Abstract
Context:
Langerhans cells (LCs) are a unique population of antigen processing cells in the epidermis and mucous membrane, which may play a role in the defence mechanism against epithelial tumors.
Aims:
To compare the distribution of LCs in oral squamous cell carcinomas (OSCC) and normal oral epithelium; and to determine whether the population of LCs in OSCC has any correlation with histological grading of these malignancies.
Settings and Design:
A cross-sectional immunohistochemical analysis of OSCC cases.
Materials and Methods:
Forty-eight randomly selected paraffin tissue blocks of OSCC cases and 30 cases of normal oral epithelium were included. Hematoxylin and eosin-stained sections of the OSCC cases were reviewed and categorized as high-grade malignant tumors or low-grade malignant tumors. Tissue sections were analyzed for density of LCs using CD1a antibody expression.
Statistical Analysis Used:
Data are expressed as percentages compared by Chi-square statistics; mean ± standard deviation, compared by Mann-Whitney-U test and Spearman's correlation tests.
Results:
LCs population was significantly higher in normal oral epithelium when compared with OSCC cases (P = 0.001). There was also a significant difference in the number of LCs per millimetre square area of tissue section between well-differentiated tumors and poorly-differentiated tumours (P = 0.03). There was a negative correlation between the population density of LCs and the grade of OSCC.
Conclusions:
These findings suggest that oral mucosal LCs are involved in immune-surveillance and immunologic impairment may characterize invasive OSCC. In addition, LCs density characterizes histological grades of OSCC, which may be of a prognostic value.
Keywords: CD1a, langerhans cells, oral squamous cell carcinoma
INTRODUCTION
The prognosis of oral squamous cell carcinoma (OSCC) is related to different factors including the histological grade, tumor size, level of involvement of neighbouring tissues, presence of metastasis at the time of diagnosis and anatomical location of the tumor.[1,2] Histological diagnosis plays an important role in the management of oral cancers and histological grade is one of the prognostic factors. This has for many decades played a central role in the determination of malignancy, the classification of neoplasms, the grading and staging of tumors and management of disease.
Langerhans cells (LCs) are a unique population of antigen processing cells in the epidermis and mucous membrane, which may play a role in the defence mechanism against epithelial tumors.[3] These cells were discovered by Paul Langerhans in 1868 and described as the stellate-shaped epidermal cells that now bear his name.[4] They are responsible for the induction of hypersensitivity reaction in the skin and mucous membrane, which may also play a role in defence mechanisms against antigenic epithelial tumors.[5] LCs are important for the presentation of tumor-associated antigens,[6] which are relevant in the recruitment of peri- and intratumoral lymphocytic infiltrates and facilitate a T-cell-mediated antitumoral immune response.[7]
The ability of the tumor cells to evade the host immune system has been attributed to low immunogenicity. This includes defects in antigen processing, transport and presentation, defects in expression of major histocompatibility complex and low expression of distinct tumor-specific antigens. These defects not only promote uncontrolled tumor growth but also represent an obstacle to successful immunotherapy.[8] Host antigen presenting cells (APCs) appear to play an important role in the presentation of tumor antigens and the induction of specific immune responses to tumors.[9]
Studies have described the defective function of some APCs including macrophages, dendritic cells and B cells in tumor bearing hosts.[10,11] Dendritic cells are the most effective APCs in the induction of primary immune response[12] and the best for the delivery of tumor-specific antigens in cancer immunotherapy.[13]
The question of failure of local immune defence in carcinogenesis where antigen presentation is a key component needs to be assessed. Do LCs in tumors, act the same way as LCs in normal tissues? Do tumors alter LC phenotype and function? LCs have been studied in a variety of cancers. However, despite the extent of investigations, the role of LCs in OSCC is still poorly understood. This study therefore investigated the distribution of LCs in OSCC compared with normal oral epithelium and also determined whether the population of LCs in OSCC has any correlation with histological grading of these malignancies.
MATERIALS AND METHODS
This was a cross-sectional immunohistochemical study of randomly selected previously diagnosed OSCC cases seen at our hospital. OSCC included squamous cell carcinoma affecting the lips, tongue, floor of the mouth, palate and buccal mucosa.
This study received ethical clearance from the Joint University/Hospital Ethical Review Committee.
The study was carried out in the Oral Pathology and Pathology Departments of the Hospital. Forty-eight tissue blocks of histologically diagnosed squamous cell carcinomas of the oral cavity seen at the hospital were retrieved and prepared for hematoxylin- eosin and immunohistochemical staining. Hematoxylin and eosin stained 3 μm thick sections of the selected biopsy specimens were reviewed by light microscopy for the confirmation of diagnosis and fresh sections were obtained for histological grading of malignancy. The histological grading was performed by two investigators independently using Bryne's system.[2] Both intra-and inter observers variability were calculated. Cases with total score ranging from 4-8 were classified as low-grade malignancy (well-differentiated) and cases with a total score from 9-16 were classified as high-grade malignancy (poorly differentiated).
The control tissue specimens from normal epithelium of the oral cavity were obtained from fresh postmortem tissues from the Pathology Department. Biopsies of normal tissue from buccal mucosa, palate, floor of mouth, tongue and lip were obtained at autopsy from 30 postmortems of previously healthy individuals matched for age and gender. The tissues were collected within 48 h of death. The cause of death for the majority was either myocardial infarction or cerebrovascular accident and subjects were excluded if there was evidence of immunosuppression or oral disease, or history of smoking, chemotherapy or radiotherapy.
The blocks were sectioned using a manually operated microtome to 3 μm thick slices, which were floated on a warm water bath and set on adhesive coated slides. The slides were placed on a warmer set at 60°C for 1 h.
Slides for immunohistochemical staining were deparaffinised by passing them through two changes of xylene for 5 min each. They were hydrated in two changes of 100% ethanol for 3 min each, 95% and 70% ethanol for 1 min each. They were then rinsed in phosphate buffered solution. Tissue sections were treated in boiling 1 mM ethylene-diamine-tetraacetic acid, pH 8.0 for 10-20 min, followed by cooling at room temperature for 20 min. Immunohistochemical staining was performed using Thermo Scientific primary antibody following manufacturer's instructions (CD1a Ab-5 Catalogue MS-1856-PO).
Photomicrographs of the slides were taken with a model Canon Power Shot A650 IS, at focal length 25 mm, width-4000 pixels, height-3000 pixels mounted on a Carl Zeiss Axio Scope-A1 light microscope set at ×400 original magnification. Each stained slide was reviewed by two investigators independently. Only cells exhibiting a densely stained cell body with at least one dendrite attached were counted. Tissue sections were divided into four quadrants and positive cells were counted at high power fields with a minimum of five high power fields per case (depending on the size of tissue section). The number of LCs was calculated per mm2 tissue section surface area.
Data analysis
The outcome variables were the mean population of LC per mm2 of tissue section area, positivity of LCs for CD1a, and the mean malignancy score. Data were expressed as percentages compared by Chi-square statistics; mean ± SD, compared by Mann-Whitney-U test. Spearman's correlation test was used to test for correlation between populations of LCs per mm2 of tissue section area and mean malignancy score of the histological grade. The level of significance was set at P < 0.05.
RESULTS
From 50 tissue specimens which were randomly selected for malignancy grading and immunohistochemistry, two cases were excluded because of insufficient area of tissue section for determination of differentiation. Therefore, 48 cases were evaluated for determination of malignancy scores using Bryne's system and were subsequently subjected to immunohistochemistry to determine LCs population, using CD1a. In addition, 30 normal oral epithelial tissue specimens were used as controls. Age and gender distribution of OSCC cases and controls are shown in Table 1.
Table 1.
Age and gender distribution of cases, subjects and controls
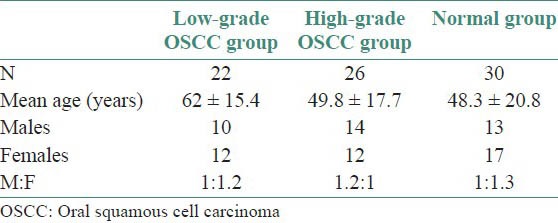
From 78 cases subjected to immunohistochemistry, 22 (28.2%) cases were of low - grade malignancy, 26 (33.3%) cases were high-grade malignancies and 30 (38.5%) were controls (normal oral epithelium). The mean malignancy score for well-differentiated cases (low-grade malignancy tumours) was 6.9 ± 1.1 and 12.5 ± 2.1 for poorly-differentiated cases (high-grade malignancy tumors). There was significant difference in the CD1a positivity and mean LCs population among the groups [Table 2]. Population of LCs was higher in well differentiated OSCC [Figure 1] than poorly differentiated OSCC [Figure 2].
Table 2.
Immunoreactivity to CD1a and mean Langerhans cells count in oral squamous cell carcinoma and controls
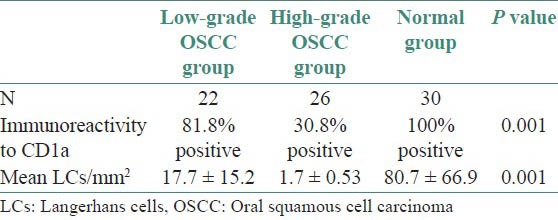
Figure 1.
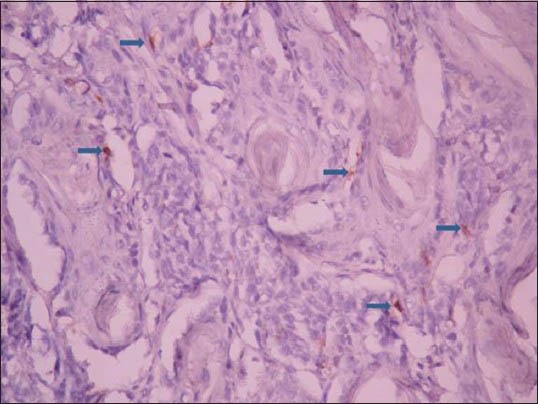
Photomicrograph showing CD1a positive Langerhans cell (arrows) in low-grade oral squamous cell carcinoma (IHC stain, ×400)
Figure 2.
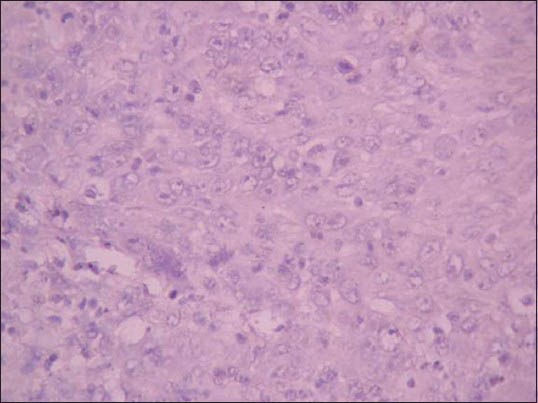
Photomicrograph showing absence of CD1a positive Langerhans cells in high-grade oral squamous cell carcinoma. (IHC stain, ×400)
DISCUSSION
The malignancy grading of OSCC was done using Bryne's system, which is a modification of previous grading systems.[14,15] This modified system has proven to be of high prognostic value, more importantly when the invasive front of the tumor is assessed for tumor differentiation.[16,17,18] The biological activity of OSCC is usually evaluated by classifying the tumors based on cellular differentiation according to a system primarily developed by Broders in 1920.[14] The grading of both biopsies and excision specimens can be used for histological diagnosis of tumors; although a higher malignancy score has been reported with the use of excision specimen compared to biopsies. However, the biopsies could still be used to predict the behaviour and prognosis significantly.[19]
The analysis of LCs population in normal oral epithelium and OSCC was done using CD1a antibody. Oral mucosa LCs have been identified by expression of a variety of different markers, including CD1a, S100 protein, human leukocyte antigen (HLA)-DR, HLA-D6, and CD36,[20,21,22] the CD1a cell membrane glycoprotein antigen is the most sensitive and specific marker of oral mucosal LCs.[23,24]
The finding of this study showed that there was a significant reduction in population of LCs in OSCC compared with normal oral epithelium. This finding is consistent with previous studies,[25,26] which compared number of LCs in OSCC with adjacent normal oral epithelium. Heerden et al.,[26] reported in their study, in which S-100 protein and HLA-DR were used for the detection of LCs, that the difference between the LCs in the tumor tissue and the adjacent or overlying epithelium was statistically significant when detection of both S-100 and HLA-DR proteins were evaluated. In contrast, another study[27] reported no appreciable difference in the population of LCs in cancer nests and adjacent epithelium except in the cases of poorly-differentiated cancer. They observed that LCs were more frequent in the adjacent epithelium than in the cancer nests in the poorly-differentiated cases.
In agreement with our finding, a previous study[28] showed 28.2% reactivity of high-grade tumors and 71.9% reactivity of low-grade tumours to S100 protein staining for LCs. Similarly, Heerden et al.,[26] reported that more LCs were generally counted in the moderately-differentiated tumors than in the poorly-differentiated tumors using both S-100 and HLA-DR detection methods although this was not statistically significant. Lack of statistical difference of LCs population between moderately and poorly-differentiated squamous cell carcinomas in their study could be attributed to the close range of malignancy scores.
The findings from this study concur with previous suggestions that OSCC is associated with a significant reduction in LCs of the oral mucosa.[11,29] In vivo chemical carcinogenesis experiments and human studies have suggested that loss of LCs during tumor promotion may impair immunologic protection against skin and mucosal tumors.[11,29,30] This study has shown that the number of LCs per millimetre square area of tissue section is significantly reduced compared with control tissue cases from normal oral epithelium.
The function of LCsin initiating immune responses by presenting antigen to lymphocytes is well-documented.[31,32,33] Studies have shown that threshold densities of LCs are required for antigen specific T-cell activation and antigens applied to skin deficient in LCs may lead to antigen specific tolerance.[34,35,36,37] The result of the present study therefore suggests that oral mucosa LCs are involved in immune-surveillance and immunologic impairment may characterize invasive OSCC. It has been established that atypical tumoral clones are able to synthesize and release a series of cytokines with immunosuppressive properties such as interferon gamma and interleukin-10.[38] Release of these substances by the tumor cells may result in reduction of the population of LCs observed in this study. On the same lines, a previous report[32] indicated that LCs tend to undergo apoptosis when unable to express their immune competent activity which promotes a substantial decrease in the LCs population.
The number of LCs was significantly reduced in high-grade oral squamous cell carcinoma and this may explain the role of immune suppression by tumour cells in their development.
These findings suggest that oral mucosal LCs may be involved in immune-surveillance and immunologic impairment may characterize invasive OSCC. In addition, LCs density may characterize histological grade of OSCC, which may be of a prognostic value. Further studies for the specific roles of these cells in categorising patients into prognostic groups are however needed to be conducted.
ACKNOWLEDGEMENTS
We thank Prof. O. I. Olopade, Prof. A. G. Falusi, Mr. O. A. Odetunde and Mr. Jide of the Genetic Research Unit - Institute of Advanced Medical Research and Training (IMRAT) University of Ibadan, Ibadan, for granting access to the use of the Immunohistochemistry Laboratory for this work.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Silveira EJ, Godoy GP, Lins RD, Arruda Mde L, Ramos CC, Freitas Rde A, et al. Correlation of clinical, histological and cytokeratin profiles of squamous cell carcinoma of the oral tongue with prognosis. Int J Surg Path. 2007;15:376–83. doi: 10.1177/1066896907304992. [DOI] [PubMed] [Google Scholar]
- 2.Bryne M. Is the invasiveness front of an oral carcinoma the most important area of prognostication? Oral Dis. 1998;4:70–7. doi: 10.1111/j.1601-0825.1998.tb00260.x. [DOI] [PubMed] [Google Scholar]
- 3.Gibson GE, O’Grady A, Kay EW, Leader M, Murphy GM. Langerhans cells in benign, premalignant and malignant skin lesions of renal transplant recipient and effect of retinoid therapy. J Eur Acad Dermatol Venereol. 1998;10:130–6. [PubMed] [Google Scholar]
- 4.Langerhans P. On the nerves of the human skin. Virchows Arch Path Anat. 1868;44:325. [Google Scholar]
- 5.Cruz PD, Jr, Bergstresser PR. Antigen processing and presentation by epidermal Langerhans cells. Induction of immunity and unresponsiveness. Dermatol Clin. 1990;8:633–47. [PubMed] [Google Scholar]
- 6.Tschachler E, Rappersberger K. Role of Langerhans cells in disease. In: Schuler G, editor. Epidermal Langerhans cells. Boca Raton: CRC press; 1991. pp. 295–316. [Google Scholar]
- 7.Streilein JW. Circuits and signals of the skin associated lymphoid tissues. J Invest Dermatol. 1985;85:10–3s. doi: 10.1111/1523-1747.ep12275413. [DOI] [PubMed] [Google Scholar]
- 8.Gabrilovic DI, Clen HL, Girgis KR, Cunningham HT, Meny GM, Nadaf S, et al. Production of vascular endothelial growth factor by human tumours inhibits the functional maturation of dendritic cells. Nat Med. 1996;2:1096–103. doi: 10.1038/nm1096-1096. [DOI] [PubMed] [Google Scholar]
- 9.Huang AY, Golumbek P, Ahmadzadeh M, Jaffee E, Pardoll D, Levitsky H. Role of bone marrow-derived cells in presenting MHC class I-restricted tumour antigens. Science. 1994;264:961–5. doi: 10.1126/science.7513904. [DOI] [PubMed] [Google Scholar]
- 10.Tan KC, Hosoi J, Grable S, Asahina A, Grastein RD. Epidermal cell presentation of tumour-associated antigens for induction of tolerance. J Immunol. 1994;153:760–7. [PubMed] [Google Scholar]
- 11.Watson GA, Lopez DM. Aberrant antigen presentation by macrophages from tumour bearing-mice is involved in the down-regulation of their T cell responses. J Immunol. 1995;155:3124–34. [PubMed] [Google Scholar]
- 12.Austin JM. The dendritic cell system and antitumor immunity. In vivo. 1993;7:193–202. [PubMed] [Google Scholar]
- 13.Knight SC, Stagg AJ. Antigen-presenting cell types. Curr Opin Immunol. 1993;5:374–82. doi: 10.1016/0952-7915(93)90056-x. [DOI] [PubMed] [Google Scholar]
- 14.Broders AC. Squamous cell epithelioma of the lip. J Am Med Assoc. 1920;74:656–64. [Google Scholar]
- 15.Anneroth G, Batsakis J, Luna M. Review of literature and a recommended system of malignancy grading in oral squamous cell carcinomas. Scand J Dent Res. 1987;95:229–49. doi: 10.1111/j.1600-0722.1987.tb01836.x. [DOI] [PubMed] [Google Scholar]
- 16.Bryne M, Koppang HS, Lillen R, Stene T, Bang G, Dabelsteen E. New malignancy grading is a better prognostic indicator than Broders’ grading in oral squamous cell carcinoma. J Oral Pathol Med. 1989;18:432–7. doi: 10.1111/j.1600-0714.1989.tb01339.x. [DOI] [PubMed] [Google Scholar]
- 17.Bryne M, Koppang HS, Lillen R. Malignancy grading of the deep invasive margins of oral squamous cell carcinoma has high prognostic value. J Pathol. 1992;166:375–81. doi: 10.1002/path.1711660409. [DOI] [PubMed] [Google Scholar]
- 18.Bello IO, Soini Y, Salo T. Prognostic evaluation of oral tongue cancer: Means, markers and perspectives (II) Oral Oncol. 2010;46:636–43. doi: 10.1016/j.oraloncology.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 19.Bigotti G, Coli A, Castangnola D. Distribution of Langerhans cells and HLA class II molecules in prostatic carcinomas of different histologic grade. Prostate. 1991;19:73–87. doi: 10.1002/pros.2990190108. [DOI] [PubMed] [Google Scholar]
- 20.Haimoto H, Hosoda S, Kato K. Differential distribution of immunoreactive S100-alpha and S100-beta proteins in normal non-nervous human tissues. Lab Invest. 1987;57:489–98. [PubMed] [Google Scholar]
- 21.Cruchley AT, Williams DM, Farthing PM, Lesch CA, Squier CA. Regional variation in Langerhans cell distribution and density in normal human oral mucosa determined using monoclonal antibodies against CD1, HLADR, HLADQ and HLADP. Oral Pathol Med. 1989;18:510–6. doi: 10.1111/j.1600-0714.1989.tb01353.x. [DOI] [PubMed] [Google Scholar]
- 22.Pimpinelli N, Borgognoni L, Ricarrdi R, Ficarra G, Mori M, Gaglioti D, et al. CD36 (OKM5)+ dendritic cells in the oral mucosa of HIV- and HIV+subjects. J Invest Dermatol. 1991;97:537–42. doi: 10.1111/1523-1747.ep12481573. [DOI] [PubMed] [Google Scholar]
- 23.Krenacs L, Tzalvivz L, Krenacs T, Boumsell L. Immunohistochemical detection of CD1a antigen in formalin-fixed and paraffin-embedded tissue sections with monoclonal antibody 010. J Pathol. 1993;171:99–104. doi: 10.1002/path.1711710206. [DOI] [PubMed] [Google Scholar]
- 24.Walling DM, Flaitz CM, Hosein FG, Montes-Walters M, Nichols CM. Effect of Epstein-Barr virus replication on Langerhans cells in pathogenesis of oral hairy leukoplakia. J Infect Dis. 2004;189:1656–63. doi: 10.1086/383132. [DOI] [PubMed] [Google Scholar]
- 25.Pitigala-Arachchi A, Crane IJ, Scully C, Prime SS. Epithelial dendritic cells in pathological human oral tissues. J Oral Pathol Med. 1989;18:11–6. doi: 10.1111/j.1600-0714.1989.tb00724.x. [DOI] [PubMed] [Google Scholar]
- 26.van Heerden WF, Raubenheimer EJ, van Rensburg EJ, le Roux R. Lack of correlation between DNA ploidy, Langerhans cell population and grading in oral squamous cell carcinoma. J Oral Pathol Med. 1995;24:61–5. doi: 10.1111/j.1600-0714.1995.tb01140.x. [DOI] [PubMed] [Google Scholar]
- 27.Kurihara K, Hashimoto N. Pathological significance of Langerhans cells in oral cancer. J Oral Pathol. 1985;14:289–98. doi: 10.1111/j.1600-0714.1985.tb00496.x. [DOI] [PubMed] [Google Scholar]
- 28.Albuquerque RL, Jr, Miguel MC, Costa AL, Souza LB. Correlation of c-erbB-2 and S-100 expression with the malignancy grading and anatomical site in oral squamous cell carcinoma. Int J Exp Path. 2003;84:254–65. doi: 10.1111/j.0959-9673.2004.00361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Odukoya O, Hawach F, Shklar G. Retardation of experimental oral cancer by topical vitamin E. Nutr Cancer. 1985;6:98–104. doi: 10.1080/01635588509513813. [DOI] [PubMed] [Google Scholar]
- 30.Daniels TE, Chou L, Greenspan JS, Grady DG, Hauck WW, Greene JC, et al. Reduction of Langerhans cells in smokeless tobacco-associated oral mucosal lesions. Oral Pathol Med. 1992;21:100–4. doi: 10.1111/j.1600-0714.1992.tb00990.x. [DOI] [PubMed] [Google Scholar]
- 31.Stingl G, Tarmarki K, Kartz SI. Origin and function of epidermal Langerhans cells. Immunol Rev. 1980;53:149–74. doi: 10.1111/j.1600-065x.1980.tb01043.x. [DOI] [PubMed] [Google Scholar]
- 32.McClellan AD, Kampgen E. Functions of myeloid and lymphoid dendritic cells. Immunol Lett. 2000;72:101–5. doi: 10.1016/s0165-2478(00)00167-x. [DOI] [PubMed] [Google Scholar]
- 33.Rowden G, Lewis MG, Sullivan AK. Ia antigen expression on human epidermal Langerhans cells. Nature. 1997;268:247–8. doi: 10.1038/268247a0. [DOI] [PubMed] [Google Scholar]
- 34.Toews GB, Bergstresser PR, Streilein JW. Epidermal Langerhans cell density determines whether contact hypersensitivity or unresponsiveness follows skin painting with DNFB. J Immunol. 1980;124:445–53. [PubMed] [Google Scholar]
- 35.Strelein JW, Toews GB, Gillian JN, Bergstresser PR. Tolerance or hypersensitivity to 2,4-dinitro-fluorobenzene: The role of Langerhans cell density within epidermis. J Invest Dermatol. 1980;74:319–22. doi: 10.1111/1523-1747.ep12543557. [DOI] [PubMed] [Google Scholar]
- 36.Seema M, Sagami Induction of suppressor T cells to DNFB contacts sensitivity by application of sensitizer through Langerhans cell-deficient skin. Arch Dermatol Res. 1981;271:361–4. doi: 10.1007/BF00409466. [DOI] [PubMed] [Google Scholar]
- 37.Streilein JW, Bergstresser PR. Langerhans cell function dictates induction of contact hypersensitivity or unresponsiveness to DNFB in Syrian hamsters. J Invest Dermatol. 1981;77:272–7. doi: 10.1111/1523-1747.ep12482453. [DOI] [PubMed] [Google Scholar]
- 38.Arany I, Adler-Storthz K, Chen Z, Tyring SK, Brysk MM. Tumour differentiation-dependent local immunity in human head and neck cancers. Cancer Lett. 1998;30:173–6. doi: 10.1016/s0304-3835(97)00432-1. [DOI] [PubMed] [Google Scholar]


