Abstract
Background:
Dysregulation of cell cycle is a fundamental hallmark of cancer progression. Cyclin D1, part of complex molecular system regulating G1-S point of cell cycle is overexpressed in variety of tumors.
Aims:
To look for its immunohistochemical expression in clinically normal mucosa from patients with and without tobacco habits, leukoplakia; and correlate its expression to individual atypical morphologic features, as seen in hematoxylin and eosin (H and E) sections of leukoplakia exhibiting dysplasia.
Materials and Methods:
We examined the expression of cyclin D1 in immunohistochemically stained sections of 15 normal buccal mucosa without any habits (group 1), 30 clinically normal mucosa from tobacco habituιs (group 2) and 30 leukoplakias exhibiting dysplasias (group 3). Descriptive statistical analysis performed. Results presented on Mean ± Standard deviation and in number (%). Adjusted Wald 95% Confidence Interval (CI) computed, percentages of morphological features assessed by Laplace estimate. Mann-Whitney U, Kruskal-Wallis test used to find the percentage expression of cyclin D1.
Results:
Expression of cyclin D1 in group 3 was significantly higher than in group 1 and 2 (P < 0.001, P = 0.028), expression in group 2 was significantly higher than in group 1 (P = 0.003) and were statistically significant. Generally expression of cyclin D1 was confined to lower one-third of epithelium and was highest in mild dysplasias. Among 13 atypical morphologic features, cyclin D1 expression consistently correlated with basilar hyperplasia.
Conclusion:
The altered pattern of cyclin D1 expression here may be an early event in conversion of normal epithelium into dysplastic epithelium and may serve as a biomarker of oral carcinogenesis. Its expression may be increased in tobacco habitués. Basilar hyperplasia should be given additional weightage in the grading system in predicting the fate of affected epithelium.
Keywords: Cell cycle, cyclin D1, epithelial dysplasia, oral carcinogenesis, tobacco
INTRODUCTION
Enormous investment of human and financial resources have failed to find a cure for cancer which remains as one of the major causes of death.[1] Disruption in cell signaling, cell cycle and mechanism to repair cell damage or eliminate dysfunctional cells can lead to excessive cell proliferation progressing to cancer.[2] It is important to understand the cell cycle defects in human oral cancer not only for biologically understanding the disease, but also to utilize this information for early diagnosis and biology based therapy.[3]
Leukoplakias are considered to be potentially malignant oral lesions. They are either idiopathic or related to habits such as tobacco and/or alcohol use.[4] In the oral mucosa the concept of two-step process of cancer development, that is, the initial presence of a precancerous lesion which later develops into cancer, is well-established.[5] Oral epithelial dysplasia is considered to be histological marker of premalignancy.[6] Traditional treatment modalities do not completely eliminate the risk of malignant transformation and there is importance of developing objective prognostic markers which will identify high risk lesions.[7]
There is a state of morphologic ambiguity in epithelial dysplasias, in which the exact mechanism that dictates the cell toward either normalcy or neoplasia is unknown. The use of molecular investigative techniques, genetic markers and immunohistochemistry will help us find specific changes occurring in the cells that may help us beyond morphologic judgments and epidemiologic uncertainties.[8]
Group of oncogenes and tumor suppressor genes which regulate specific pathways of cell growth or cell death have been identified by research on cell proliferation and programmed cell death.[9] Chromosome 11q13 which harbors cyclin D1 protooncogene encodes a 45 kDa cell cycle regulatory protein, which is a part of complex molecular system regulating the G1-S transition point of cell cycle.[10] Complexes of cyclin D1 with Cyclin-dependent kinases has a critical role in the cell cycle and determines the molecular on-off switch for the cell cycle by phosphorylating the retinoblastoma protein. Retinoblastoma protein when hypophosphorylated prevents cells from replicating by forming a tight inactive complex with transcription factor E2F. Phosphorylation of the retinoblastoma protein dissociates the complex and releases the inhibition on E2F transcriptional activity which may lead to dysregulated cell proliferation, thus contributing to oncogenesis.[11]
Because of self-sufficiency of growth signals in cancer cells, the concentration of cyclin D1 goes up which results in activation of cyclin D1 complex in turn causing phosphorylation of retinoblastoma protein, progressing the cell through various stages of cell cycle. As a result of amplification and rearrangement, there is overexpression of cyclin D1 which drives the cells through G1-S transition in an uncontrolled manner.[12] Number of epithelial tumors including carcinoma of breast, bladder, lung, head and neck carcinomas and certain B lymphocyte malignancies and parathyroid tumors show cyclin D1 gene amplification.[13]
This study was an attempt to determine the expression of cyclin D1 in normal mucosa of subjects with and without tobacco smoking habit and in patients with leukoplakia. Also to determine which of the individual dysplastic features could probably be more predictive of malignant transformation by correlating Cyclin D1 expression with individual atypical morphological features as seen in hematoxylin and eosin (H and E) stained sections of dysplastic epithelium.
MATERIALS AND METHODS
The materials for the present study comprised a total of 75 biopsy specimens [Table 1]. Fifteen biopsies were of normal buccal mucosa from volunteers without any tobacco smoking or chewing habits (group 1), 30 biopsies of normal buccal mucosa from volunteers with smoking habit (cigarette, beedi) (group 2) and 30 cases were of leukoplakia exhibiting dysplasia (group 3) [Table 2]. Biopsies were taken after informed consent was obtained and the study was reviewed and approved by the ethical committee. All the tissues were fixed in 10% formalin, routine processing was done and tissues embedded in paraffin wax. All diagnosis was reviewed by two pathologists using sections routinely stained with H and E, the serial sections of the same was stained by immunohistochemical reagent cyclin D1 primary antibody (Novocastra, UK) using immune polymer assay/labeled polymeric method/enhanced polymer staining method.
Table 1.
Basic characteristics of the study
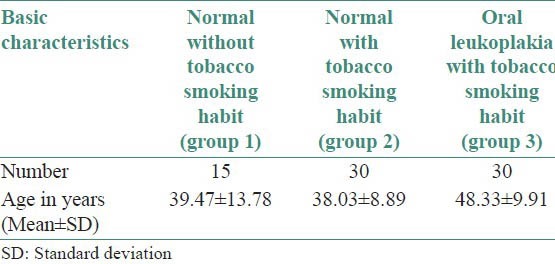
Table 2.
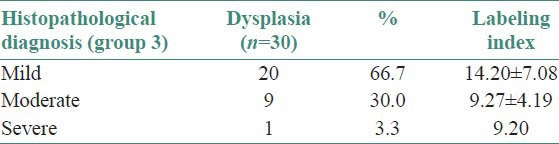
Histopathological diagnosis of dysplasia cases
Criterias for selection of samples for group 2 and 3 were as follows:
Inclusion criteria
The subject should be a smoker of either cigarette or beedi, habit duration of minimum 5 years, biopsy from clinically normal buccal mucosa for group 1 and 2, and only those leukoplakias exhibiting dysplasia were included in group 3.
Exclusion criteria
Leukoplakias with adjacent squamous cell carcinoma or any other lesion, nonsmokers, tobacco chewers and leukoplakias without any evidence of dysplasia.
Antibodies used (Novocastra, UK)
Monoclonal mouse antihuman Cyclin D1 primary antibody ready to use (clone P2D11F11, RTU-CYCLIN D1-GM).
Novolink polymer detection system ready to use RE7140-K containing following reagents:
- Peroxidase block RE7101
- Protein block RE7102
- Post primary block RE7111
- Novolink Polymer RE 7112
- Novolink 3, 3’- diaminobenzidine (DAB) RE 7230-K
- Novolink DAB substrate buffer RE 7143
- Hematoxylin RE 7107.
Procedure
Three micrometer thick sections were cut and mounted on 3-aminopropyltriethoxysilane coated glass slides and incubated overnight at 55-60°C. The sections were deparaffinized in two changes of xylene for 5 min each and were hydrated, cleared through different grades of isopropyl alcohol 100-50% and bought to distilled water. The tissues were then incubated with 3% hydrogen peroxide for 5 min to block the endogenous peroxidase activity. The tissues were then washed with tris buffer saline and subjected to antigen retrieval in boiling ethylenediaminotetraacetic acid (EDTA) buffer for 45 min in a pressure cooker without closing the lid. The solution was allowed to cool to room temperature.
The slides were then incubated with protein block for 5 min followed by incubation with monoclonal mouse antihuman Cyclin D1 primary antibody (ready to use, Clone P2D11F11, RTU-CYCLIN D1-GM) for 45 min. The slides were then washed with tris buffer saline and incubated with post primary block for 30 min and washed followed by incubation with Novolink polymer for 30 min. Subsequently, the slides were incubated with fresh Diaminobenzidine (DAB) chromogen for 1 min and counter stained with hematoxylin. The slides were then dehydrated, cleared and mounted with DPX. A negative control was achieved by omitting the primary antibody. Sections of oral squamous cell carcinoma with known cyclin D1 expression were used as positive control.
Cells were considered to be positive for cyclin D1 protein antigens if there was any staining of the nucleus, regardless of the staining intensity. The expression pattern of cyclin D1 was studied in the entire length of normal and dysplastic epithelium along with its preceding serial sections stained with H and E. The expression of cyclin D1 was taken into consideration in the dysplastic epithelium and the corresponding H and E sections were reviewed for individual atypical features corresponding to the expression of cyclin D1 in immunohistochemical slides.
A comparative study of expression patterns of cyclin D1 was made between normal epithelium of subjects with and without tobacco smoking habits and dysplastic epithelium. Positively stained epithelial cells in normal and dysplastic epithelium were counted in at least four randomly selected fields at a magnification of ×400. The percentage of positive cells were measured and recorded with a labeling index (LI; the number of cells with unequivocal nuclear staining divided by the total number of cells counted).[14] A comparative correlation study was done.
Statistical methods
Descriptive statistical analysis has been carried out in the present study. Results on continuous measurements are presented as Mean ± standard deviation (SD) and results on categorical measurements are presented in number (%). Adjusted Wald 95% Confidence Interval (CI) has been computed and percentages of morphological features are assessed by Laplace estimate. Mann-Whitney U test and Kruskal-Wallis test has been used to find the percentage expression of cyclin D1. The statistical software namely Statistical Package for Social Sciences (SPSS) 15.0, Stata 8.0, MedCalc 9.0.1, and Systat 11.0 were used for the analysis of the data and Microsoft Word and Excel have been used to generate tables.
RESULTS
Detectable cyclin D1 expression, light-dark brown staining, confined to cell nuclei was found in basal and parabasal layers of the epithelium in 12 of 15 cases of clinically normal mucosa without tobacco smoking habit (group 1). The expression was confined mainly to parabasal layer with only few cells in the basal layer showing positivity [Figures 1 and 2]. The mean LI determined was 3.48% [Table 3].
Figure 1.
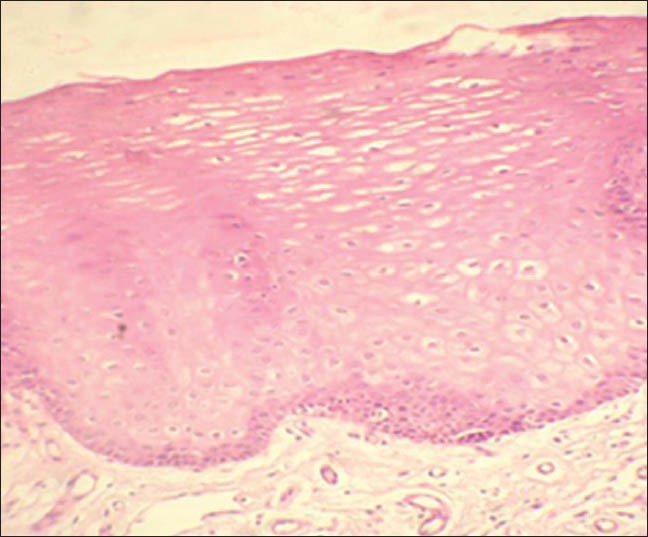
Sections showing normal epithelium and connective tissue (group 1). (H&E stain, ×200)
Figure 2.
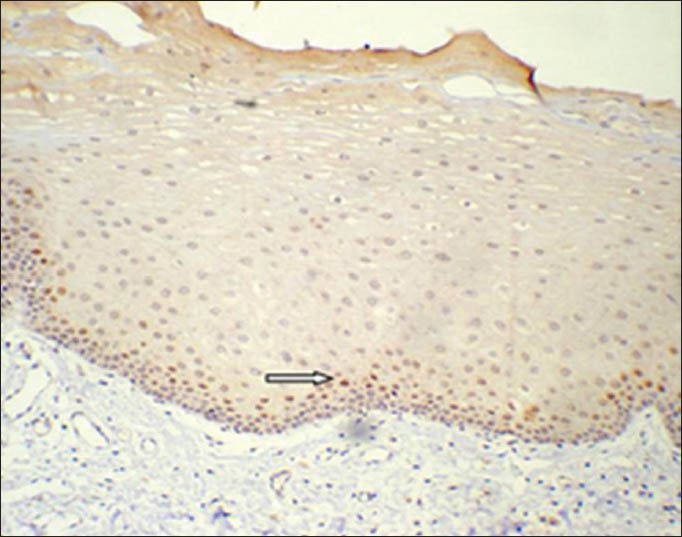
Corresponding immunohistochemical localization of cyclin D1 in normal epithelium. (IHC stain, ×200)
Table 3.
Mean Labeling index of three groups and their comparative analysis
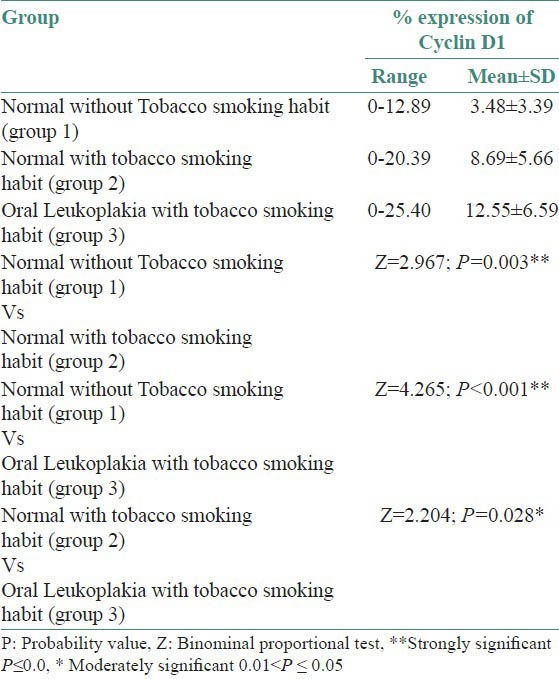
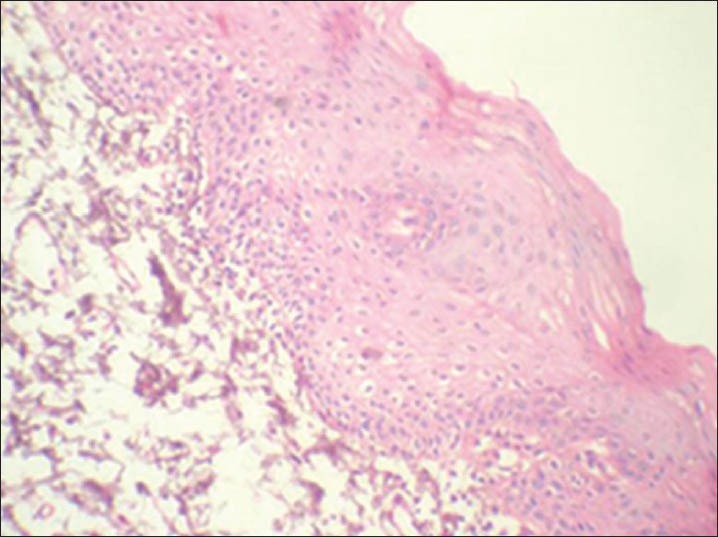
Cyclin D1 expression in group 2 was similar to group 1, but the expression was also detected in lower spinous layers of the epithelium in 26 of 30 cases [Figures 3 and 4]. The mean LI determined was 8.69% [Table 3].
Figure 3.
Sections showing epithelium and connective tissue (group 2) (H&E stain, ×200)
Figure 4.
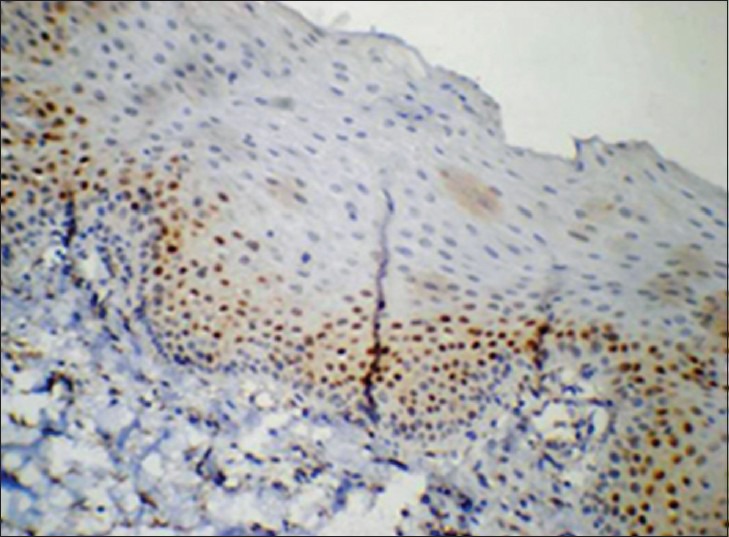
Corresponding immunohistochemical localization of cyclin D1 in group 2. (IHC stain, ×200)
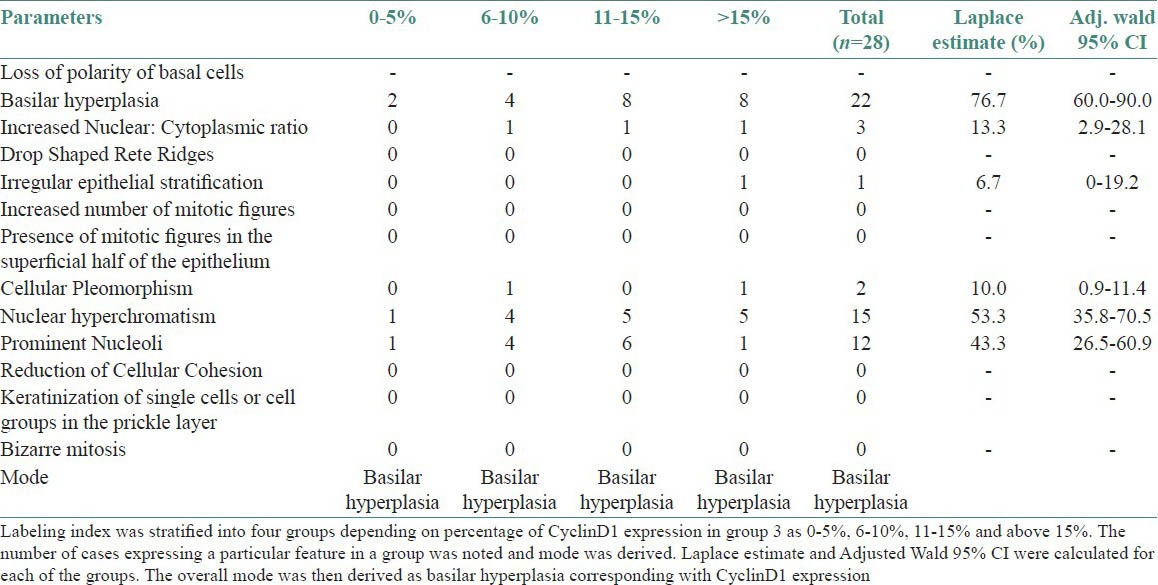
Cyclin D1 expression in group 3 was observed in lower and upper spinous layers of the epithelium in 28 of 30 cases. LI determined was 12.55% [Table 3]. LI was stratified into four groups depending on percentage of cyclin D1 expression in group 3 as 0-5, 6-10, 11-15, and above 15%. The number of cases expressing a particular feature in a group was noted and mode was derived. Laplace estimate and Adjusted Wald 95% CI were calculated for each of the groups. The overall mode was then derived as basilar hyperplasia corresponding with cyclin D1 expression [Table 4].
Table 4.
Cyclin D1 expression with individual atypical morphological features in preinvasive dysplastic oral epithelium (group 3) (n=28)
In mild dysplasia cases (group 3) the positive expression was mainly seen in parabasal and lower spinous layer, with few cells in basal layer showing positivity [Figures 5 and 6]. In moderate and severe dysplasia cases (group 3) the positive expression was mainly seen in parabasal, lower and upper spinous layers with few cells in basal layer showing positivity. LI (14.20 ± 7.08) was highest in mild dysplasia cases compared to moderate (9.27 ± 4.19) and severe (9.20) dysplasias [Table 2].
Figure 5.
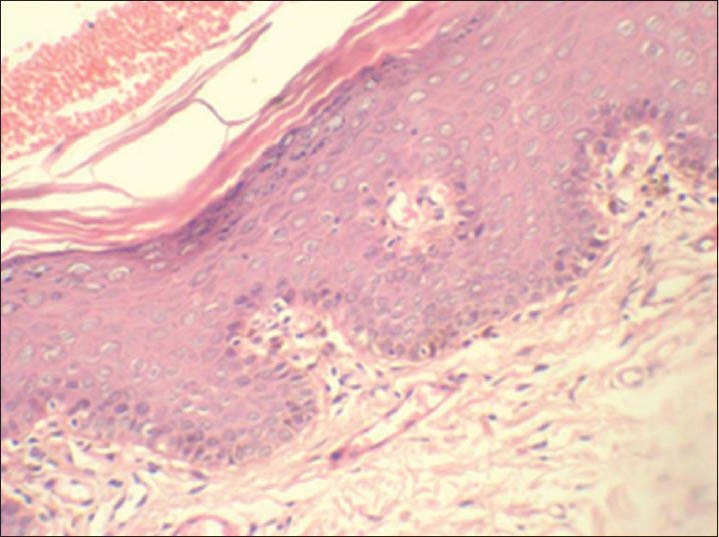
Sections showing epithelium with mild dysplasia (group 3). (H&E stain, ×200)
Figure 6.
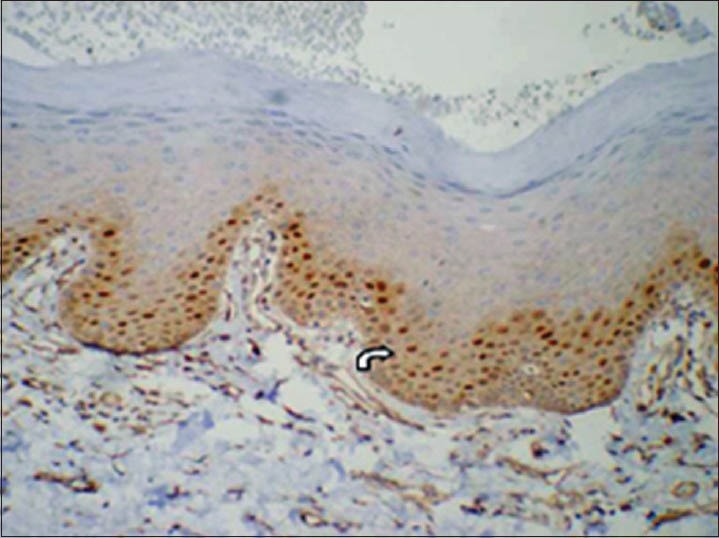
Corresponding immunohistochemical localization of cyclin D1 in epithelium with mild dysplasia (group 3). (IHC stain, ×200)
Comparison of percentage expression of cyclin D1 between the three groups revealed an increased labeling index in leukoplakia exhibiting dysplasia (group 3). There was strongly significant statistical expression pattern when compared between group 1 and 3 (P < 0.001)**, 1 and 2 (P = 0.003)** and moderately significant statistical expression pattern when compared between 2 and 3 (P = 0.028)*.
Labeling index determination and corresponding atypical morphological features were noted in the preceding H and E sections (group 3). The most common atypical morphological feature as seen in the H and E sections of dysplasia correlating with Cyclin D1 expression was basilar hyperplasia [Figures 7 and 8]. Of the 13 atypical morphologic features studied, Laplace estimate for basilar hyperplasia was 76.7% followed by nuclear hyperchromatism in 53.3% [Figures 7], prominent nucleoli in 43.3%, increased nuclear cytoplasmic ratio in 13.3%, and cellular pleomorphism in 10.0%.
Figure 7.
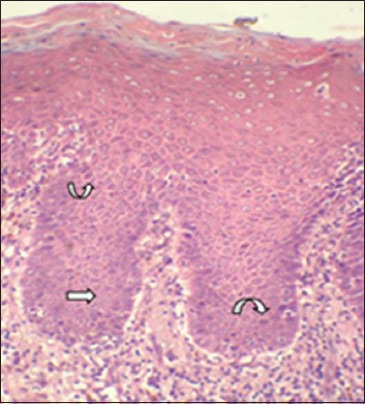
Photomicrograph showing basilar hyperplasia (straight arrow) and hyperchromtic nuclei in group3 (curved arrow). (H&E stain, ×200)
Figure 8.
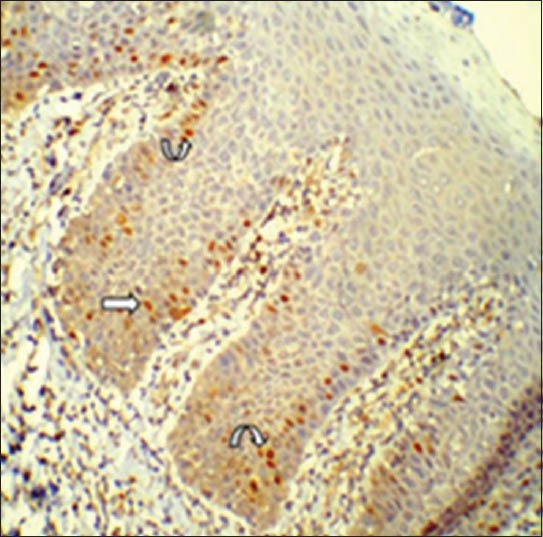
The corresponding image showing cyclin D1 immunohistochemical expression in sections with basilar hyperplasia (straight arrow) and hyperchromatic nuclei in group 3 (curved arrow). (IHC stain, ×200)
Similarly Adjusted Wald 95% CI for basilar hyperplasia was 60.0-90.0%, compared with nuclear hyperchromatism 35.8-70.5%, prominent nucleoli 26.5-60.9%, increased nuclear cytoplasmic ratio 2.9-28.1% and cellular pleomorphism 0.9-11.4%. Both by Laplace estimate and Adjusted Wald 95% CI, basilar hyperplasia was found to be the most important parameter among all the atypical morphological features studied in dysplasias.
DISCUSSION
The challenge within the field of oral precancer is to predict which lesions will eventually develop into carcinomas. Various studies have been done to predict the malignant potential in the dysplastic epithelium. Malignant progression is a multistep process. Escape of the cancer cells from cell cycle regulation reflects an important aspect of this multistep process. Thus, immunohistochemically detectable proliferation markers could be of great value in predicting the behavior of the lesion.
Cyclin D1 is a cell cycle protein expressed in G1-S phase of the cell cycle. This protein is overexpressed in variety of neoplasms and there is increasing evidence that, in head and neck tumors there is aberrant control of cell cycle proteins-cyclins.
Liu et al.,[15] studied the expression of cyclin D1 in normal epithelium and dysplastic leukoplakias, and concluded that the cyclin D1 expression in normal epithelia was limited to the germinative layer, that is, basal and parabasal layers. Parabasal cells showed higher LI than did basal epithelial cells. The expression of cyclin D1 in low-grade dysplasia (mild, moderate) was restricted to basal and suprabasal layers and in high-grade dysplasia (severe) positively stained cells were also observed in the superficial layers.
Our study was in concordance with the study of Liu et al., in that the expression of cyclin D1 in group 1 was mainly confined to cells in parabasal layer with few cells in basal layer showing positivity. However, in contrast to the above study, the expression of cyclin D1 in our study was also detected in layers above parabasal in both mild and moderate dysplasia (group 3).
Cyclin D1 expression was studied and LI was determined for each group. Group 1 - the average LI was 3.48%, Group 2 - the average LI was 8.69%, Group 3 - the average LI was 12.55%. The LI increased from group 1 to 3. There was strongly significant statistical expression when LI was compared between group 1 and 3 (P< 0.001) and group 1 and 2 (P= 0.003) and moderately significant statistical expression when compared between group 2 and 3 (P = 0.028). It means that the expression pattern in group 3 was significantly higher than in group 1 and 2 and the expression pattern in group 2 was significantly higher than in group 1.
We hypothesized that the increased LI in group 2 may be habit-related and there may be certain molecular changes occurring within the mucosa which is apparently looking normal. And hence changes seen at the molecular level need not transform into clinically or histopathologically evident morphological changes. And the expression of cyclin D1 here may be an early event in conversion of normal epithelium into dysplastic epithelium.
Our findings also showed that the LI was highest in mild dysplasia (14.20 ± 7.08) as compared to moderate dysplasia (9.27 ± 4.19) and severe dysplasia (9.20) (group 3), and the expression of Cyclin D1 was mainly seen in the lower one-third of the epithelium. Hence suggesting that its expression may be an early event in carcinogenesis, provided we assume that carcinogenesis is always preceded by precancerous state, which means that normal epithelium transforms through various stages of dysplasias before undergoing malignant transformation.
There was no increase in the LI of cyclin D1 with the grades of dysplasia and no statistical significance was obtained between the grades of dysplasia. This was in accordance with study conducted by Shintani et al.,[16] to detect the expression of G1 cyclins (Cyclin D1, E) in oral epithelial dysplasias. The study concluded that the LI of cyclin D1 was higher in epithelial dysplasia cases compared to normal epithelium, and there was no significant correlation of cyclin D1 with the degree of dysplasia.
The importance of individual criteria in predicting malignant transformation has been dealt by studies of epithelial dysplasia adjacent to oral carcinomas based on an assumption that dysplastic feature in this region were truly premalignant. The highest frequencies of dysplastic feature in this region were basal cell hyperplasia, nuclear hyperchromatism and perturbation in epithelial maturation.[5] Our results were in concordance with this study in that, basilar hyperplasia followed by nuclear hyperchromatism were the most common dysplastic features which correlated with cyclin D1 expression.
To the best of our knowledge no literature correlates cyclin D1 expression to individual atypical features neither in oral epithelial dysplasia nor in dysplasia of other areas of the body. When the cyclin D1 expression was correlated to the H and E sections of dysplasia, the most common individual atypical feature that correlated with cyclin D1 expression was basilar hyperplasia which was closely followed by nuclear hyperchromatism, prominent nucleoli, increased nuclear cytoplasmic ratio and cellular pleomorphism. Both by Laplace estimate and Adjusted Wald 95% CI, basilar hyperplasia was found to be the most important atypical morphologic feature, which was consistently correlating with cyclin D1 expression, again suggesting that the expression of cyclin D1 is an early event in carcinogenesis.
In this study we also tried to assess the significance of cyclin D1 expression by correlating with the age, type, and duration of habit. There was no statistical significance found in any of the above parameters.
CONCLUSION
The expression of cyclin D1 increased in subjects with clinically normal mucosa with smoking habit (group 2), compared to normal mucosa of subjects without tobacco smoking habit (group 1) suggesting that the habit may play an important role in the increased expression of cyclin D1 and there may be certain molecular changes occurring within the mucosa which is apparently looking normal. And hence changes seen at the molecular level need not transform into clinically or histopathologically evident morphological changes. And the expression of cyclin D1 here may be an early event in conversion of non-dysplastic lesion into a dysplastic lesion and may serve as biomarker of oral carcinogenesis.
The recognition of events that contribute to malignant transformation, their prognostic significance, is of utmost importance for the development of additional therapeutic and preventive strategies in head and neck cancer. Basilar hyperplasia should be given additional weightage and importance in grading of dysplasias. Additional studies with different types of tobacco habit, large sample size, and long-term follow-up and other objective methods would further help to assess the significance of -cyclin D1 protein in oral carcinogenesis.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Balmain A, Quintanilla M, Brown K, Ramsden M. An approach to the molecular mechanisms of the cancer induction. J Pathol. 1986;149:3–8. doi: 10.1002/path.1711490103. [DOI] [PubMed] [Google Scholar]
- 2.Scully C, Field JK, Tanzawa H. Genetic aberrations in oral or head and neck squamous cell carcinoma 2: Chromosomal aberrations. Oral Oncol. 2000;36:311–27. doi: 10.1016/s1368-8375(00)00021-x. [DOI] [PubMed] [Google Scholar]
- 3.Todd R, Hinds PW, Munger K, Rustqi AK, Opitz OG, Suliman Y, et al. Cell cycle dysregulation in oral cancer. Crit Rev Oral Biol Med. 2002;13:51–61. doi: 10.1177/154411130201300106. [DOI] [PubMed] [Google Scholar]
- 4.Scully C. Oral precancer: Preventive and medical approaches to management. Eur J Cancer B Oral Oncol. 1995;31B:16–26. doi: 10.1016/0964-1955(94)00049-a. [DOI] [PubMed] [Google Scholar]
- 5.Reibel J. Prognosis of oral premalignant lesions: Significance of clinical histopathological and Molecular biological characteristics. Crit Rev Oral Biol Med. 2003;14:47–62. doi: 10.1177/154411130301400105. [DOI] [PubMed] [Google Scholar]
- 6.Lumerman H, Freedman P, Kerpel S. Oral epithelial dysplasia and the development of invasive squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;79:321–9. doi: 10.1016/s1079-2104(05)80226-4. [DOI] [PubMed] [Google Scholar]
- 7.Warnakulasuriya S. Lack of molecular markers to predict malignant potential of oral precancer. J Pathol. 2000;190(4):407–9. doi: 10.1002/(SICI)1096-9896(200003)190:4<407::AID-PATH546>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 8.Abbey LM, Kaugars GE, Gunsolley JC, Burns JC, Page DG, Svirsky JA. The effect of clinical information on the histopathologic diagnosis of oral epithelial dysplasia. Oral Surg Oral Med Oral Pathol. 1998;85:74–7. doi: 10.1016/s1079-2104(98)90401-2. [DOI] [PubMed] [Google Scholar]
- 9.Kirsh DG, Kastan MB. Tumor suppressor p53: Implications for tumor development and prognosis. J Clin Oncol. 1998;16:3158–68. doi: 10.1200/JCO.1998.16.9.3158. [DOI] [PubMed] [Google Scholar]
- 10.Mate JL, Ariza A, Aracil C, Lopez D, Isamat M, Perez-Piteira J, et al. Cyclin D1 overexpression in non small cell lung carcinoma: Correlation with KI-67 labelling index and poor cytoplasmic differentiation. J Pathol. 1996;180:395–9. doi: 10.1002/(SICI)1096-9896(199612)180:4<395::AID-PATH688>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 11.Oliver RJ, Macdonald DG. G1 cyclins in oral epithelial dysplasia. J Oral Pathol Med. 2001;30:80–6. doi: 10.1034/j.1600-0714.2001.300203.x. [DOI] [PubMed] [Google Scholar]
- 12.Prabhu SR, Dhafthary DK, Wilson DF, Johnson NW. 1st ed. New Delhi (India): Oxford Medical Publications; 1992. Oral Disease in Tropics; p. 402. [Google Scholar]
- 13.Serrano M, Hannon GJ, Beach D. A new regulatory motif in cell cycle control causing specific inhibition of cyclinD/CDK4. Nature. 1993;366:704–7. doi: 10.1038/366704a0. [DOI] [PubMed] [Google Scholar]
- 14.Shenoy MK, Narayan TV, Shreedhar B, Mohanty L. Correlation of p53 expression with individual atypical morphological features in preinvasive dysplastic oral epithelium-An immunohistochemical study. J Oral Health Res. 2011;2:20–7. [Google Scholar]
- 15.Liu SC, Sautre ER, Clapper ML, Feldman RS, Levin L, Chen SY, et al. Markers of cell proliferation in normal epithelia and dysplastic leukoplakias of the oral cavity. Cancer Epidemiol Biomarkers Prev. 1998;7:597–603. [PubMed] [Google Scholar]
- 16.Shintani S, Mihara M, Nakahara Y, Kiyota A, Ueyama Y, Matsumura T, et al. Expression of cell cycle control proteins in normal epithelium, premalignant and malignant lesions of oral cavity. Oral Oncol. 2002;38:235–43. doi: 10.1016/s1368-8375(01)00048-3. [DOI] [PubMed] [Google Scholar]


