Abstract
The lifetime and efficacy of a subcutaneously implanted glucose biosensor could be greatly improved by a self-cleaning membrane capable of periodic physical removal of adhered cells associated with the foreign body reaction. Previously, we reported thermoresponsive double network nanocomposite (DNNC) membrane comprised of poly(N-isopropylacrylamide) (PNIPAAm) and embedded polysiloxane nanoparticles. When the membrane was thermally cycled above and below its volume phase transition temperature (VPTT, ~33–35 °C), the associated deswelling and reswelling, respectively, led to in vitro cell release. Herein, this membrane design was tailored to meet the specific demands of a subcutaneously implanted glucose biosensor and critical functional properties were assessed. First, N-vinylpyrrolidone (NVP) comonomer increased the VPTT to ~38 °C so that the membrane would be swollen and thus more permeable to glucose in the “off-state” (i.e. no heating) while residing in the subcutaneous tissue (~35 °C). Second, glucose diffusion kinetics though the DNNC membrane was experimentally measured in its deswollen and reswollen states. A cylindrical DNNC membrane with dimensions considered suitable for implantation (1.5×5 mm, diameter × length) was used to model the glucose diffusion lag time. In addition, the DNNC cylinder was used to observe dimensional changes associated with deswelling and reswelling. Non-cytotoxicity was confirmed and self-cleaning was assessed in vitro in terms of thermally-driven cell release to confirm the potential of the DNNC membrane to control biofouling.
Keywords: thermoresponsive, hydrogel, nanocomposite, glucose diffusion, biofouling, cell release
Introduction
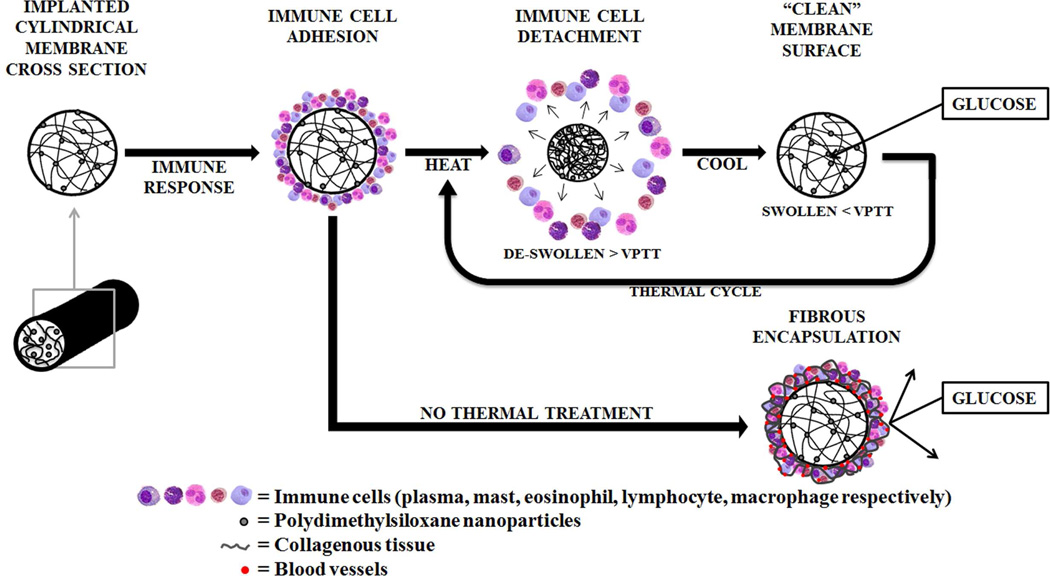
In the United States alone, diabetes mellitus affects ~26 million individuals and is projected to increase to ~36 million by 2030.1 Poor monitoring of glucose levels leads to an increase in hypoor hyperglycemic events as well as serious long-term complications (e.g. blindness and heart disease) or even death.2 Presently, a finger-prick test is most commonly used to provide intermittent blood sugar measurements. However, a subcutaneously implanted glucose biosensor could offer a continuous and more convenient method to monitor glucose levels. Unfortunately, membrane biofouling severely limits the lifetime and accuracy of subcutaneous or transdermal sensors.3–4 Upon implantation of a sensor, a foreign body reaction is triggered that results in the attachment of proteins and cells to the surrounding membrane and, eventually, the formation of a fibrous capsule3–4 (Figure 1). Membrane biofouling will inhibit glucose diffusion to the sensor thereby causing its failure. In this way, commercially available transdermal continuous glucose monitoring (CGM) systems are limited to a 3–7 day lifetime. Approaches to control membrane biofouling have largely focused on passive or “anti-fouling” membranes such as those based on poly(ethylene glycol)diacrylate (PEG-DA),5 poly(hydroxyethylmethacrylate) (PHEMA)6 and poly(tetrafluoroethylene) (PTFE).7–8 In contrast, the self-cleaning membrane reported herein relies on an active or “foul-releasing” mechanism to physically remove adsorbed cells.
Figure 1.
Biofouling of a membrane surrounding an implanted biosensor compromises glucose diffusion. The double network nanocomposite (DNNC) membrane described herein is designed to exhibit “self-cleaning” when thermally cycling above its volume phase transition temperature (VPTT).
Thermoresponsive PNIPAAm hydrogels undergo deswelling and reswelling when heated above and cooled below, respectively, their volume phase transition temperature (VPTT) (~33–35 °C). This process has been shown to cause the release of cultured cells in vitro9–14 If utilized as a membrane for an implanted glucose biosensor, self-cleaning may be accomplished via transdermal thermal cycling. Conventional single network (SN) PNIPAAm hydrogels prepared via copolymerization of NIPAAm and a crosslinker such as N,N’-methylenebisacrylamide (BIS) exhibit slow deswelling and reswelling kinetics (i.e. thermosensitivity) as well as poor mechanical properties.15–16 When used as a self-cleaning membrane, the PNIPAAm hydrogel requires enhanced thermosensitivity (for self-cleaning) as well as robust mechanical properties (for surgical insertion). Recently, we reported a double network nanocomposite (DNNC) hydrogel comprised of an interpenetrating, asymmetrically crosslinked PNIPAAm matrix with polysiloxane nanoparticles (~200 nm diameter) embedded during formation of the first network.17 This DNNC hydrogel exhibited significantly improved thermosensitivity in terms of both the rate and the extent of deswelling and reswelling versus a conventional PNIPAAm hydrogel. Furthermore, the DNNC hydrogel exhibited improved modulus and strength.
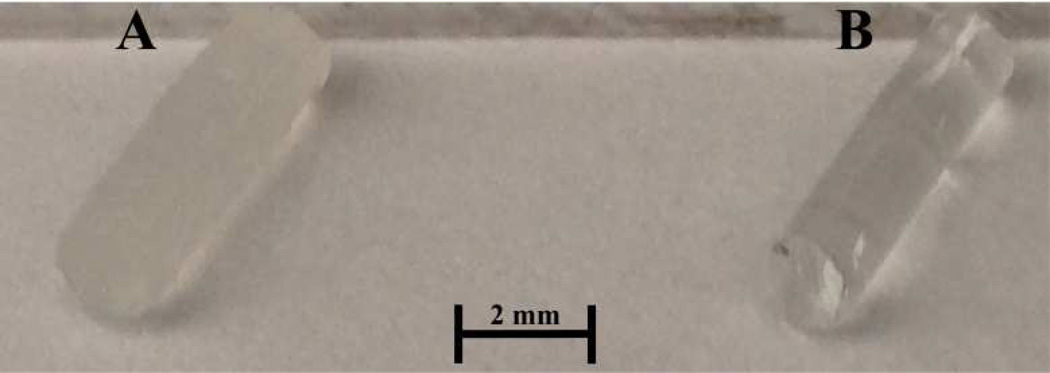
Extending the utility of this DNNC hydrogel as a self-cleaning membrane for an implanted glucose biosensor requires further refinement and is addressed in this study. First, the VPTT of the DNNC membrane was increased to ~38°C. In the subcutaneous tissue of the wrist, a likely location for an implanted sensor, the body temperature is ~35 °C.18–19 Thus, a membrane with a VPTT ~38°C in the “off-state” will be fully swollen for optimal glucose diffusion. When undergoing self-cleaning (“on-state”), the membrane would begin to deswell via transdermal heating. Copolymerization of NIPAAm with a hydrophilic comonomer is known to increase the VPTT of the resulting hydrogel.20–21 Previously, we demonstrated that addition of 1–2 wt% N-vinylpyrrolidone (NVP) comonomer (based on NIPAAm wt) produced analogous single network nanocomposite (SNNC) hydrogels with a VPTT of ~38°C.22 Thus, NVP was similarly incorporated into the DNNC hydrogels. Second, glucose diffusion through a planar DNNC membrane was measured at temperatures above and below the VPTT. Third, a membrane with a geometry suitable for implantation was considered to be a cylindrical rod (~1.5 mm×5 mm, diameter×length) (Figure 2). A finite element model was constructed for the DNNC hydrogel cylinders to estimate the glucose diffusion lag time before achieving equilibrium with its external environment at varying glucose concentrations. Since size and geometry also affect thermosensitivity23–24 which are critical to fast and efficient self-cleaning, the thermosensitivity of the DNNC hydrogel cylinders was assessed by measuring the change in diameter with temperature. Finally, cytocompatibility was assessed and thermally-induced in vitro cellular detachment was observed using planar DNNC hydrogels.
Figure 2.
DNNC [A] and PEG-DA [B] cylindrical membranes fabricated with a diameter of ~1.5 mm and length of 5 mm.
Materials and methods
Materials
NIPAAm (97%), NVP, PEG-DA (MW 575 g/mol), ammonium hydroxide (NH4OH), sodium chloride (NaCl), sodium phosphate-dibasis (Na2HPO4), potassium phosphate-monobasis (KH2PO4), hydrochloric acid (HCl), sodium hydroxide (NaOH), newborn calf serum (NCS), antibiotic antimycotic solution (100X) - stabilized bioreagent sterile filtered with 10,000 units penicillin and 10 mg streptomycin A, sterile Dulbecco’s phosphate buffered saline (PBS), HEPES (≥ 99.5%), and Dulbecco’s Modified Eagle’s Medium (DMEM) –1000 mg dL−1 glucose and L-glutamine without sodium bicarbonate and phenol red were purchased from Sigma- Aldrich (St. Louis, MO). Potassium chloride (KCl) and D-glucose anhydrous was purchased from Fisher Scientific (Pittsburgh, PA). Potassium persulfate (K2S2O8) was purchased from Mallinchrodt Chemicals. N,N’-methylenebisacrylamide (BIS, 99%) was purchased from Acros Organics (Geel, Belgium). 2-Hydroxy-2-methyl-1-phenyl-1-propanone (Darocur 1173) and 1-[4-(2-Hydroxy)-phenyl]-2-hydroxy-2-methyl-1-propane-1-one (Irgacure 2959) was purchased from Ciba Specialty Chemicals (Tarrytown, NY). Octamethylcyclotetrasiloxane (D4) and 1,3,5,7-tetramethyl- 1,3,5,7-tetravinylcyclotetrasiloxane (D4Vi) came from Gelest, Inc. Dodecylbenzene-sulfonic acid (DBSA, BIO-SOFT® S-101) came from Stepan Co. (Northfield, IL). The Slide-A-Lyzer dialysis cassettes (MWCO 10,000) and lactate dehydrogenase (LDH) cytotoxicity assay kit were obtained from Pierce (Rockford, IL). For hydrogel fabrication and other experiments, deionized water (DI H2O) with a resistance of 18 MPΩ·cm (Millipore, Billerica, MA) was used. 3T3 H2B-GFP mouse fibroblast cell line was a kind gift from Peter Ghazal at the Division of Pathway Medicine at the University of Edinburgh. Cell culture media was pH adjusted with 1 M HCl and 1 M NaOH, verified with a pH meter (420 A+, Orion; electrode 5990-30, Cole-Parmer, Vernon Hills, IL) and sterilized by 0.2 µm filtration (sterile 90 mm filter unit, Nalgene Filtration Products).
Preparation of Polysiloxane Nanoparticles
Polysiloxane colloidal nanoparticles with an average diameter of ~200 nm were prepared via emulsion polymerization and purified via dialysis as previously reported.14 The final emulsion was 4.8 wt% solids.
Preparation of PEG-DA Hydrogels
Precursor solutions were formed by votexing DI-H2O, PEG-DA (100 %v/v) and Darocur 1173 (1% v/v) for 1 min.
Planar sheets
Planar hydrogel sheets (~1 mm thick per electronic caliper measurements) were prepared by pipetting the precursor solution between two clamped glass slides (75×50 mm) separated by polycarbonate spacers (1 mm thick) and exposing the mold to longwave ultraviolet (UV) light (UVP UV-Transilluminator, 6 mW cm−2, λpeak = 365 nm) for 2 min at room temperature (RT). Hydrogel sheets were removed from their molds, rinsed with DI H2O and soaked in a Petri dish containing DI H2O (60 mL) for 24 hours.
Cylinders
Cylindrical hydrogels (~1.5 mm×5 mm, diameter×length per electronic caliper) were prepared by pipetting the precursor solution into a hollow cylindrical glass mold (inside diameter = 1.0 mm, length = 15 mm) with one end sealed by Parafilm. After sealing the other end of the mold, it was likewise exposed to longwave UV light as above at RT for 3 sec. The cylindrical hydrogel was removed from the mold, rinsed with DI H2O and immersed in a Petri dish containing DI H2O (60 mL) for 24 hours. A clean razor blade was used to equally trim the ends to reduce the length to 5 mm.
Preparation of Thermoresponsive DNNC Hydrogels
DNNC hydrogels were prepared by sequential formation of a relatively tightly crosslinked 1st network containing polysiloxane nanoparticles (2 wt% solid nanoparticles based on NIPAAm weight) and a loosely crosslinked 2nd network.17 The “1st network precursor solution” was formed by combining NIPAAm monomer (1.0 g), NVP co-monomer (0.16 g), BIS crosslinker (0.04 g), polysiloxane nanoparticle emulsion (0.485 g), Irgacure-2959 photoinitiator (0.08 g) and DI H2O (6.54 g). The “2nd network precursor solution” was formed by combining NIPAAm (6.0 g), NVP (0.96 g), BIS (0.012 g), Irgacure 2959 (0.24 g), and DI H2O (21.0 g).
Planar sheets
Planar hydrogel sheets (1 mm thick) were produced by pipetting the 1st network precursor solution into a mold consisting of two clamped glass slides (75×50 mm) separated by 1 mm thick polycarbonate spacers. The mold was then immersed into an ice water bath (~7 °C) and exposed to longwave UV light for 30 min. The resulting single network nanocomposite (SNNC) sheet was removed from the mold, rinsed with DI H2O and then soaked in DI H2O at RT for 2 days with daily water changes. The SNNC sheet was then transferred into a covered Petri dish containing the 2nd network precursor solution for 24 hours at RT. Next, the planar hydrogel was placed into a rectangular mold (1.5 mm thick), photocured for 30 min and finally soaked in DI H2O as above.
Cylinders
Cylindrical hydrogels (~1.5 mm×5 mm, diameter×length) were prepared by pipetting the precursor solution into a cylindrical glass mold (inside diameter = 1.0 mm, length = 15 mm) as above. The mold was immersed in an ice water bath (~7 °C) and exposed for 10 min to longwave UV light. Cylindrical hydrogels were removed from their molds, rinsed with DI H2O, and soaked in a Petri dish containing DI H2O (60 mL) for 2 days at RT with daily water changes. A SNNC cylindrical hydrogel was then transferred into a Petri dish containing the 2nd network precursor solution for 24 hours at RT. The cylindrical hydrogel was then placed into a second cylindrical mold (diameter = 1.5 mm, length = 15 mm), submerged in an ice water bath (~7 °C), exposed for 10 min to longwave UV light and soaked in DI H2O as above. A clean razor blade was used to trim ends to reduce the cylindrical length to 5 mm. The final diameter was measured via calipers.
Differential Scanning Calorimetry (DSC)
The VPTT of swollen hydrogels was determined by differential scanning calorimetry (DSC, TA Instruments Q100). Water-swollen hydrogels were blotted with a Kim Wipe and a small piece sealed in a hermetic pan. After cooling to −50 °C, the temperature was increased to 50 °C at a rate of 3 °C /min for 2 cycles. The resulting endothermic phase transition peak is characterized by the initial temperature at which the endotherm starts (To) and the peak temperature of the endotherm (Tmax). Reported data are from the 2nd cycle.
Glucose Diffusion
Planar hydrogel strips (1 cm × 1 cm × 1 mm) were placed in a side-by-side diffusion cell (PermeGear, Bethlehem, PA) positioned atop a stir plate. The donor chamber contained 3 mL of glucose solution (~1,000 mg dL−1) and the receptor chamber contained 3 mL of DI H2O. Chamber solutions were stirred with Teflon-coated stir bars (800 rpm) to maintain constant solution concentrations. A water jacket maintained the designated temperature (25, 35 and 40 °C) throughout the system. Every 20 min (for a total time of 3 hours), 50 µL aliquots were removed via pipette from each chamber and glucose concentration determined with a YSI 2700 Select Biochemistry Analyzer (YSI Incorporated, Yellow Springs, OH). The diffusion coefficients were calculated using Fick’s second law of diffusion.
Glucose Diffusion Lag Time
A computational model of the DNNC hydrogels was developed using COMSOL Multiphysics® software (COMSOL, Inc., Los Angeles, CA). Conducting a time-dependent transport of diluted species study, a geometric cylinder (1.5 mm×5 mm, diameter×length) was constructed with a maximum and minimum free tetrahedral mesh element size of 0.382 mm and 0.0249 mm, respectively. The simulation began with a DNNC hydrogel internal glucose quantity of 0 mg dL−1 and external glucose levels of 60, 80, 160 and 300 mg dL−1. The average glucose concentration within the cylindrical hydrogel was assessed every second for 1 hour for each external glucose concentrations. The diffusion lag time was defined as the time required for the hydrogel internal glucose concentration to fall within 5% of the external glucose concentration.
Thermosensitivity
Three cylindrical DNNC hydrogels (~1.5 mm×3 mm, diameter×length) were vertically attached to a single Petri dish with a small amount of optical adhesive (Norland Optical Adhesive 61) to the base of one end. To hydrate the affixed cylinders, the Petri dish was filled with DI H2O for at least 12 hours at RT prior to thermally cycling. The Petri dish was positioned atop a heating plate under a non-inverted bright field microscope (Nikon Eclipse LV 100D, Nikon America Inc., Melville, NY) with a 5X objective. Images were taken every 5 min as the hydrogels were thermally cycled between 25 and 40 °C for 5 cycles. The average rate of heating to 40 °C was ~1.06 °C/min and passive cooling to 25 °C was ~0.28 °C/min. Thus, each cycle consisted of a 1 hour heating period followed by 1 hour of passive cooling. Cylinder diameters were recorded with Nikon® NIS Elements imaging software.
Cytocompatiblity
DNNC hydrogel cytocompatibility was assessed by measuring LDH concentrations released by 3T3 H2B-GFP mouse fibroblast cells 24 hours after cell seeding versus that of two cytocompatible controls – a PEG-DA hydrogel as well as tissue culture plastic (i.e. polystyrene, PS). Planar DNNC and PEG-DA hydrogel sheets were prepared as described above. Four 6 mm discs were punched from each sheet and then sterilized by immersion in 80% ethanol for 45 min. The hydrogel discs were sequentially washed 3X (30 min each) with sterile DMEM (40% NCS), submerged in DMEM (40% NCS) for 24 hours and transferred to a sterile 12-well plate. Next, 3T3 H2B-GFP mouse fibroblast cells [suspended in DMEM (40% NCS) containing antimycotics and antibiotics], were seeded onto each of the hydrogel surfaces and into the empty tissue culture plastic wells at a concentration of ~6,500 cells cm−2. Cells were allowed to incubate for 24 hours at ~37 °C (T < VPTT; swollen state) with 5% CO2. Finally, the media surrounding the hydrogel discs or from the empty wells was extracted and assessed for LDH levels per the manufacturer’s protocol. The relative LDH activity was calculated by normalizing PEG-DA and DNNC sample absorptions to that of polystyrene.
“Self-Cleaning” Behavior in Vitro
Planar DNNC and PEG-DA hydrogel sheets (2 cm × 2 cm × ~1 mm) were sterilized by immersion in 80% ethanol for 45 min. All specimens were then washed 3X for 30 min each with sterile DMEM (40% NCS). DNNC and PEG-DA hydrogel sheets were submerged for 48 and 96 hours, respectively, in DMEM (40% NCS). Next, in a sterile plastic Petri dish, DNNC and PEG hydrogel sheets were inoculated with 3T3 H2B-GFP mouse fibroblast cells stained with a lipophilic indocarbocyanine dye (DiI) and suspended in DMEM (40% NCS) containing antimycotics and antibiotics at a concentration of ~30,000 cells/mL. For DNNC and PEG hydrogels, cells were allowed to incubate for 4 hours and 72 hours, respectively, at ~35 °C (T < VPTT; swollen state) with 5% CO2 before imaging. The Petri dish was transferred to the enclosed microscope stage which contained two heating pads (Minco) connected to thermistors controlled via a temperature feedback system (LabView). Hydrogel surface images were captured every 20 sec for 10 min with an inverted bright field microscope (Nikon Eclipse TE 2000-S, Nikon America Inc., Melville, NY) with a 10X objective as the temperature was increased from ~35 °C to ~39.5 °C (T > VPTT) at a rate of ~0.41 °C/min (i.e. ~10 minutes).
Results and Discussion
VPTT
By incorporating low levels of NVP comonomer (1.6 wt% based on NIPAAm monomer weight) into the 1st and 2nd network precursor solutions, the VPTT of the DNNC hydrogel was successfully increased. Per the DSC thermogram (Figure S1), To and Tmax were equal to 36.5 and 39.5 °C, respectively. Thus, at subcutaneous body temperature of the wrist (~35 °C), the DNNC hydrogel are expected to be swollen in the absence of external heating (i.e. “off-state”).
Glucose Diffusion
A side-by-side diffusion cell system was used to study glucose diffusion through the DNNC membrane 25 °C (T < VPTT), 35 °C (body temperature) and 40 °C (T > VPPT). Fick’s second law of diffusion was used to calculate the diffusion coefficients at each temperature:
where c is the concentration within the hydrogel, t is the time, D is the diffusion coefficient and x is the diffusion distance.25–28 Assuming that each solution preserved a uniform concentration and that each element concentrations were equal at the hydrogel membrane surface as in the bulk volume of each chamber, the equation may be simplified to:
where Qt is the overall quantity of glucose transferred through the hydrogel until the specified time, t, A refers to the hydrogel area exposed to the donor or receptor chambers, C1 is the initial solute concentration of the donor chamber, and L is the measured hydrogel membrane thickness. Table 1 reveals the influence of temperature on the diffusion coefficients (D) of the DNNC hydrogel. At 25 °C (T < VPTT), the swollen state of the hydrogel facilitates glucose diffusion. In contrast, when heated to 40 °C (T > VPTT), the hydrogel is deswollen and glucose diffusion is thus substantially slowed. While still below the measured To of the VPTT (~36.5 °C), glucose diffusion at 35 °C (body temperature) began to decrease somewhat indicating that some deswelling may have occurred. However, D of glucose through the dermis and epidermis has been reported as 2.64 ± 0.42×10−6 cm2/s and 0.075 ± 0.05×10−6 cm2/s, respectively.29 Thus, D of glucose through the hydrogel (1.88 ± 0.01×10−6 cm2/s) is within the functional range. Furthermore, D of a PEG-DA (MW 575 g/mol) hydrogel was previously determined to be 1.59 ± 0.42×10−6 cm2/s 30 and such materials are noted to not significantly impede glucose transfer to encapsulated biosensors.31 Thus, in the “off-state”, glucose diffusion through the DNNC hydrogel to the enclosed sensor is expected to be satisfactory.
Table 1.
Glucose diffusion coefficients (D) of the DNNC hydrogel (VPTT ~38 °C) at varying temperatures.
| Temperature (°C) | Membrane Behavior | Diffusion Coefficient (cm2/s) |
|---|---|---|
| 25 (T < VPTT) | Swollen | 2.73 ± 0.01 × 10−6 |
| 35 (body temperature) | Swollen | 1.88 ± 0.01 × 10−6 |
| 40 (T > VPTT) | Deswollen | 1.03 ± 0.01 × 10−6 |
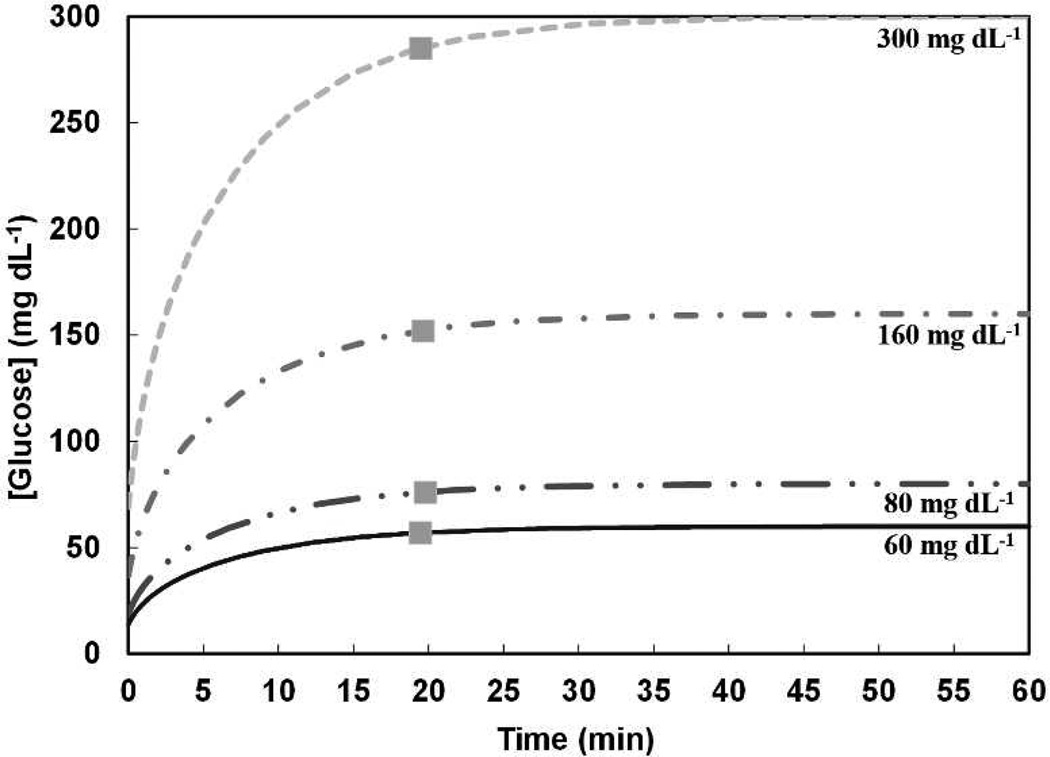
A COMSOL Multiphysics® computational model was utilized to evaluate the glucose diffusion lag time for a DNNC cylindrical hydrogel. The simulation began with an initial glucose quantity of 0 mg dL−1 within the hydrogel and the hydrogel then suspended in an environment with a constant glucose level of varying concentrations: 60, 80, 160, and 300 mg dL−1. These concentrations represent low, normal, high and very high physiologically glucose levels, respectively.32 Figure 3 expresses the average glucose concentration within the cylindrical hydrogel cavity every second for 1 hour at each of the external glucose concentrations. Physiological lag times upwards of 15 min have been reported between glucose changes in the interstitial fluid (ISF) and in the blood.33–37 For the DNNC cylindrical hydrogel (diameter 1.5 mm, length 5 mm), an average lag time of 19.59 ± 0.13 minutes was observed and thus somewhat exceeds physiological lag. To reduce the lag time, the cylinder diameter may be reduced. For instance, when the model was applied to a DNNC cylindrical hydrogel with a reduced diameter (diameter 350 µm, length 5 mm), a lag time of less than 5 min was determined (Figure S2).
Figure 3.
A computational model was utilized to determine the average glucose concentration inside a DNNC cylindrical hydrogel at 35 °C for constant environmental glucose levels of 300, 160, 80 and 60 mg dL−1. The glucose diffusion lag time ( ) marks when the average internal hydrogel glucose concentration is 95% to that of the external environment.
) marks when the average internal hydrogel glucose concentration is 95% to that of the external environment.
Thermosensitivity
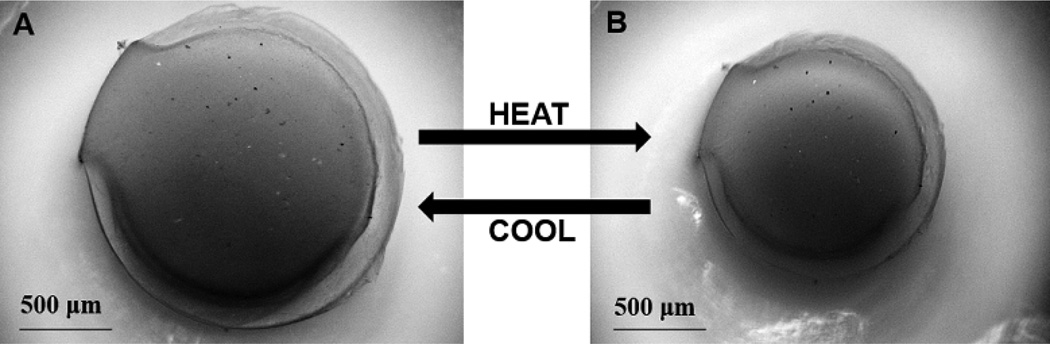
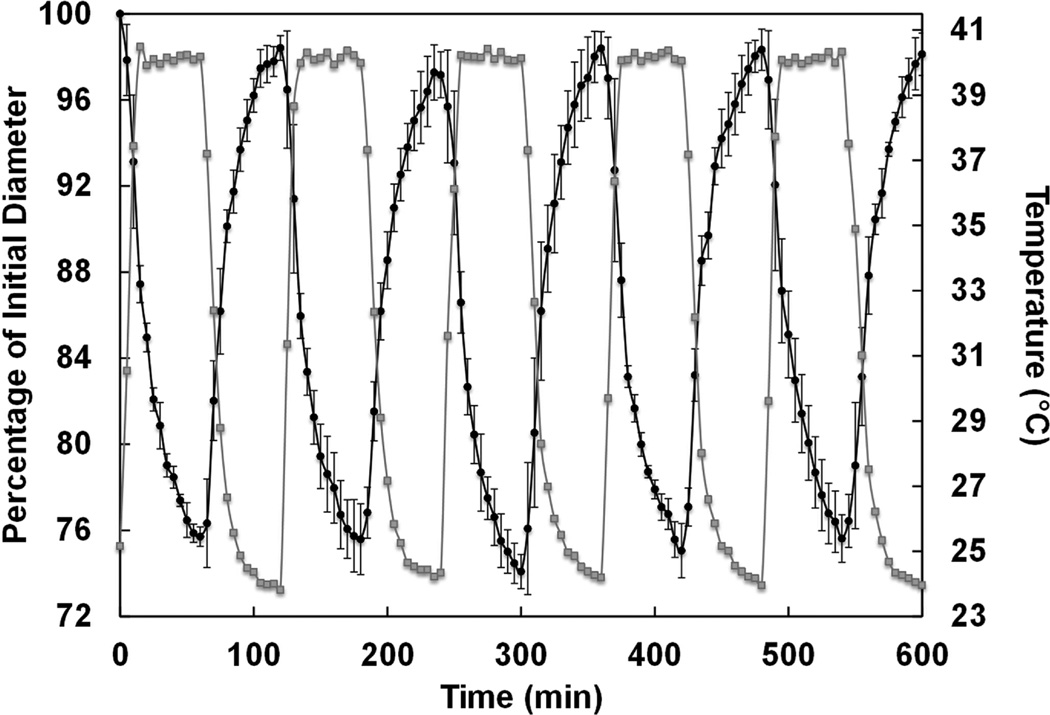
The extent and rate at which the DNNC cylindrical hydrogel deswells and reswells upon cyclically heating (T > VPTT) and cooling (T < VPTT) is important for its ability to function as a self-cleaning membrane for a subcutaneous glucose biosensor. First, the extent of deswelling is critical as this is the driving force behind physical removal of adhered cells on the membrane. 9, 14 When the temperature was increased and maintained at ~40 °C for 1 hour, the diameter of a vertically affixed DNNC cylinder decreased to ~25% of its initial swollen state diameter at RT (Figure 4). After returning to 25 °C for a period of 1 hour, the diameter returned to within 5% of its initial measured swollen state diameter. Second, the membrane must be able to undergo cyclical deswelling/reswelling while being thermally cycled. This behavior was confirmed by subjecting a vertically affixed DNNC cylinder to cyclical heating (~1.06 °C/min) and cooling (~0.28 °C/min) over a 10 hour period (Figure 5). Here, diameters achieved in the swollen and deswollen states remained very consistent.
Figure 4.
Bright field microscopy images of a vertically affixed DNNC cylinder in its swollen state at 25 °C (T < VPTT) [A] and deswollen state at 40 °C (T > VPTT) [B].
Figure 5.
Diameter change during thermal cycling of a vertically affixed DNNC cylinder over a 10 hour time period. Diameter change (black) and temperature change (grey).
Cytocompatibility
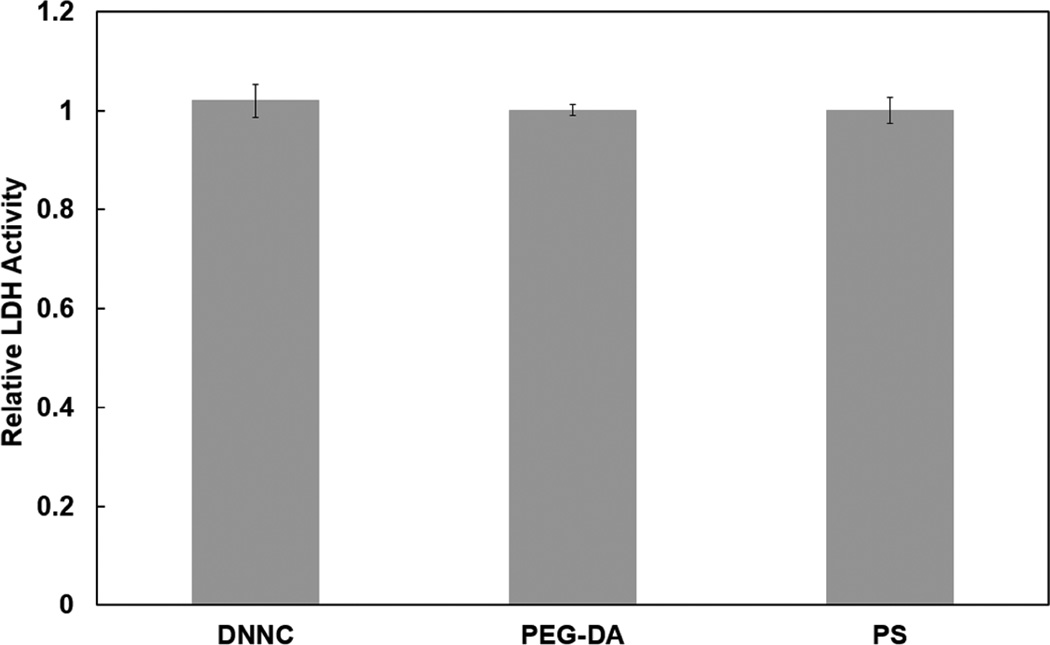
Cytocompatibility is essential to the utility of a self-cleaning membrane for a subcutaneously implanted glucose biosensor. The cytocompatibility of the DNNC hydrogel was determined via LDH activity assays (Figure 6). LDH is a soluble cytosolic enzyme that is released into the culture medium due to apoptosis or necrosis.38 LDH levels released by 3T3 H2B-GFP mouse fibroblast cells 24 hours post-seeding were measured for the DNNC hydrogel and compared to that of non-cytotoxic PEG-DA hydrogel and tissue culture plastic (i.e. PS). No statistical difference in normalized levels of exogenous LDH activity was observed. Thus, the DNNC hydrogel exhibits low cytotoxicity toward fibroblast cells similar to that of PEG-DA hydrogels.
Figure 6.
Relative LDH activity after 24 hours for PEG-DA, DNNC, and polystyrene (PS) inoculated with 3T3 H2B-GFP mouse fibroblast cells.
“Self-Cleaning” in Vitro
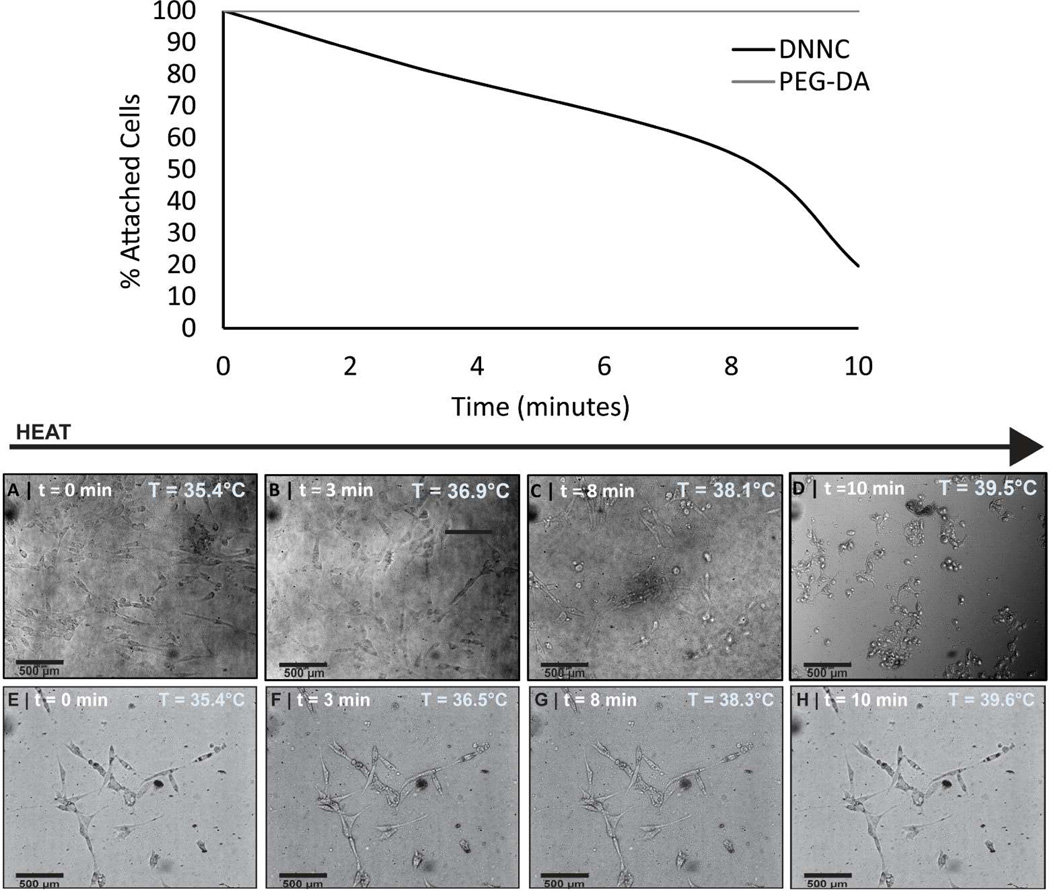
The ability of the DNNC hydrogel to release adhered cells (i.e. self-clean) when induced to deswell with thermally heating was assessed in vitro against a non-thermoresponsive PEG-DA hydrogel control (Figure 7). To achieve adequate adhesion of fibroblasts, the DNNC and PEG hydrogel sheets were first exposed to DMEM (40% NCS) for 48 and 96 hours, respectively. Such surface conditioning with protein is a common strategy to facilitate cellular adhesion.39 This protocol also parallels the way in which an implanted surface first adsorbs proteins prior to the adhesion of cells.3 A longer conditioning period for PEG hydrogels was determined to be required and can be attributed to the protein repulsive nature of PEG.40–41 Initially, at ~35.4 °C (T < VPTT), both the DNNC and PEG hydrogels were swollen and adhered fibroblasts exhibit a characteristic spread morphology. Heating to a temperature of ~39.5 °C was chosen as it is above the onset of the VPTT of the DNNC hydrogel and is below ~41 °C where protein denaturation may occur. Upon heating, the DNNC hydrogel underwent deswelling and fibroblasts display a round cell morphology indicative of end stages of detachment. In contrast, the PEG hydrogel does not undergo appreciable deswelling and cells remained adhered. The percentage of attached cells on DNNC and PEG-DA hydrogels was assessed from four frames each taken while heating a single membrane from ~35 °C to ~39.5 °C (T > VPTT) over 10 minutes (Figure 7). Thus, while the PEG hydrogel was more resistant to cellular adhesion, cell release was thermally triggered for the DNNC hydrogel.
Figure 7.
Bright field microscopy frames of DNNC [A-D] and PEG-DA [E-H] membranes seeded with 3T3 H2B-GFP mouse fibroblast cells incubated at ~35 °C (T < VPTT) and then heated to ~39.5 °C (T > VPTT). [A-D] demonstrate the detachment of cells due to deswelling of DNNC membrane. The graph depicts the percentage of attached cells to either DNNC or PEG-DA membranes as the temperature was increased from ~35 °C to ~39.5 °C over a ~10 minute period.
Conclusions
A thermoresponsive DNNC hydrogel design was refined and evaluated for its ability to function as a self-cleaning membrane for a subcutaneously implanted glucose biosensor. The VPTT was adjusted to ~38 °C with NVP comonomer such that the membrane would be swollen at body temperature (35 °C, wrist subcutaneous tissue) to maximize glucose diffusion. Thus, when heated about the VPTT, the membrane would undergo reversible deswelling, which should detach adhered cells from its surface. Furthermore, the non-degradable nature of PNIPAAm hydrogels42–43 is expected to be advantageous to maintain membrane functionality and to sustain containment of sensing materials.
The measured glucose diffusion coefficient (D) for the DNNC membrane was within the physiological range at 35 °C but decreased substantially when the membrane was heated to ~40 °C and deswollen. Consequently, during this phase of self-cleaning, glucose measurements would not be effective. A cylindrical rod (~1.5 mm×5 mm, diameter×length) was considered to be a suitable geometry for implantation. Based on a finite element model, glucose diffusion lag time for the DNNC hydrogel cylinders was estimated to be ~19 min. However, when a reduced diameter (350 µm) was considered, the lag time was reduced to ~5 min.
Thermosensitivity, critical to self-cleaning efficacy, of the DNNC cylindrical hydrogel was assessed by measuring the change in diameter when in a deswollen (T > VPTT) versus swollen state (T < VPTT). Over a 10 hour period of thermal cycling, the diameter of the deswollen cylindrical hydrogel returned to within 5% of the original swollen diameter.
In vitro, fibroblast cells exhibited minimal cytotoxicity and release from a planar DNNC hydrogel upon deswelling by heating above the VPTT while cells remained adhered to the non-thermoresponsive PEG-DA surface. In future studies, the release of other cell types as well as the adsorption of proteins will be conducted to thoroughly assess the self-cleaning behavior of the DNNC membrane.
Supplementary Material
Acknowledgments
Funding from the NIH/NIDDK (1R01DK095101-01A1) is gratefully acknowledged. We thank Prof. Michael McShane (Texas A&M University) for the use of his YSI 2700 Select Biochemistry Analyzer. Finally, we thank Prof. Arum Han (Texas A&M University) for the use of his Nikon Eclipse LV 100D non-inverted bright field microscope.
Footnotes
Supporting Information Available. Supporting information includes: Figure S1: DSC thermogram of the DNNC hydrogel; Figure S2: computational glucose diffusion model of 350 Wm diameter cylindrical DNNC hydrogel; Figure S3: percentage of attached cells to DNNC and PEG-DA hydrogels as the temperature was increased from ~35 °C to ~39.5 °C. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Shaw JE, Sicree RA, Zimmet PZ. Diabetes Res. Clin. Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Rubin RR, Peyrot M. Diabetes Metab. Res. Rev. 1999;15:205–2018. doi: 10.1002/(sici)1520-7560(199905/06)15:3<205::aid-dmrr29>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 3.Frost M, Meyerhoff ME. Anal. Chem. 2006;78:7370–7377. doi: 10.1021/ac069475k. [DOI] [PubMed] [Google Scholar]
- 4.Wisniewski N, Reichert M. Colloids Surf. B. 2000;18:197–219. doi: 10.1016/s0927-7765(99)00148-4. [DOI] [PubMed] [Google Scholar]
- 5.Quinn CA, Connor RE, Heller A. Biomaterials. 1997;18:1665–1670. doi: 10.1016/s0142-9612(97)00125-7. [DOI] [PubMed] [Google Scholar]
- 6.Marshall AJ, Ratner BD. AIChE J. 2005;51:1221–1232. [Google Scholar]
- 7.Bota PCS, Collie AMB, Puolakkainen P, Vernon RB, Sage EH, Ratner BD, Stayton PS. J. Biomed. Mater. Res. Part A. 2010;95:649–657. doi: 10.1002/jbm.a.32893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brauker JH, Carr-Brendel VE, Martinson LA, Crudele J, Johnston WD, Johnson RC. J. Biomed. Mater. Res. Part A. 1995;29:1517–1524. doi: 10.1002/jbm.820291208. [DOI] [PubMed] [Google Scholar]
- 9.Okano T, Yamada N, Okuhara M, Sakai H, Sakurai Y. Biomaterials. 1995;16:297–303. doi: 10.1016/0142-9612(95)93257-e. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi J, Okano T. Sci. Technol. Adv. Mater. 2010;11:014111. doi: 10.1088/1468-6996/11/1/014111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang J, Yamato M, Nishida K, Ohki T, Kanzaki M, Sekine H, Shimizu T, Okano T. J. Controlled Release. 2006;116:193–203. doi: 10.1016/j.jconrel.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 12.Tang Z, Akiyama Y, Okano T. Polymers. 2012;4:1478–1498. [Google Scholar]
- 13.Gant RM, Hou Y, Grunlan MA, Cote GL. J. Biomed. Mater. Res. Part A. 2009;90:695–701. doi: 10.1002/jbm.a.32135. [DOI] [PubMed] [Google Scholar]
- 14.Hou Y, Matthews AR, Smitherman AM, Bulick AS, Hahn MS, Hou H, Han A, Grunlan MA. Biomaterials. 2008;29:3175–3184. doi: 10.1016/j.biomaterials.2008.04.024. [DOI] [PubMed] [Google Scholar]
- 15.Haraguchi K, Li HJ. Macromolecules. 2006;39:1898–1905. [Google Scholar]
- 16.Zhang XZ, Xu XD, Cheng SX, Zhuo RX. Soft Matter. 2008;4:385–391. doi: 10.1039/b713803m. [DOI] [PubMed] [Google Scholar]
- 17.Fei R, George JT, Park J, Grunlan MA. Soft Matter. 2011;8:481–487. doi: 10.1039/C1SM06105D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Montgomery LD, Williams BA. Ann. Biomed. Eng. 1976;4:209–219. doi: 10.1007/BF02584515. [DOI] [PubMed] [Google Scholar]
- 19.Werner J, Buse M. J. Appl. Physiol. 1988;65:1110–1118. doi: 10.1152/jappl.1988.65.3.1110. [DOI] [PubMed] [Google Scholar]
- 20.Erbil C, Aras S, Uyanik N. J. Polym. Sci. Part A: Polym. Chem. 1999;37:1847–1855. [Google Scholar]
- 21.Feil H, Bae YH, Feijen J, Kim SW. Macromolecules. 1993;26:2496–2500. [Google Scholar]
- 22.Gant RM, Abraham AA, Hou Y, Cummins BM, Grunlan MA, Cote GL. Acta Biomater. 2010:2903–2910. doi: 10.1016/j.actbio.2010.01.039. [DOI] [PubMed] [Google Scholar]
- 23.Wu C, Zhou S. Macromolecules. 1997;30:574–576. [Google Scholar]
- 24.Bao L-R, Cheng X, Huang XD, Guo LJ, Pang SW, Yee AF. J. Vac. Sci. Technol. B. 2002;20:2881–2886. [Google Scholar]
- 25.Hannoun BJ, Stephanopoulos G. Biotechnol. Bioeng. 1986;28:829–835. doi: 10.1002/bit.260280609. [DOI] [PubMed] [Google Scholar]
- 26.Teixeira JA, Mota M, Venncio A. Chem. Eng. J. 1994;56:B9–B14. [Google Scholar]
- 27.Venancio A, Teixeira JA. Biotechnol. Tech. 1997;11:183–185. [Google Scholar]
- 28.Zhang W, Furusaki S. Biochem. Eng. J. 2001;9:73–82. [Google Scholar]
- 29.Khalil E, Kretsos K, Kasting GB. Pharm. Res. 2006;23:1227–1234. doi: 10.1007/s11095-006-0141-9. [DOI] [PubMed] [Google Scholar]
- 30.Russell RJ, Axel AC, Shields KL, Pishko MV. Polymer. 2001;42:4893–4901. [Google Scholar]
- 31.Quinn CP, Pishko MV, Schmidtke DW, Ishikawa M, Wagner JG, Raskin P, Hubbell JA, Heller A. The American journal of physiology. 1995;269:E155–E161. doi: 10.1152/ajpendo.1995.269.1.E155. [DOI] [PubMed] [Google Scholar]
- 32.Tuchin VV. Handbook of Optical Sensing of Glucose in Biological Fluids and Tissues. p xxxii. Boca Raton: CRC Press; 2009. p. 709. [Google Scholar]
- 33.Aussedat B, Dupire-Angel M, Gifford R, Klein JC, Wilson GS, Reach G. The American journal of physiology. 2000;278:E716–E728. doi: 10.1152/ajpendo.2000.278.4.E716. [DOI] [PubMed] [Google Scholar]
- 34.Baker DA, Gough DA. Anal. Chem. 1996;68:1292–1297. doi: 10.1021/ac960030d. [DOI] [PubMed] [Google Scholar]
- 35.Heise T, Koschinsky T, Heinemann L, Lodwig V. Diabetes Technol. Ther. 2003;5:563–571. doi: 10.1089/152091503322250587. [DOI] [PubMed] [Google Scholar]
- 36.Rebrin K, Steil GM. Diabetes Technol. Ther. 2000;2:461–472. doi: 10.1089/15209150050194332. [DOI] [PubMed] [Google Scholar]
- 37.Rebrin K, Steil GM, van Antwerp WP, Mastrototaro JJ. The American journal of physiology. 1999;277:E561–E571. doi: 10.1152/ajpendo.1999.277.3.E561. [DOI] [PubMed] [Google Scholar]
- 38.Renner K, Amberger A, Konwalinka G, Kofler R, Gnaiger E. Biochim. Biophys. Acta, Molecular Cell Res. 2003;1642:115–123. doi: 10.1016/s0167-4889(03)00105-8. [DOI] [PubMed] [Google Scholar]
- 39.Grinnell F, Feld MK. J Biol Chem. 1982;257:4888–4893. [PubMed] [Google Scholar]
- 40.Gombotz WR, Guanghui W, Hoffman AS. J. Biomed. Mater. Res. 1991;25:1547–1562. doi: 10.1002/jbm.820251211. [DOI] [PubMed] [Google Scholar]
- 41.Jeon SI, Andrade JD. J. Colloid Interface Sci. 1991;142:159–166. [Google Scholar]
- 42.Patenaude M, Hoare T. Biomacromolecules. 2012;13:369–378. doi: 10.1021/bm2013982. [DOI] [PubMed] [Google Scholar]
- 43.Klouda L, Mikos AG. Eur. J. Pharm. Biopharm. 2008;68:34–45. doi: 10.1016/j.ejpb.2007.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.