Abstract
Background:
Xylene in one of the non-substitutable chemical used in histology laboratories. However, it is known to have many toxic effects. The toxic effects of xylene include heart and kidney injuries, some fatal blood dyscrasia and other less dangerous problems, such as skin erythema, drying, scaling and secondary infections. The exposure and handling of xylene is maximum during deparaffinizing tissue sections.
Aims:
The aim of this study was to evaluate the efficacy of 1.7% dishwashing soap (DWS) solution as a deparaffinizing agent for hematoxylin and eosin (H and E) staining and compare it with xylene.
Materials and Methods:
Sixty sections of 4 μm were obtained from 30 formalin-fixed paraffin-embedded (FFPE) tissues and were considered in two different groups, groups A and B. Slides in group A were stained with routine H and E staining procedure; whereas, slides in group B were stained using 1.7% DWS as a deparaffinizing agent.
Statistical Analysis Used:
Wilcoxon matched-pairs signed rank test was used to calculate the test of significance (P-value significant at ≤0.05).
Results and Conclusion:
1.7% DWS was found to be an effective alternative deparaffinizing agent to xylene and meanwhile facilitating as less biohazardous, economical and a faster deparaffinizing agent.
Keywords: Deparaffinization, hematoxylin and eosin, liquid dishwashing solution, xylene
INTRODUCTION
Many sophisticated, immunological and molecular biological techniques have been introduced into pathological practice during past few decades that helps in precise diagnosis. However, hematoxylin and eosin (H and E) stained paraffin sections still remain the most widely used technique for routine diagnostic work. Though for the last 150 years, the method of preparing these sections has remained largely unchanged.[1] In addition to H and E, the components in the H and E staining procedure are xylene and graded alcohol which are used to carry out the intermediate steps of deparaffinization, rehydration and dehydration of tissue sections during the staining.[2] The problems associated with this age old procedure are the toxicity of reagents used particularly xylene, cost containment, problem of disposal of hazardous substances and polluting working environment.[3]
Xylene is an aromatic hydrocarbon which is extremely biohazardous. The histopathological laboratory technicians are routinely exposed to xylene during procedures like tissue processing, clearing, staining, placing a cover slip and cleaning tissue processors. The exposure and handling of xylene is maximum during dewaxing of sections. The main effects of inhaling xylene vapor are depression of the central nervous system with symptoms such as headache, dizziness, nausea and vomiting. Long-term exposure may lead to irritability, insomnia agitation, extreme tiredness, tremors, impaired concentration and short-term memory.[4,5] The other toxic effects of xylene include acute neurotoxicity, heart and kidney pathologies, some fatal blood dyscrasia, skin erythema drying and scaling of skin. All these effects are caused by depletion of mitochondrial adenosine triphosphate (ATP) in the affected cells.[6,7,8]
Many substitute chemicals like aliphatic and aromatic hydrocarbons, limonene reagents and mineral oil mixtures have been used to substitute xylene as a clearing agent during tissue processing.
Falkeholm et al.,[1] and Buesa and Peshkov[6] have shown the advantages of hot dishwashing soap (DWS) solution for deparaffinization of tissue sections for H and E staining and some special staining procedures like periodic acid-Schiff (PAS) staining and Van Gieson staining. Henwood[9] has also successfully used hot DWS solution for dewaxing in immunohistochemistry.
Therefore, this study was conducted to determine the applicability of hot DWS solution as deparaffinizing agent in H and E staining.
MATERIALS AND METHODS
Thirty formalin-fixed paraffin-embedded (FFPE) tissues were used for the study. Two sections of 4 μm were obtained from each of 30 FFPE tissues and were considered in two different groups, groups A and B. Slides in group A were stained with conventional H and E staining procedure using xylene as deparaffinizing agent and slides in group B were stained using 1.7% DWS solution at 90°C as deparaffinizing agent. The DWS solution was prepared by dissolving 25 ml of household DWS (Pril Dishwashing Liquid) in 1,500 ml of distilled water for a 1.7% solution. The protocol followed for groups A and B are given in Tables 1 and 2, respectively.
Table 1.
Routine H and E staining using xylene as dewaxing agent
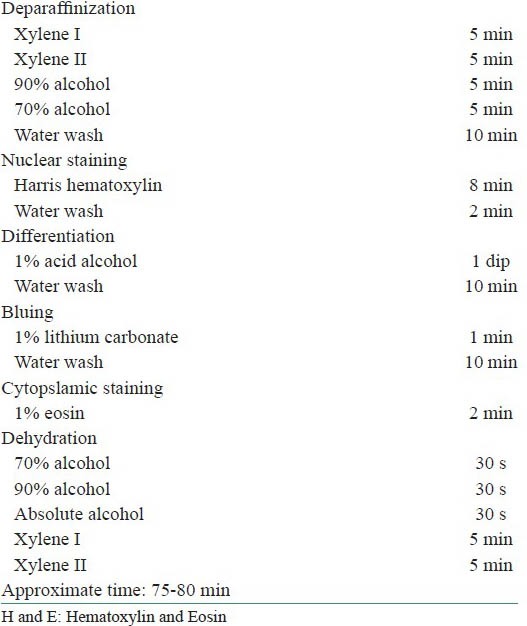
Table 2.
Xylene free staining using 1.7% DWS as dewaxing agent

Slides in each group were scored by three different oral pathologists referred as O1, O2 and O3 (Two HODs of renowned colleges and an Associate Professor) and scored considering the following criteria:
Nuclear staining : Adequate = 1, inadequate = 0
Cytoplasmic staining : Adequate = 1, inadequate = 0
Clarity of staining : Present = 1, absent = 0
Uniformity of staining : Present = 1, absent = 0
Crispness of staining : Present = 1, absent = 0
The scores for each slide were totalled. A score of ≤2 was graded as inadequate for diagnosis, slides with scores 3-5 were considered as adequate for diagnosis.
STATISTICS
The mean value for each grading criteria was calculated for the three observers. Wilcoxon-matched-pairs signed rank test was used to calculate the test of significance (P ≤ 0.05).
RESULTS
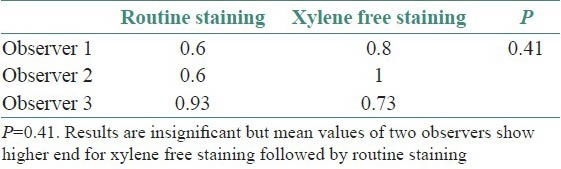
The results for all the grading criteria were found to be statistically insignificant with slightly higher mean values for xylene free staining. Also the percentage of adequacy of diagnosis was found to be higher for O1 and O2 (86 and 100%, respectively) for xylene free staining as compared to routine H and E staining (73 and 86%) [Tables 3–8, Figures 1 and 2].
Table 3.
Nuclear staining
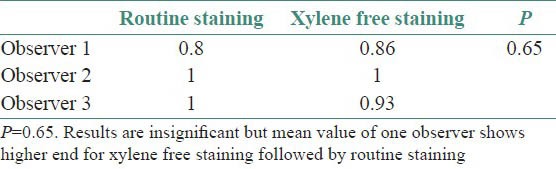
Table 8.
Percentage of adequacy for diagnosis
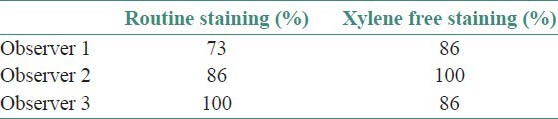
Figure 1.
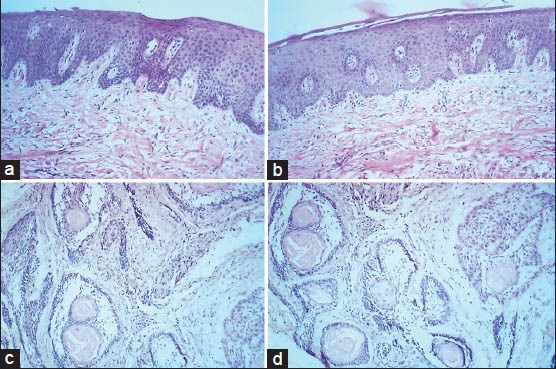
Comparison of photomicrographs of sections stained with routine H and E staining in (a) epithelium and connective tissue and (c) Ameloblastoma (×100), with xylene free H and E staining in (b) epithelium and connective tissue and (d) Ameloblastoma (×100)
Figure 2.
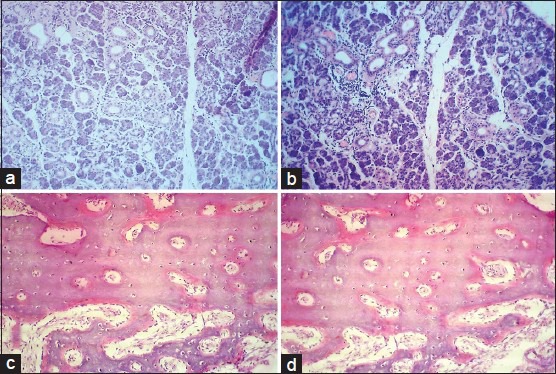
Comparison of photomicrographs of slides stained with routine H and E staining in (a) salivary gland tissue and (c) bony tissue (×100), with xylene free H and E staining in (b) salivary gland tissue and (d) bony tissue (×100)
Table 4.
Cytoplasmic staining
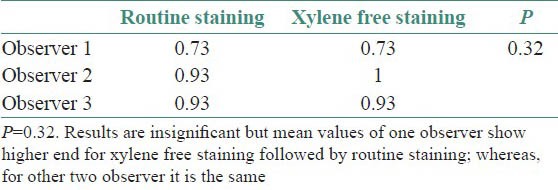
Table 5.
Clarity of staining
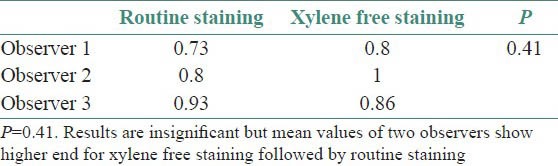
Table 6.
Uniformity of staining
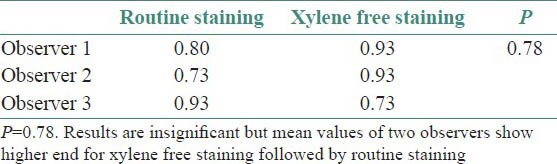
Table 7.
Crispness of staining
DISCUSSION
Despite the known toxicity of xylene, it is widely used in histopathology laboratory without any monitoring for the exposure and any standardized method for disposal. Many xylene substitutes are commercially available and have been used during tissue processing. However, during H and E staining procedure, xylene is still being used liberally.
Xylene is a colorless, sweet smelling liquid or gas occurring naturally in petroleum, coal and wood tar. It is named so as it is found in crude wood spirit (Gr. Xylon means wood). Other than occupational exposure, humans can get exposed to xylene via soil contamination from leaking underground tanks containing petroleum products. It may remain in soil and underground water for months or more before getting broken down to other chemicals. It evaporates easily in air and breaks down by sunlight into less harmful chemicals. It can be smelled in air at 0.08-3.7 parts per million (ppm) and can be tasted in water at 0.53-1.8 ppm.[10] Exposure to xylene can occur via inhalation, ingestion and eye or skin contact. It is primarily metabolized in liver by oxidation of methyl group and conjugation with glycine to yield methyl hippuric acid, which is excreted in urine. Very small amount is exhaled as such and usually there is low potential for accumulation within the body.[11,12] Xylene causes health effects from both acute (<14 days) and chronic (>365 days) exposure. The current Occupational Safety and Health Administration (OSHA) permissible exposure limit for xylene is 100 ppm as an 8 hour time weighted average (TWA) concentration.[5]
Liquid DWS is readily available and is much cheaper as compared to xylene. It is composed of sodium lauryl sulfate, sodium dodecyl benzene sulfonate, cocamidopropyl betaine and nonionic surfactants.[7] The concentration of these components is already monitored by the manufacturer. In our study, we have used DWS in a dilution of 1.7%, hence there are least chances of toxicity to the laboratory personnel.
Though the results were found to be statistically insignificant, the mean values of two observers for clarity of staining, uniformity of staining and crispness of staining showed higher end for xylene free staining as compared to routine H and E staining. The mean values of one observer for nuclear staining and cytoplasmic staining showed higher end for xylene free staining. Even the percentage of adequacy for diagnosis was again higher for xylene free staining for two observers out of three.
The approximate time taken for xylene free staining (45-50 min) is also about half the time taken for the routine H and E staining (75-80 min). Apart from being nontoxic and taking less time for staining, other advantages of using DWS as deparaffinizing agent are its low cost (750 times cheaper), easy handling and easy disposal. The only safety concern is the possibility of finger burns while handling hot DWS solution.
CONCLUSION
Considering the toxicity of xylene, it is desirable to minimize its use in histopathology laboratory without compromising the staining quality and hence the appropriate diagnosis. Our study shows that staining quality of xylene free H and E staining using hot DWS solution as deparaffinizing agent is equivalent to routine H and E staining. This will help us in maintaining a favorable and healthy laboratory environment.
Although we have significant positive results in our hands, further studies are required including larger sample size and multiple observers.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Falkeholm L, Grant CA, Magnusson A, Mollur E. Xylene-free method for histological preparation: A Multicentre Evaluation. Lab Invest. 2001;81:1213–21. doi: 10.1038/labinvest.3780335. [DOI] [PubMed] [Google Scholar]
- 2.Bancroft JD, Gamble M. 6th ed. Philadalphia, USA: Churchill Livingstone Elsevier; 2011. Theory and Practice of Histologic Techniques. [Google Scholar]
- 3.Dapson JC, Dapson RW. 4th ed. Battle Creek: Anatech; 2005. Hazardous materials in histopathology laboratory, regulations, risks, handeling and disposal. [Google Scholar]
- 4.OSHA (Occupational safety and health administration) 2005 Air contaminants Occupational safety and Health Administration. [Last accessed on 2009 Dec 16]. Available from: http://www.osha.gov/comp-links.html .
- 5.Kandyala R, Raghvendra SP, Rajasekharan ST. Xylene: An overview of its health hazards and preventive measures. J Oral Maxillofac Pathol. 2001;4:1–5. doi: 10.4103/0973-029X.64299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buesa RJ, Peshkov MV. Histology without xylene. Ann Diagn Pathol. 2009;13:246–56. doi: 10.1016/j.anndiagpath.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 7.Ankle MR, Joshi PS. A study to evaluate the efficacy of xylene free hematoxylin and eosin staining procedure as compared to the conventional hematoxylin and eosin staining: An experimental study. J Oral Maxillofac Pathol. 2011;15:161–7. doi: 10.4103/0973-029X.84482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Revilla AS, Pestana CR, Pardo- Andreu GL, Santos AC, Uyemura SA, Gonzales ME, et al. Potential toxicity of toluene and xylene evoked by mitochondrial uncoupling. Toxicol In Vitro. 2007;21:782–8. doi: 10.1016/j.tiv.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 9.Henwood AF. The application of detergent dewaxing and rehydration to immunohistochemistry. Biotech Histochem. 2012;87:46–50. doi: 10.3109/10520295.2010.518891. [DOI] [PubMed] [Google Scholar]
- 10.Mike F, John FR, Wilson JD, Margaret F, Daniel P, Lisa I. Atlanta, Georgia: Agency for Toxic Substances and Disease Registry; 1995. Toxicological profile for xylene. US department of Health Services, public health services, Agency for toxic substance and disease registry. [Google Scholar]
- 11.Sedivec V, Flek J. Exposure test for xylenes. Int Arch Occup Environ Health. 1976;37:219–32. doi: 10.1007/BF00378420. [DOI] [PubMed] [Google Scholar]
- 12.Ogata M, Tomokuni K, Takatsuka Y. Urinary excretion of hippuric acid and m- or p-methylhippuric acid in the urine of persons exposed to vapours of toluene and m- or p-xylene as a test of exposure. Br J Int Med. 1970;27:43–50. doi: 10.1136/oem.27.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]


