Abstract
Aim and Objective:
The aim of this study was to investigate the expression of Src protein (an osteoclastic factor) in peripheral and central giant cell granulomas (PGCG and CGCGs) of the jaws and the relationship between the expression of this protein and the clinical behavior of these two lesions.
Materials and Methods:
Thirty cases of PGCG and 30 cases of CGCG were immunohistochemically stained with Src. A staining-intensity-distribution (SID) score (proportion of stained cells × staining intensity) was used to evaluate immunoreactivity of the protein. Data were analyzed using statistical package for social sciences (SPSS) 17.0.
Results:
There were no significant differences in the Src expression and the SID score between PGCG and CGCG. Furthermore, Spearman's rank correlation coefficient showed that there was a significant correlation between Src expression and SID score within both PGCG and CGCG (P < 0.001; r = 0.87 and 0.75, respectively).
Conclusion:
The findings of this study suggest that the multinucleated giant cells share some similarities with osteoclasts and Src protein can be used as a new therapeutic target to inhibit osteoclastic activity. In addition, differences in immunoreactivity of this osteoclastic protein do not reflect different clinical behaviors of PGCG and CGCG.
Keywords: Central giant cell granuloma, immunohistochemistry, peripheral giant cell granuloma, Src
INTRODUCTION
Giant cell granuloma is a relatively common tumor-like lesion of the oral cavity. The peripheral variant of giant cell granuloma (PGCG) is probably a reactive lesion and is caused by local irritation or trauma. It occurs absolutely on the gingiva or edentulous alveolar ridge.[1] Central giant cell granuloma (CGCG) is less common than the peripheral variant and grows centrally in the jaws.[2,3]
Histologically, both PGCGs and CGCGs are characterized by the presence of multinucleated giant cells (MGCs) in a background of mononuclear mesenchymal cells.[1,4,5]
In spite of this histopathological similarity, CGCG and PGCG have a distinctive biologic performances and it is not certain whether these two lesions are the same.[1,5,6] The PGCG rarely causes the underlying bone erosion and recurs infrequently,[1,5,7] while CGCG has higher growth and recurrence rates and causes root resorption and cortical perforation.[1,5,8]
The histogenesis of MGCs (hallmark of these lesions) has been subject of much debate.[1,9] There is some evidence that MGCs show characteristics of macrophages, endothelial cells, fibroblasts, myofibroblasts or osteoclast progenitor cells.[10,11]
Previous studies revealed that MGCs in CGCG exhibit osteoclastic factors like receptor activator of nuclear factor κB (RANK), tartrate-resistant acid phosphatase (TRAP), glucocorticoid receptor (GR) α, and Src.[10,11,12,13] RANK signaling pathway is the critical regulatory constituent of osteoclast formation, activation, and survival. The Src protein, which is required for osteoclast activation in vitro, promotes RANK pathway to induce cytoskeletal rearrangements and motility.[13,14,15]
Considering the fundamental role of RANK/TRAF6-Src pathway in osteoclastogenesis, we evaluated Src expression by immunohistochemistry to clarify whether MGCs have osteoclastic phenotype and immunohistochemical divergence is associated with different behaviors of CGCG and PGCG.
MATERIALS AND METHODS
Tissue samples
Formalin-fixed, paraffin-embedded biopsy materials of 30 cases of PGCG and 30 cases of CGCG with sufficient clinical and radiographic records were selected from laboratory archives of the Oral Pathology Department, Faculty of Dentistry.
None of the patients had received pharmacological treatment (e.g. calcitonin or corticosteroids) prior to the biopsy. Cases with histopathological similarity to CGCG or PGCG, but clinically distinct, such as brown tumor of hyperparathyroidism, cherubism and aneurysmal bone cyst were excluded.
Immunohistochemistry and staining evaluation
The 4 μm-thick sections were placed onto slides. Sections were deparaffinized in xylene, hydrated through graded alcohol, and then rinsed in 0.01 M phosphate buffered saline (PBS; pH 7.4). Endogenous peroxidase activity was blocked by 3% hydrogen peroxide for 5 min. The sections were microwaved for 10 min in 10 mM sodium citrate buffer (pH 6.0) at 100°C for antigen retrieval. After cooling for 20 min, the slides were washed in PBS and preincubated with 10% bovine serum albumin (BSA) for 10 min in order to decrease background staining.
Sections were incubated for 2 h at 37°C with 1:100 diluted rabbit anti-Src antibody (Sigma, St Louis, MO, USA). PBS was applied instead of the primary antibody in negative controls. The standard streptavidin-biotin-peroxidase complex method was performed to detect the primary antibody with the use of extravidin-peroxidase staining kit according to manufacturer's instructions (Sigma, St Louis, MO, USA). Reaction products were visualized using 0.3% diaminobenzidine (Dako Cytomation) solution. Subsequently, sections were counterstained with Harris hematoxylin for 1 min and mounted.
For immunohistochemical analysis, two independent observers performed blind specimen assessment, with an agreement of 92%. Because of different cross-sectional dimensions for each sample, eight high power (×400 magnification) non-overlapping fields, limited to relatively cellular areas containing giant cells, were randomly selected to obtain the maximum number of high power fields common to all samples. The cells with a clearly defined immunostaining were counted. The count was divided by the total number of cells in each field. The mean of the eight fields was taken as the Src expression estimation for each sample. Moreover, the proportion of stained cells and the intensity of overall staining were assessed for each field. The proportion of stained cells in each field was assessed as: 0, no stained cells; 1, 25% stained cells; 2, 25-50% stained cells; and 3, more than 50% stained cells. Staining intensity was graded as: 0, negative staining; 1, light staining; 2, moderate staining; and 3, intense staining. A staining-intensity-distribution (SID) score was computed for each sample as follows: The score of the proportion of stained cells for each field was multiplied by the score of the staining intensity in that field to provide an SID score for the field. The average of the eight fields was the SID score for the sample. To enhance the confidence level of positive staining, sections with too weak and/or equivocal staining were considered negatively stained.[5,10,16]
Statistical analysis
The collected data were analyzed using the Statistical Package for Social Sciences (SPSS) 17.0 (SPSS, Chicago, IL). Interobserver reproducibility for both Src expression and SID score were determined through six double evaluations for each measurement. Results were expressed as the mean ± standard error of the mean (SEM). To compare the Src expression and the SID score, the nonparametric Mann-Whitney U-test was used. Spearman's rank correlation coefficient was used to determine the correlations amongst Src expression and the SID score in each group. Significance was established at a P < 0.05.
RESULTS
Clinical and histopathologic profile of the patients
Complete records and biopsy materials from 30 cases of PGCG (18 female and 12 male), ranging in age from 7 to 70 (mean 34) years, and 30 cases of CGCG (18 female and 12 male), ranging in age from 9 to 75 (mean 34) years were assessed. These findings indicated that age and sex distributions were relatively similar in PGCG and CGCG and 60% of cases of both PGCG and CGCG occurred in females.
PGCG was found in the gingiva of maxilla and mandible in the same proportion. Fourteen cases of PGCG were located in the anterior regions (47% of cases) and 16 cases occurred in the posterior regions of the jaws (53% of cases). Twenty-one cases of CGCG were located in the maxilla (70% of cases) and nine cases were found in the mandible (30% of cases). Fifty-seven percent were in the posterior regions and 43% were in the anterior regions of the jaws. These results showed that CGCG occurred more commonly in the maxilla and posterior regions of the jaws.
The basic histopathologic patterns for both PGCG and CGCG were similar and showed classical histopathologic features described in the literature [Figure 1a].
Figure 1.
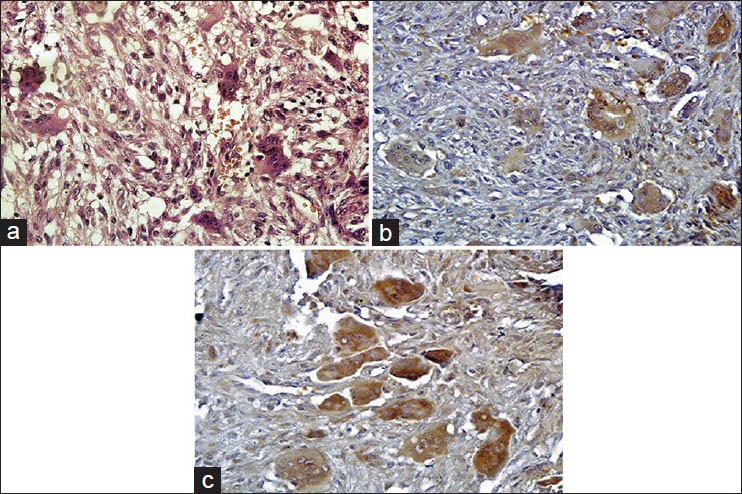
(a) Hematoxylin and Eosin stained section in which the stroma surrounding the multinuclear giant cells is cellular and extravasated red blood cells are seen (H&E stain, ×400), (b) Immunohistochemical staining for peripheral giant cell granuloma showing multinucleated giant cells (MGCs) expressing Src protein (IHC stain, ×400) and (c) Immunohistochemical staining for central giant cell granuloma demonstrating Src positive MGCs (IHC stain, ×400)
All the lesions consisted of ovoid to spindle-shaped stromal cells admixed with MGCs. The size, shape, and number of MGCs varied within each lesion and from lesion to lesion. Numerous capillaries and abundant hemorrhage were present throughout the lesions. Areas of reactive bone formation were also found within the lesions.
Detection of Src
In this study, immunohistochemical evaluation confirmed the presence of Src in both the PGCG [Figure 1b] and the CGCG [Figure 1c]. Mann-Whitney U-test did not show statistically significant difference neither in the Src expression (P = 0.057) nor the SID score (P = 0.09) between PGCG and CGCG [Figure 2]. However, Src expression was considerably higher in CGCG [Table 1]. Spearman's rank correlation coefficient showed a significant correlation between Src expression and SID score in both the PGCG (r = 0.87, P < 0.001) and the CGCG (r = 0.75, P < 0.001) [Figure 3].
Figure 2.
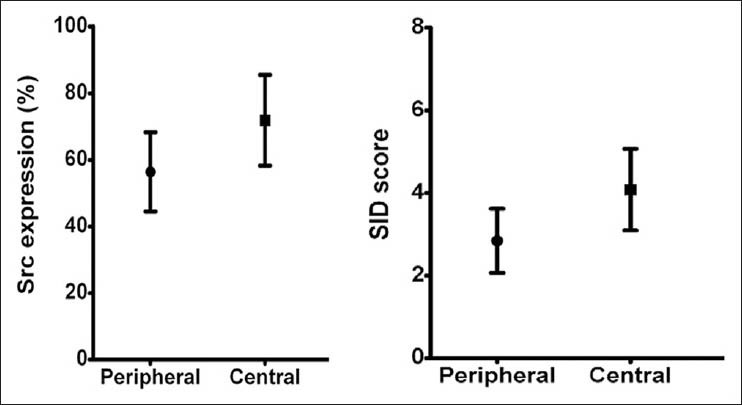
The means of Src expression and staining-intensity-distribution (SID) score with error bars in two groups with confidence interval of 95%
Table 1.
Src expression and staining-intensity-distribution score in PGCG and CGCG
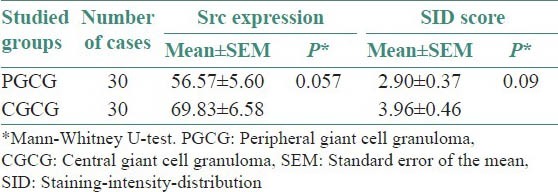
Figure 3.
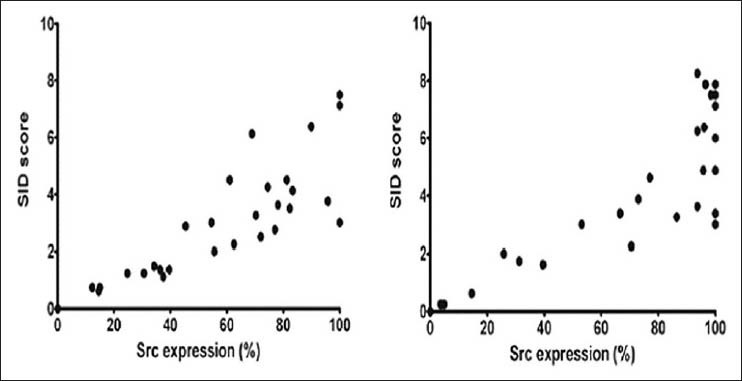
Correlation between Src expression and SID score in (a) PGCG (r = 0.87, P < 0.001) and (b) CGCG (r = 0.75, P < 0.001)
DISCUSSION
PGCGs and CGCGs of the jaws are characterized by the presence of MGCs in a background of spindle-shaped mesenchymal cells and monocyte macrophages. The spindle-shaped cells are related to fibroblasts.[1] Sources of monocytes are provided in GCG while imperfect vascular channels are forming during angiogenesis. It has been suggested that the spindle cells which are the proliferating population of the lesion, recruit monocyte macrophage precursors and stimulate their differentiation into osteoclastic giant cells by activating RANK/RANK ligand signaling pathway.[1,10] Src is a member of a family of non-receptor tyrosine kinases. This protein is activated downstream of RANK (a member of the tumor necrosis factor (TNF) receptor superfamily) and induces osteoclast formation, function, and survival.[14,17] The Src knockout mice developed osteoclasts which fail to resorb bone. These abnormal osteoclasts have aberrant cytoskeletal organization and lack polarization of the cell responsible for bone resorption.[14] and mature osteoclasts. These findings are in agreement with the results of Liu et al., who demonstrated that the osteoclastic markers, TRAP, and RANK are expressed in the MGCs of both PGCG and CGCG.[11] Lim and Gibbins also have suggested that the MGCs in CGCG are similar to osteoclasts and express TRAP 5 and MB1.[18] Likewise, Src expression in cherubism, giant cell tumor, and CGCG has been shown by Wang et al.[13]
Pathogenesis of CGCG and PGCG of the jaws has been subject of disagreement for many years. Histopathological and immunohistochemical parameters have been evaluated in some studies to determine reliable markers related to their different biologic behavior.[3,5] Moreover, results have revealed that the use of quantitative methods helps to find hidden aspects of diseases that may be ignored in routine histolopathological assessments. However, because of the limited number of studies evaluating differences between these lesions, coming to a firm conclusion is difficult.[5]
The results of this study showed no statistically significant difference for both Src expression and SID score between PGCG and CGCG, indicating that their biologic diversities are not supported by different Src expression patterns and PGCG is the peripheral variant of CGCG.
Souza et al., also concluded that the expressions of p53, Mouse Double Minute 2 homolog (MDM2), Proliferating Cell Nuclear Antigen (PCNA), and ki-67 in PGCG and CGCG do not reflect different clinical behavior of these lesions.[19] Tiffee and Aufdemorte similarly could not demonstrate different expressions of TRAP and factor XIII in PGCG and CGCG.[20] However, Flórez-Moreno et al., demonstrated that both nuclear morphometric parameters of MGC and CD68 immunoreactivity are different between PGCG and CGCG.[5]
In this study, Src expression was considerably higher in CGCG although it was not statistically significant. Further investigations with more sample size will make the concept clearer.
CONCLUSION
The findings of the present study suggest a role of Src (an osteoclastic factor) in resorptive activity of the MGCs in both PGCG and CGCGs of the jaws. Therefore, treatment strategies targeting osteoclasts via blocking RANK/Src activity may be useful for nonsurgical treatment of PGCG and CGCG and provide a new therapeutic target to inhibit osteoclastic activity. In addition, relatively similar immunohistochemical expressions of Src protein in PGCG and CGCG show that Src protein is not associated with different clinical behaviors of peripheral and CGCGs of the jaws.
Footnotes
Source of Support: Tabriz University of Medical Sciences.
Conflict of Interest: None declared.
REFERENCES
- 1.Neville BW, Damm DD, Allen CM, Bouquot JE. 3rd ed. St. Louis: Saunders Elsevier; 2009. Oral and maxillofacial pathology; p. 520. (553-554). 627-628. [Google Scholar]
- 2.Kruse-Losler B, Diallo R, Gaertner C, Mischke KL, Joos U, Kleinheinz J. Central giant cell granuloma of the jaws: A clinical, radiologic, and histopathologic study of 26 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:346–54. doi: 10.1016/j.tripleo.2005.02.060. [DOI] [PubMed] [Google Scholar]
- 3.Torabinia N, Razavi SM, Shokrolahi Z. A comparative immunohistochemical evaluation of CD68 and TRAP protein expression in central and peripheral giant cell granulomas of the jaws. J Oral Pathol Med. 2011;40:334–7. doi: 10.1111/j.1600-0714.2010.00944.x. [DOI] [PubMed] [Google Scholar]
- 4.Regezi JA, Sciubba JJ, Jordan RC. 5th ed. St Louis: Saunders Elsevier; 2008. Oral pathology: Clinical Pathologic Correlations; pp. 165–166. 292. [Google Scholar]
- 5.Flórez-Moreno GA, Henao-Ruiz M, Santa-Sáenz DM, Castañeda-Peláez DA, Tobón-Arroyave SI. Cytomorphometric and immunohistochemical comparison between central and peripheral giant cell lesions of the jaws. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:625–32. doi: 10.1016/j.tripleo.2007.08.032. [DOI] [PubMed] [Google Scholar]
- 6.Giansanti JS, Waldron CA. Peripheral giant cell granuloma: Review of 720 cases. J Oral Surg. 1969;27:787–91. [PubMed] [Google Scholar]
- 7.Pandolfi PJ, Felefli S, Flaitz CM, Johnson JV. An aggressive peripheral giant cell granuloma in a child. J Clin Pediatr Dent. 1999;23:353–5. [PubMed] [Google Scholar]
- 8.Whitaker SB, Waldron CA. Central giant cell lesions of the jaws. A clinical, radiologic, and histopathologic study. Oral Surg Oral Med Oral Pathol. 1993;75:199–208. doi: 10.1016/0030-4220(93)90094-k. [DOI] [PubMed] [Google Scholar]
- 9.Vered M, Nasrallah W, Buchner A, Dayan D. Stromal myofibroblasts in central giant cell granuloma of the jaws cannot distinguish between non-aggressive and aggressive lesions. J Oral Pathol Med. 2007;36:495–500. doi: 10.1111/j.1600-0714.2007.00541.x. [DOI] [PubMed] [Google Scholar]
- 10.Tobón-Arroyave SI, Franco-González LM, Isaza-Guzmán DM, Florez-Moreno GA, Bravo-Va´squez T, Castan˜eda-Pela´ez DA, et al. Immunohistochemical expression of RANK, GRalpha and CTR in central giant cell granuloma of the jaws. Oral Oncol. 2005;41:480–8. doi: 10.1016/j.oraloncology.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 11.Liu B, Yu SF, Li TJ. Multinucleated giant cells in various forms of giant cell containing lesions of the jaws express features of osteoclasts. J Oral Pathol Med. 2003;32:367–75. doi: 10.1034/j.1600-0714.2003.00126.x. [DOI] [PubMed] [Google Scholar]
- 12.Roux S, Amazit L, Meduri G, Guiochon-Mantel A, Milgrom E, Mariette X. RANK (receptor activator of nuclear factor kappa B) and RANK ligand are expressed in giant cell tumors of bone. Am J Clin Pathol. 2002;117:210–6. doi: 10.1309/BPET-F2PE-P2BD-J3P3. [DOI] [PubMed] [Google Scholar]
- 13.Wang C, Song Y, Peng B, Fan M, Li J, Zhu S, et al. Expression of c-Src and comparison of cytologic features in cherubism, central giant cell granuloma and giant cell tumors. Oncol Rep. 2006;15:589–94. [PubMed] [Google Scholar]
- 14.Xing L, Venegas AM, Chen A, Garrett-Beal L, Boyce BF, Varmus HE, et al. Genetic evidence for a role for Src family kinases in TNF family receptor signaling and cell survival. Genes Dev. 2001;15:241–53. doi: 10.1101/gad.840301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suda T, Takahashi N, Udagawa N, Jimi E, Gillespie MT, Martin TJ. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr Rev. 1999;20:345–57. doi: 10.1210/edrv.20.3.0367. [DOI] [PubMed] [Google Scholar]
- 16.Tobón-Arroyave SI, Mideros-Simarra SM, Castaño-Ramírez LM, Flórez-Moreno GA, Isaza-Guzmán DM. Overexpression of matrix metalloproteinase (MMP)-1 and -9 in central giant cell lesions of the jaws: Implications for clinical behavior. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;110:755–63. doi: 10.1016/j.tripleo.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 17.Li J, Sarosi I, Yan XQ, Morony S, Capparelli C, Tan H, et al. RANK is the intrinsic hematopoietic cell surface receptor that controls osteoclastogenesis and regulation of bone mass and calcium metabolism. Proc Natl Acad Sci USA. 2000;97:1566–71. doi: 10.1073/pnas.97.4.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lim L, Gibbins JR. Immunohistochemical and ultrastructural evidence of a modified microvasculature in the giant cell granuloma of the jaws. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;79:190–8. doi: 10.1016/s1079-2104(05)80281-1. [DOI] [PubMed] [Google Scholar]
- 19.Souza PEA, Mesquita RA, Gomez RS. Evaluation of p53, PCNA, Ki-67, MDM2 and AgNOR in oral peripheral and central giant cell lesions. Oral Dis. 2000;6:35–9. doi: 10.1111/j.1601-0825.2000.tb00319.x. [DOI] [PubMed] [Google Scholar]
- 20.Tiffee JC, Aufdemorte TB. Markers for macrophage and osteoclast lineages in giant cell lesions of the oral cavity. J Oral Maxillofac Surg. 1997;55:1108–12. doi: 10.1016/s0278-2391(97)90291-3. [DOI] [PubMed] [Google Scholar]


