pseudotyping with MV glycoproteins were (i) the identification of H protein cytoplasmic tail truncation mutants that allowed pseudotyping while retaining the fusion helper function, and (ii) the determination of the optimal ratio of F to H expression plasmids in the packaging cells in order to produce high titer vector stocks. Although it had been established earlier that more F than H mRNA is present in MV-infected cells, the extent of titer reduction observed when we applied suboptimal F:H ratios, was unexpected.24,32 Obviously, a fine-tuned balance of the relative amounts of F and H has to be maintained in the packaging cells to allow efficient formation of pseudotyped HIV-1 particles (also see Supplementary Figure S3).
The recently published lentiviral cell–targeting system based on the co-incorporation of Sindbis virus glycoproteins and a complete antibody molecule also used CD20 as the target molecule.15 Data from that study show that transduction of 293T-CD20 cells using these vectors yielded titers similar to those produced by the MV glycoprotein–based targeting vectors in our study. However, the study involving Sindbis virus showed a higher background transduction on control cells. Also, in that study, transductions were not performed on mixed cultures of target and nontarget cells, and the experiments involving transduction of primary human B lymphocytes lacked some important controls, like the demonstration that the CD20-negative lymphocytes can be transduced with a lentiviral vector after stimulation with lipopolysaccharide alone. However, at least in mice, lipopolysaccharide stimulation is insufficient to allow lentiviral vector–mediated gene transfer into T lymphocytes.33,34
In contrast to Yang et al.15 we achieved a surprisingly efficient transduction of primary human B lymphocytes using the CD20-targeted vector, achieving >70% GFP-positive cells with a single transduction cycle. Difficulties in achieving efficient transduction of human primary B lymphocytes have been described earlier. All the studies were in agreement about VSV-G being the optimal envelope for B-cell transduction, but even under carefully optimized conditions transduction levels never rose higher than 20%.33,35 When the same culture and activation conditions were applied as in our study, only 2.3% transduced B cells were obtained.33 Therefore, the MV glycoproteins modified with an αCD20-scAb are much more efficient in mediating gene transfer into primary human B lymphocytes than the VSV-G protein is.
One possible explanation for this observation is an additional stimulatory signal induced by CD20 binding. This hypothesis was substantiated by the observation that the αCD20-HIV vector was able to transduce unstimulated primary human B cells; to our knowledge, this has not been demonstrated before with any type of retro- or lentiviral vector. It has been previously shown that lentiviral vectors that display cytokines, such as interleukin-7, thrombopoietin, or stem cell factor on their surface induce mitogenic stimuli in resting lymphocytes or hematopoietic stem cells, and thereby enhance lentiviral vector transduction.36,37 There is increasing evidence suggesting a physiological role for CD20 as a regulator of cell growth and differentiation. 38 Indeed, the binding of most monoclonal antibodies to CD20 generates transmembrane signals, and some antibodies activate G0-arrested B cells to transition into G1.38,39 The multivalent CD20 binding, as it occurs with lentiviral vector particles, possibly further potentiates this effect that requires cross-linking of CD20 molecules.40
Independent of the molecular mechanism that causes the efficient B-lymphocyte transduction, the lentiviral CD20-targeted vectors described in this study are available for a number of applications ranging from the genetic modification of B cells for investigating basic questions in immunology to therapeutic strategies such as the treatment of inherited B-cell disorders or lymphomas. Importantly, this novel targeting strategy can be easily adapted to many target molecules of interest by extending the H protein with appropriate specificity domains or scAbs of choice. It is now possible, therefore, to tailor lentiviral vectors for highly selective gene transfer into specific target cell populations with an unprecedented degree of efficiency.
MATERIALS AND METHODS
Plasmid constructs
The method of cloning of the glycoprotein H and F genes derived from the recombinant attenuated MV strain Edmonston B into the pCG expression plasmid under the control of the cytomegalovirus promoter has been described previously.41 The pCG-HcΔ14 and pCG-FcΔ24 plasmids were generated by replacing the respective glycoprotein gene with the HcΔ14 (PacI/SpeI) or FcΔ24 (NarI/PacI) coding region, removed from plasmids peHcΔ14 and p(+)MV-FcΔ24, respectively.42 Cloning of all other F and H cytoplasmic tail mutants has been described.17 The pCG-H-αCD20 plasmid was constructed by subcloning the PacI/ NheI fragment that codes for the truncated cytoplasmic tail of HcΔ18 into pCG-HmutXαCD20-6His.22 For generating the plasmid pCG-H-αEGFR, the αCD20-scAb coding region in pCG-H-αCD20 was replaced after SfiI/NotI digestion by the EGF ligand coding region removed from pE-Mo.43 The HIV-1 transfer vector plasmid pS-CD34TK39-W was constructed by PCR amplification of the cd34tk39 coding region of plasmid M71tCD34tk39m,27 and subsequent ligation of the PCR fragment into the AscI/SbfI restriction sites of the lentiviral transfer plasmid pSEW,44 thereby replacing the gfp gene.
Cell lines
HEK-293T (ICLC HTL04001), HT1080 (ATCC CCL-121), A-431 (ATCC CRL-1555), and CHO-K1 (ATCC CCL-61) cells were grown in Dulbecco’s modified Eagle medium supplemented with 10% fetal calf serum (FCS) and 1% glutamine. Daudi (ECACC 85011437), Raji (ATCC CCL-86), K-562 (ATCC CCL-243), and A3.01 (ref. 45) cells were grown in RPMI 1640 supplemented with 10% FCS and 1% glutamine. The cell lines CHO-SLAM, CHO-CD46 (previously termed CHO-BC1), CHO-EGFR, CHO-CD20, and HT1080-CD20 have been described previously. 46,47 CHO-SLAM cells (kindly donated by Y. Yanagi) were cultivated in RPMI 1640 supplemented with 10% FCS, 1% glutamine, and 0.5 mg/ ml G418. The other cell lines were grown in Dulbecco’s modified Eagle medium supplemented with 10% FCS, 1% glutamine and 1.2 mg/ml G418 (CHO-CD46), or 1.0 mg/ml G418 (CHO-EGFR), or 3 μg/ml puromycin (HT1080-CD20, CHO-CD20).
Primary human cells
Primary human B cells were isolated from fresh human peripheral blood mononuclear cells using the Dynal B cell negative isolation kit (Invitrogen, Karlsruhe, Germany) in accordance with the manufacturer’s instructions. Isolated B cells were either activated for 48 hours in RPMI 1640 supplemented with 10% FCS, 1% glutamine, 0.5% streptomycin/penicillin, 25 mmol/l HEPES, as well as 300 ng/ml CD40 ligand, 50 ng/ml interleukin-2, 10 ng/ml interleukin-4, and 10 ng/ml interleukin-10, or used directly for transduction. FACS analysis demonstrated that unstimulated, in contrast to stimulated, B cells were negative for the activation marker CD69.
In addition, a mixture of primary human CD20+/CD19+ B and CD3+ T cells was collected during immunomagnetic purification of peripheral blood stem cells from three healthy donors. Briefly, apheresis products were harvested after granulocyte-colony stimulating factor stimulation and performed using a COBE Spectra (Gambro, Lakewood, CO). After platelet reduction, cells were labeled with anti-CD3 and anti-CD19 antibodies (CD3+ CD19+ Microbeads; Miltenyi/Biotec, Bergisch-Gladbach, Germany) for 30 minutes. Next, T- and B cells were depleted immunomagnetically on the CliniMacs system (Miltenyi/Biotec, Bergisch-Gladbach, Germany) under good manufacturing practice conditions in accordance with the manufacturer’s instruction. While the positive fractions including stem cells, monocytes, natural killer cells, and dendritic cells were used for haploidentical stem cell transplantation, the negative fractions consisting of a mixture of highly purified CD19+ B and CD3+ T cells were finally activated as described earlier. Informed consent was given by the donors, and use of the samples has been approved by the University Hospital Ethics Committee (Frankfurt, Germany).
Flow cytometry
Flow cytometry was performed on the Galaxy flow cytometry system (DakoCytomation, Hamburg, Germany). Adherent cells were detached by incubation with 100 μl phosphate-buffered saline–trypsin solution. Cells in suspension were then washed in 1 ml FACS washing buffer (phosphate-buffered saline, 1% FCS, 0.1% NaN3), and incubated with a 1:10 dilution in phosphate-buffered saline of the mouse antihuman CD20/PE-Cy5 antibody (BD Pharmingen, Heidelbeg, Germany) to detect CD20-positive cells, the mouse anti-human CD19/PE antibody (DakoCytomation, Hamburg, Germany) to detect CD19-positive cells, and the mouse anti-human CD34/PE antibody (EuroBioSciences GmbH, Friesoythe, Germany) to detect CD34-positive cells. After two stages of washing, the cells were finally resuspended in 200 μl phosphate-buffered saline/1% paraformaldehyde. The data were analyzed using the FloMax program version 2.0 (Partec, Münster, Germany).
Production of vector particles
Vector particles were generated by transient transfection of HEK-293T cells. Twenty-four hours before transfection, 6.5 × 106 HEK-293T cells were seeded into a T75 flask. One hour before transfection the medium was replaced with 7 ml fresh medium. In total, 8.0 μg of the two plasmids encoding either F or H protein variants of MV, 6.72 μg of the packaging plasmid pCMVΔR8.9,48 and 11.27 μg of the transfer vector plasmid (pS-CD34TK39-W, pSEW, or pHR′-CMV-GFP49) were mixed. The plasmid DNA was filled up with H2O (Sigma-Aldrich, Taufkirchen, Germany) to 450 μl. Next, 50 μl of 2.5 mol/l CaCl2 (Sigma-Aldrich) solution was added. While vortexing the DNA-CaCl2 solution, 500 μl 2× HEPES buffer saline buffer [281 m mol/l NaCl; 100 m mol/l HEPES; 1.5 mmol/l Na2HPO4 (Sigma-Aldrich)] was added dropwise. The precipitate was then added to the cells. After 17 hours the medium was replaced with 12 ml of fresh medium. Twenty-four hours afterwards, the cell supernatant containing the pseudotyped lentiviral vector particles was filtered (0.45-μm filter), and 300 μl thereof was directly used for transduction. The remaining supernatant was concentrated by centrifugation at 3,450g and 4 °C for at least 24 hours. The pellet was resuspended in 120 μl FCS-free medium. Vector particles pseudotyped with the VSV-G protein or the Env protein of MoMLV were produced by cotransfection of 4.55 μg of the plasmid pMD.G2 (Didier Trono, Tronolab, Lausanne, Switzerland) encoding VSV-G, or pHIT123 (ref. 50) encoding MoMLV Env, respectively, along with 8.45 μg of pCMVΔR8.9 and 13.00 μg of transfer plasmid.
The αCD20-HIV and VSV-G-HIV vector stocks were titrated on HT1080-CD20 cells for determining titers and calculating the applied MOIs.
Western blot analysis
Vector particle–producing 293T cells were lysed in lysis buffer [50 mmol/l Tris pH 8.0; 62.5 mmol/l EDTA; 1% NP-40; 0.4% deoxycholate; 40 μl/ml protease inhibitor cocktail (25×; Roche, Mannheim, Germany)]. Vector particles released from these cells were concentrated and purified over a 20% sucrose cushion. Cell lysates or purified vector particles were denatured by incubation for 10 minutes at 95 °C in 2× urea sample buffer (5% sodium dodecyl sulfate, 8 mol/l urea, 200 mmol/l Tris–HCl, 0.1 mmol/l EDTA, 0.03% bromphenol blue, 2.5% dithiothreitol, pH 8.0), separated by electrophoresis on 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis gels, and electrotransferred onto nitrocellulose membranes (Amersham Biosciences, Freiburg, Germany). The membranes were incubated with rabbit anti-F (1:1,000; F 431) polyclonal serum, rabbit anti-H (1:2,000; H 606) polyclonal serum, or mouse anti-p24 (clone 38/8.7.47, 1:1,000; Gentaur; Aachen, Germany) monoclonal antibody, to detect F, H, or p24 Gag, respectively. The respective secondary antibodies conjugated with horseradish peroxidase (1:2,000; DakoCytomation, Hamburg, Germany) were used. Signals were developed using SuperSignal West Pico Luminol kit (Pierce, Bonn, Germany) for the blots for detection of H or F. For detection of p24, the Amersham ECL Plus Western Blotting detection reagent (GE Healthcare, München, Germany) was used.
Transduction of adherent cell lines
For transduction, ~1.5 × 105 cells were seeded into a single well of a 24-well plate. The next day, vector particle stocks were serially diluted in 1:10 steps and a total of 250 μl of the dilutions, including 2.0 μg Polybrene, were added per well, incubated for 2.5–3 hours, and then replaced with 1 ml of fresh medium. After 48–72 hours, titers were calculated by determining the number of GFP fluorescent cells under the fluorescence microscope. Alternatively, titers were determined by FACS analysis based on the indicated percentage of green fluorescent cells.
Transduction of suspension cells
Fibronectin-coated 48-well plates were precoated with half the number of vector particles used for transduction, diluted in 120 μl of medium. The plate was then centrifuged at 860g and 4 °C for 20 minutes. The rest of the vector particles were diluted in 180 μl medium supplemented with 1.2 μg protamine sulfate containing 1 × 105 cells (suspension cell lines) or 5.0 × 104 primary cells, and added to the wells precoated with the vector particles. This was followed by centrifugation at 430g and 32 °C for 90 minutes. After 2 hours of incubation at 37 °C in a cell culture incubator, 700 μl medium per well was added. Seventy-two hours after transduction, the fraction of GFP-fluorescent cells was determined by FACS analysis.
PCR assays
For detection of integrated vector sequences, cellular genomic DNA was isolated 6 days after transduction, using the DNeasy Tissue Kit (Qiagen GmbH, Hilden, Germany). For the first step of Alu-PCR, 90 ng of genomic DNA was applied under standard PCR conditions (Taq DNA Polymerase; 5 PRIME GmbH, Hamburg, Germany) after initial denaturation (3 minutes 95 °C) in 35 PCR cycles (30 seconds 95 °C, 30 seconds 60 °C, 2 minutes 68 °C) with primers ALUs (5′-AAA CCC ACG CAT GAC ACA ACA CTG-3′) and HIV-AluPCRas (5′-CGG GCG CCA CTG CTA GAG ATT TT-3′). Subsequently, 1/10 of the product was used for the specific LTR-PCR (30 cycles; 30 seconds 95 °C, 30 seconds 60 °C, 40 seconds 68 °C) with primers SEW-LTR1s (5′-ACT GGA AGG GCT AAT TCA CTC C-3′) and SEW-LTR1as (5′-TGC TAG AGA TTT TCC ACA CTG ACT-3′). For detection of cellular β-actin gene, 90 ng of genomic DNA was applied under standard PCR conditions after initial denaturation (3 minutes 94°) in 35 PCR cycles (1 minute 94 °C, 1 minute 58.8 °C, 2 minutes 68 °C) C with primers β-Act-for (5′-ATG ATA TCG CCG CGC TCG TCG TC-3′) and β-Act-rev (5′-TTC TCG CGG TTG GCC TTG GGG TTC AG-3′). Amplification products were 236 bp for HIV-1-derived sequences and 390 bp for β-actin sequences.
Supplementary Material
HT1080 cells transduced by HIV-1 vector particles pseudo-typed with F and H protein variants of MV.
CD20-dependent membrane fusion at neutral pH.
Titer enhancement of αCD20-HIV vector particles.
Transduction of various cell lines with HIV-1 vector particles pseudotyped with modified MV glycoproteins.
Transduction of primary human B cells from various donors.
Acknowledgments
This work was supported by grant BU 1301/2-1 of the priority program “Mechanisms of gene vector entry and persistence” of the Deutsche Forschungsgemeinschaft to C.J.B. and K.C., and National Institutes of Health grant CA90636 to R.C. S.F. was supported by the graduate study program “GK1172 Biologicals” of the Johann Wolfgang Goethe-University Frankfurt a. M. We thank Ulrike Müller (University of Heidelberg) for a critical reading of the manuscript. This work was performed in Langen, Germany.
References
- 1.Cockrell AS, Kafri T. Gene delivery by lentivirus vectors. Mol Biotechnol. 2007;36:184–204. doi: 10.1007/s12033-007-0010-8. [DOI] [PubMed] [Google Scholar]
- 2.Levine BL, Humeau LM, Boyer J, MacGregor RR, Rebello T, Lu X, et al. Gene transfer in humans using a conditionally replicating lentiviral vector. Proc Natl Acad Sci USA. 2006;103:17372–17377. doi: 10.1073/pnas.0608138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown BD, Venneri MA, Zingale A, Sergi SL, Naldini L. Endogenous microRNA regulation suppresses transgene expression in hematopoietic lineages and enables stable gene transfer. Nat Med. 2006;12:585–591. doi: 10.1038/nm1398. [DOI] [PubMed] [Google Scholar]
- 4.Schnierle BS, Stitz J, Bosch V, Nocken F, Merget-Millitzer H, Engelstadter M, et al. Pseudotyping of murine leukemia virus with the envelope glycoproteins of HIV generates a retroviral vector with specificity of infection for CD4-expressing cells. Proc Natl Acad Sci USA. 1997;94:8640–8645. doi: 10.1073/pnas.94.16.8640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miletic H, Fischer YH, Neumann H, Hans V, Stenzel W, Giroglou T, et al. Selective transduction of malignant glioma by lentiviral vectors pseudotyped with lymphocytic choriomeningitis virus glycoproteins. Hum Gene Ther. 2004;15:1091–1100. doi: 10.1089/hum.2004.15.1091. [DOI] [PubMed] [Google Scholar]
- 6.Sakalian M, Hunter E. Molecular events in the assembly of retrovirus particles. Adv Exp Med Biol. 1998;440:329–339. doi: 10.1007/978-1-4615-5331-1_43. [DOI] [PubMed] [Google Scholar]
- 7.Sandrin V, Muriaux D, Darlix JL, Cosset FL. Intracellular trafficking of Gag and Env proteins and their interactions modulate pseudotyping of retroviruses. J Virol. 2004;78:7153–7164. doi: 10.1128/JVI.78.13.7153-7164.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christodoulopoulos I, Cannon PM. Sequences in the cytoplasmic tail of the gibbon ape leukemia virus envelope protein that prevent its incorporation into lentivirus vectors. J Virol. 2001;75:4129–4138. doi: 10.1128/JVI.75.9.4129-4138.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merten CA, Stitz J, Braun G, Poeschla EM, Cichutek K, Buchholz CJ. Directed evolution of retrovirus envelope protein cytoplasmic tails guided by functional incorporation into lentivirus particles. J Virol. 2005;79:834–840. doi: 10.1128/JVI.79.2.834-840.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buchholz CJ, Duerner LJ, Funke S, Schneider IC. Retroviral display and high throughput screening. Comb Chem High Throughput Screen. 2008;11:99–110. doi: 10.2174/138620708783744543. [DOI] [PubMed] [Google Scholar]
- 11.Sandrin V, Russell SJ, Cosset FL. Targeting retroviral and lentiviral vectors. Curr Top Microbiol Immunol. 2003;281:137–178. doi: 10.1007/978-3-642-19012-4_4. [DOI] [PubMed] [Google Scholar]
- 12.Hartl I, Schneider RM, Sun Y, Medvedovska J, Chadwick MP, Russell SJ, et al. Library-based selection of retroviruses selectively spreading through matrix metalloprotease-positive cells. Gene Ther. 2005;12:918–926. doi: 10.1038/sj.gt.3302467. [DOI] [PubMed] [Google Scholar]
- 13.Szecsi J, Drury R, Josserand V, Grange MP, Boson B, Hartl I, et al. Targeted retroviral vectors displaying a cleavage site-engineered hemagglutinin (HA) through HA-protease interactions. Mol Ther. 2006;14:735–744. doi: 10.1016/j.ymthe.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 14.Morizono K, Xie Y, Ringpis GE, Johnson M, Nassanian H, Lee B, et al. Lentiviral vector retargeting to P-glycoprotein on metastatic melanoma through intravenous injection. Nat Med. 2005;11:346–352. doi: 10.1038/nm1192. [DOI] [PubMed] [Google Scholar]
- 15.Yang L, Bailey L, Baltimore D, Wang P. Targeting lentiviral vectors to specific cell types in vivo. Proc Natl Acad Sci USA. 2006;103:11479–11484. doi: 10.1073/pnas.0604993103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yanagi Y, Takeda M, Ohno S. Measles virus: cellular receptors, tropism and pathogenesis. J Gen Virol. 2006;87:2767–2779. doi: 10.1099/vir.0.82221-0. [DOI] [PubMed] [Google Scholar]
- 17.Moll M, Klenk HD, Maisner A. Importance of the cytoplasmic tails of the measles virus glycoproteins for fusogenic activity and the generation of recombinant measles viruses. J Virol. 2002;76:7174–7186. doi: 10.1128/JVI.76.14.7174-7186.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vongpunsawad S, Oezgun N, Braun W, Cattaneo R. Selectively receptor-blind measles viruses: identification of residues necessary for SLAM- or CD46-induced fusion and their localization on a new hemagglutinin structural model. J Virol. 2004;78:302–313. doi: 10.1128/JVI.78.1.302-313.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakamura T, Peng KW, Harvey M, Greiner S, Lorimer IA, James CD, et al. Rescue and propagation of fully retargeted oncolytic measles viruses. Nat Biotechnol. 2005;23:209–214. doi: 10.1038/nbt1060. [DOI] [PubMed] [Google Scholar]
- 20.Hammond AL, Plemper RK, Zhang J, Schneider U, Russell SJ, Cattaneo R. Single-chain antibody displayed on a recombinant measles virus confers entry through the tumor-associated carcinoembryonic antigen. J Virol. 2001;75:2087–2096. doi: 10.1128/JVI.75.5.2087-2096.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paraskevakou G, Allen C, Nakamura T, Zollman P, James CD, Peng KW, et al. Epidermal growth factor receptor (EGFR)-retargeted measles virus strains effectively target EGFR- or EGFRvIII expressing gliomas. Mol Ther. 2007;15:677–686. doi: 10.1038/sj.mt.6300105. [DOI] [PubMed] [Google Scholar]
- 22.Ungerechts G, Springfeld C, Frenzke ME, Lampe J, Johnston PB, Parker WB, et al. Lymphoma chemovirotherapy: CD20-targeted and convertase-armed measles virus can synergize with fludarabine. Cancer Res. 2007;67:10939–10947. doi: 10.1158/0008-5472.CAN-07-1252. [DOI] [PubMed] [Google Scholar]
- 23.Liu ML, Winther BL, Kay MA. Pseudotransduction of hepatocytes by using concentrated pseudotyped vesicular stomatitis virus G glycoprotein (VSV-G)- Moloney murine leukemia virus-derived retrovirus vectors: comparison of VSV-G and amphotropic vectors for hepatic gene transfer. J Virol. 1996;70:2497–2502. doi: 10.1128/jvi.70.4.2497-2502.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cattaneo R, Rebmann G, Schmid A, Baczko K, ter Meulen V, Billeter MA. Altered transcription of a defective measles virus genome derived from a diseased human brain. EMBO J. 1987;6:681–688. doi: 10.1002/j.1460-2075.1987.tb04808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blakely BT, Rossi FM, Tillotson B, Palmer M, Estelles A, Blau HM. Epidermal growth factor receptor dimerization monitored in live cells. Nat Biotechnol. 2000;18:218–222. doi: 10.1038/72686. [DOI] [PubMed] [Google Scholar]
- 26.Haigler H, Ash JF, Singer SJ, Cohen S. Visualization by fluorescence of the binding and internalization of epidermal growth factor in human carcinoma cells A-431. Proc Natl Acad Sci USA. 1978;75:3317–3321. doi: 10.1073/pnas.75.7.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Junker K, Koehl U, Zimmerman S, Stein S, Schwabe D, Klingebiel T, et al. Kinetics of cell death in T lymphocytes genetically modified with two novel suicide fusion genes. Gene Ther. 2003;10:1189–1197. doi: 10.1038/sj.gt.3301977. [DOI] [PubMed] [Google Scholar]
- 28.Gallardo HF, Tan C, Ory D, Sadelain M. Recombinant retroviruses pseudotyped with the vesicular stomatitis virus G glycoprotein mediate both stable gene transfer and pseudotransduction in human peripheral blood lymphocytes. Blood. 1997;90:952–957. [PubMed] [Google Scholar]
- 29.Chun TW, Stuyver L, Mizell SB, Ehler LA, Mican JA, Baseler M, et al. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci USA. 1997;94:13193–13197. doi: 10.1073/pnas.94.24.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cosset FL, Morling FJ, Takeuchi Y, Weiss RA, Collins MK, Russell SJ. Retroviral retargeting by envelopes expressing an N-terminal binding domain. J Virol. 1995;69:6314–6322. doi: 10.1128/jvi.69.10.6314-6322.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Glennie MJ, French RR, Cragg MS, Taylor RP. Mechanisms of killing by anti-CD20 monoclonal antibodies. Mol Immunol. 2007;44:3823–3837. doi: 10.1016/j.molimm.2007.06.151. [DOI] [PubMed] [Google Scholar]
- 32.Plumet S, Duprex WP, Gerlier D. Dynamics of viral RNA synthesis during measles virus infection. J Virol. 2005;79:6900–6908. doi: 10.1128/JVI.79.11.6900-6908.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bovia F, Salmon P, Matthes T, Kvell K, Nguyen TH, Werner-Favre C, et al. Efficient transduction of primary human B lymphocytes and nondividing myeloma B cells with HIV-1-derived lentiviral vectors. Blood. 2003;101:1727–1733. doi: 10.1182/blood-2001-12-0249. [DOI] [PubMed] [Google Scholar]
- 34.Zubler RH. Naive and memory B cells in T-cell-dependent and T-independent responses. Springer Semin Immunopathol. 2001;23:405–419. doi: 10.1007/s281-001-8167-7. [DOI] [PubMed] [Google Scholar]
- 35.Janssens W, Chuah MK, Naldini L, Follenzi A, Collen D, Saint-Remy JM, et al. Efficiency of onco-retroviral and lentiviral gene transfer into primary mouse and human B-lymphocytes is pseudotype dependent. Hum Gene Ther. 2003;14:263–276. doi: 10.1089/10430340360535814. [DOI] [PubMed] [Google Scholar]
- 36.Verhoeyen E, Dardalhon V, Ducrey-Rundquist O, Trono D, Taylor N, Cosset FL. IL-7 surface-engineered lentiviral vectors promote survival and efficient gene transfer in resting primary T lymphocytes. Blood. 2003;101:2167–2174. doi: 10.1182/blood-2002-07-2224. [DOI] [PubMed] [Google Scholar]
- 37.Verhoeyen E, Wiznerowicz M, Olivier D, Izac B, Trono D, Dubart-Kupperschmitt A, et al. Novel lentiviral vectors displaying “early-acting cytokines” selectively promote survival and transduction of NOD/SCID repopulating human hematopoietic stem cells. Blood. 2005;106:3386–3395. doi: 10.1182/blood-2004-12-4736. [DOI] [PubMed] [Google Scholar]
- 38.Tedder TF, Engel P. CD20: a regulator of cell-cycle progression of B lymphocytes. Immunol Today. 1994;15:450–454. doi: 10.1016/0167-5699(94)90276-3. [DOI] [PubMed] [Google Scholar]
- 39.Smeland E, Godal T, Ruud E, Beiske K, Funderud S, Clark EA, et al. The specific induction of myc protooncogene expression in normal human B cells is not a sufficient event for acquisition of competence to proliferate. Proc Natl Acad Sci USA. 1985;82:6255–6259. doi: 10.1073/pnas.82.18.6255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clark EA, Shu G, Ledbetter JA. Role of the Bp35 cell surface polypeptide in human B-cell activation. Proc Natl Acad Sci USA. 1985;82:1766–1770. doi: 10.1073/pnas.82.6.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cathomen T, Buchholz CJ, Spielhofer P, Cattaneo R. Preferential initiation at the second AUG of the measles virus F mRNA: a role for the long untranslated region. Virology. 1995;214:628–632. doi: 10.1006/viro.1995.0075. [DOI] [PubMed] [Google Scholar]
- 42.Cathomen T, Naim HY, Cattaneo R. Measles viruses with altered envelope protein cytoplasmic tails gain cell fusion competence. J Virol. 1998;72:1224–1234. doi: 10.1128/jvi.72.2.1224-1234.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buchholz CJ, Peng KW, Morling FJ, Zhang J, Cosset FL, Russell SJ. In vivo selection of protease cleavage sites from retrovirus display libraries. Nat Biotechnol. 1998;16:951–954. doi: 10.1038/nbt1098-951. [DOI] [PubMed] [Google Scholar]
- 44.Demaison C, Parsley K, Brouns G, Scherr M, Battmer K, Kinnon C, et al. High-level transduction and gene expression in hematopoietic repopulating cells using a human immunodeficiency [correction of imunodeficiency] virus type 1-based lentiviral vector containing an internal spleen focus forming virus promoter. Hum Gene Ther. 2002;13:803–813. doi: 10.1089/10430340252898984. [DOI] [PubMed] [Google Scholar]
- 45.Folks T, Benn S, Rabson A, Theodore T, Hoggan MD, Martin M, et al. Characterization of a continuous T-cell line susceptible to the cytopathic effects of the acquired immunodeficiency syndrome (AIDS)-associated retrovirus. Proc Natl Acad Sci USA. 1985;82:4539–4543. doi: 10.1073/pnas.82.13.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bucheit AD, Kumar S, Grote DM, Lin Y, von MV, Cattaneo RB, et al. An oncolytic measles virus engineered to enter cells through the CD20 antigen. Mol Ther. 2003;7:62–72. doi: 10.1016/s1525-0016(02)00033-3. [DOI] [PubMed] [Google Scholar]
- 47.Nakamura T, Peng KW, Vongpunsawad S, Harvey M, Mizuguchi H, Hayakawa T, et al. Antibody-targeted cell fusion. Nat Biotechnol. 2004;22:331–336. doi: 10.1038/nbt942. [DOI] [PubMed] [Google Scholar]
- 48.Zufferey R, Nagy D, Mandel RJ, Naldini L, Trono D. Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat Biotechnol. 1997;15:871–875. doi: 10.1038/nbt0997-871. [DOI] [PubMed] [Google Scholar]
- 49.Miyoshi H, Takahashi M, Gage FH, Verma IM. Stable and efficient gene transfer into the retina using an HIV-based lentiviral vector. Proc Natl Acad Sci USA. 1997;94:10319–10323. doi: 10.1073/pnas.94.19.10319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Soneoka Y, Cannon PM, Ramsdale EE, Griffiths JC, Romano G, Kingsman SM, et al. A transient three-plasmid expression system for the production of high titer retroviral vectors. Nucleic Acids Res. 1995;23:628–633. doi: 10.1093/nar/23.4.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
HT1080 cells transduced by HIV-1 vector particles pseudo-typed with F and H protein variants of MV.
CD20-dependent membrane fusion at neutral pH.
Titer enhancement of αCD20-HIV vector particles.
Transduction of various cell lines with HIV-1 vector particles pseudotyped with modified MV glycoproteins.
Transduction of primary human B cells from various donors.