Abstract
Background:
Angiogenesis is the formation of new vessels from preexisting ones which takes place by capillary sprouting. It is seen in healing, at sites of injury and collateral circulation in ischemia. It is also seen in tumors, as once the original blood supply of a tumor is exhausted it cannot grow without further blood supply. Also as the angiogenic capacity of a tumor increases, its microvasculature, that is, microvessel density (MVD) also increases. Based on this literary evidence we carried out an immunohistochemical (IHC) study to observe the relationship between the expression of vascular endothelial growth factor (VEGF) [angiogenesis] and CD 34 [MVD] in oral squamous cell carcinoma (OSCC).
Aim:
To evaluate the correlation between expression of VEGF and CD 34, the role of MVD in progression of OSCC and to compare the degree of angiogenesis in different grades of OSCC.
Settings and Design:
In this study we observed the relation between angiogenesis and MVD and the overall effect of this on oral cancer.
Materials and Methods:
Thirty-three cases of OSCC were stained with hematoxylin and eosin, (H and E) to confirm the diagnosis and immunohistochemically using VEGF and CD 34 antibody. The slides were evaluated for positivity and intensity of staining.
Statistical Analysis:
The result was subjected to statistical analysis using analysis of variance (ANOVA) test and Fisher's exact test.
Results:
VEGF positivity as well as MVD was found to be independent of the grade of the tumor. Tumor MVD was found to be independent of expression of VEGF.
Keywords: Angiogenesis, microvessel density, neovascularization, oral squamous cell carcinoma
INTRODUCTION
Oral squamous cell carcinoma (OSCC) is the most common malignant, aggressive neoplasm to affect the maxillofacial region. It has a multifactorial etiology due to the fact that there is a complex signaling pattern.[1]
Angiogenesis, an essential step in tumor growth and metastasis, is the formation of new vessels from preexisting ones which takes place by capillary sprouting. Once the original blood supply of a tumor is exhausted it cannot grow beyond 1-2 mm without further blood supply. The initial 1-2 mm zone represents the maximum distance across which oxygen and nutrients can diffuse from blood vessels. Beyond this size, the tumor fails to enlarge without vascularization because hypoxia induces apoptosis.[1]
Angiogenic properties are correlated with tumor aggressiveness, and intratumor microvessel density (MVD) has been found to be an independent prognostic factor.[1]
The new blood vessels provide the principal route by which tumor cells leave the primary tumor site and enter the circulation. Therefore, angiogenesis is essential for tumor progression and metastasis. Although angiogenesis is difficult to measure directly in human tumors, there is increasing evidence that MVD may be considered as an indirect marker of neoangiogenesis. The most common antibodies used for microvessel staining so far are those against Von Willebrand Factor (Factor VIII), CD31 and CD34.[2]
Vascular Endothelial Growth Factor (VEGF) is considered the first factor which maintains its position as the most critical driver of vascular formation and is required to initiate the formation of immature vessels. During their initial growth period, however, tumors do not initiate angiogenesis. This vascular quiescence is terminated by the “angiogenic switch”, that is, the activation of angiogenic factors. The “angiogenic switch” is “on” when the net balance is tipped in favor of angiogenesis and “off” when effect of proangiogenic molecules is balanced by that of antiangiogenic molecules.[3]
Thus, this present study provides evidence for a strong correlation between VEGF and oral cancer. It also provides a basis to study the relation between VEGF and MVD. These tumor markers can not only be used to study progression and prognosis of the disease, but also to develop newer antiangiogenic drugs to prevent and treat cancer, that are used as an adjunct to the currently available modalities.
MATERIALS AND METHODS
The present study was carried out on 33 archival tissues which included 12 cases of well differentiated OSCC, six cases of moderately differentiated OSCC and 15 cases of poorly differentiated OSCC. Formalin fixed-paraffin embedded tissues were sectioned at 3-4 micron thick sections. Routine staining protocols were carried out for hematoxylin and eosin (H and E) and immunohistochemical (IHC) technique for VEGF and CD 34.
Determination of VEGF expression and MVD
VEGF expression was determined by IHC using anti-VEGF antibody (DAKO) to assess localization, intensity and area of stained cells. Intensity of stain was scored as follows:
0 - No staining
1 - Mild staining
2 - Moderate staining
3 - Intense staining
Images were recorded using the Sony Cyber shot (DSC-W570, Japan) with a 10× apochromatic objective.
To determine MVD as specified by Weidner et al.,[4] any brown staining endothelial cell or cell cluster that was clearly separated from adjacent microvessels, tumor cells and other connective tissue was considered a single countable microvessel. Vessel lumens, although usually present were not necessary for a structure to be defined as a microvessel and red cells were not used to define a vessel lumen. Microvessel counts were determined blindly as the investigator was blinded to this variable. After selecting three microscopic fields of highest neovascularization or hot spots, under low magnification, individual microvessels were counted manually using freehand draw option in the image analysis software, Image Drafter and counted at 40× magnification. Images of these selected fields were captured along with marked microvessels.
RESULTS
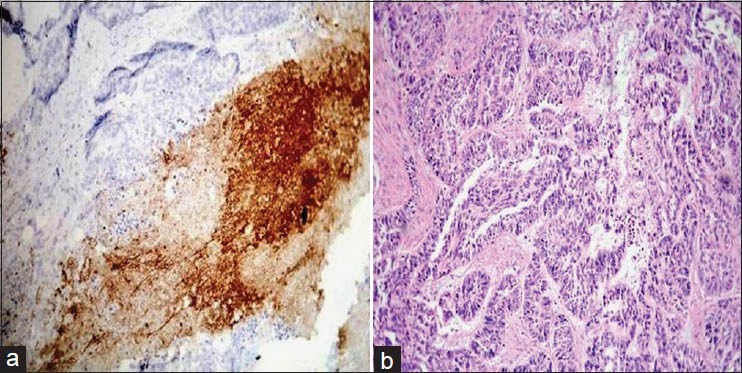
Using the above criteria, the following results were obtained. Table 1 and Figure 1 show the histopathological grades vs the intensity of staining with VEGF. Out of 15 cases of poorly differentiated squamous cell carcinoma, four cases showed negative staining with VEGF [Figure 2a and b], six cases showed a staining intensity of 1+ with VEGF, four cases showed a staining intensity of 2+ and one case showed a staining intensity of 3+ [Figure 3a and b]. In cases of moderately differentiated squamous cell carcinoma all six cases showed a staining intensity of 1+ [Figure 4a and b]. Out of 12 cases of well differentiated squamous cell carcinoma, eight cases showed a staining intensity of 1+ and four cases showed a staining intensity of 2+ with VEGF [Figure 5a and b]. By using Fisher's exact test P value was 0.094, since P > 0.05, therefore, there is no association between tumor grade and VEGF staining intensity.
Table 1.
Distribution of cases with respect to histopathological grades and VEGF staining intensity
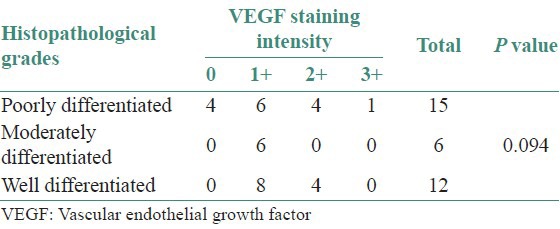
Figure 1.
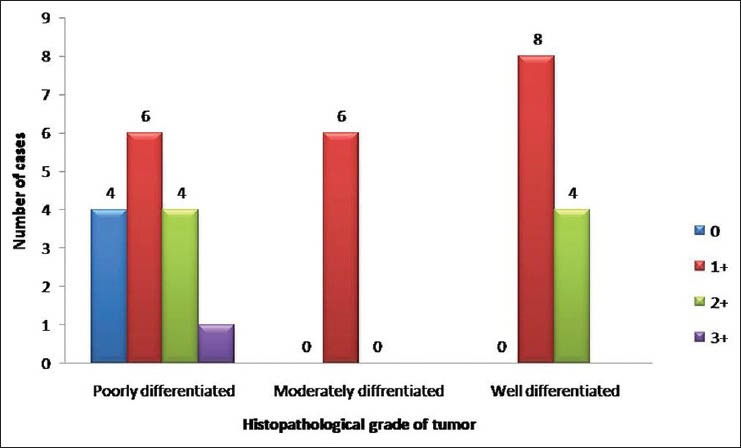
Distribution of cases with respect to histopathological grades and vascular endothelial growth factor staining intensity
Figure 2.
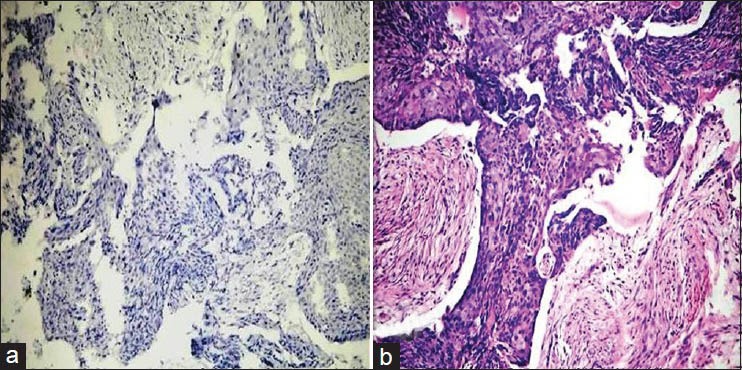
(a) Histopathological image shows poorly differentiated oral squamous cell carcinoma: VEGF staining intensity 0 (IHC stain, ×100). (b) Histopathological image shows poorly differentiated oral squamous cell carcinoma. (H&E stain, ×100)
Figure 3.
(a) Histopathological image shows poorly differentiated oral squamous cell carcinoma: VEGF staining intensity 3× (IHC stain, ×100). (b) Histopathological image shows poorly differentiated oral squamous cell carcinoma. (H&E stain, ×100)
Figure 4.
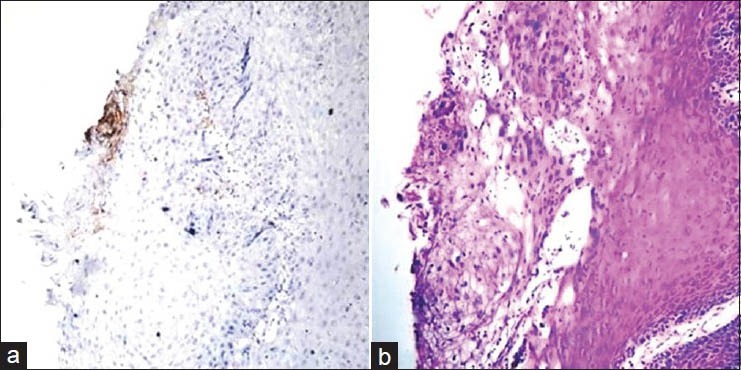
(a) Histopathological image shows moderately differentiated oral squamous cell carcinoma: VEGF staining intensity 1+ (IHC stain, ×100). (b) Histopathological image shows moderately differentiated oral squamous cell carcinoma. (H&E stain, ×100)
Figure 5.
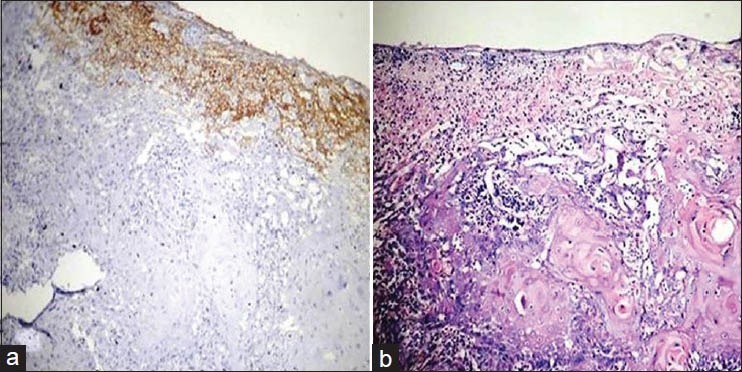
(a) Histopathological image shows well differentiated oral squamous cell carcinoma: VEGF staining intensity 2× (IHC stain, ×100) (b) Histopathological image shows well differentiated oral squamous cell carcinoma.(H&E stain, ×100)
Table 2 and Figure 6 show the comparison of MVD count with respect to grade of the tumor. Poorly differentiated OSCC shows an average count of 9.64 with SD of ±4.22 [Figure 7]. Moderately differentiated OSCC shows an average of 5.63 with SD of ±3.04 [Figure 8]. Well differentiated OSCC shows an average count of 12.08 with SD of ±5.39 [Figure 9]. By using analysis of variance (ANOVA) test P value is 0.027, that is, <0.05. Therefore, the results are statistically significant. No correlation is seen between the MVD counts and the grade of the tumor. However, it is observed that well-differentiated OSCC showed the maximum MVD.
Table 2.
Comparison of MVD count with respect to grade of the tumor
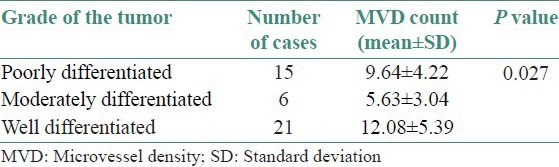
Figure 6.
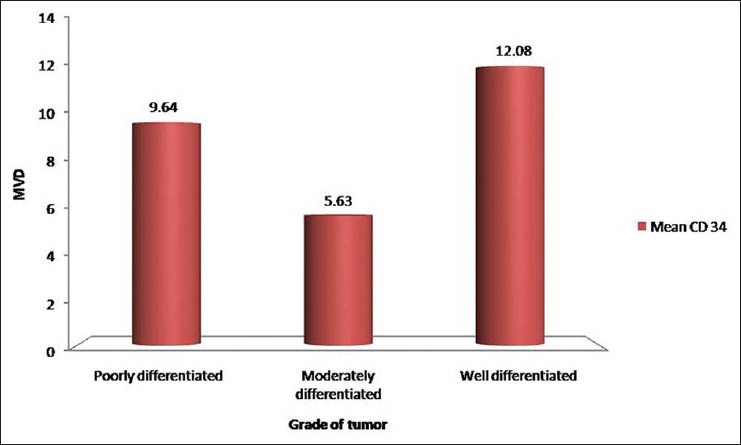
Comparison of microvessel density count with respect to grade of the tumor
Figure 7.
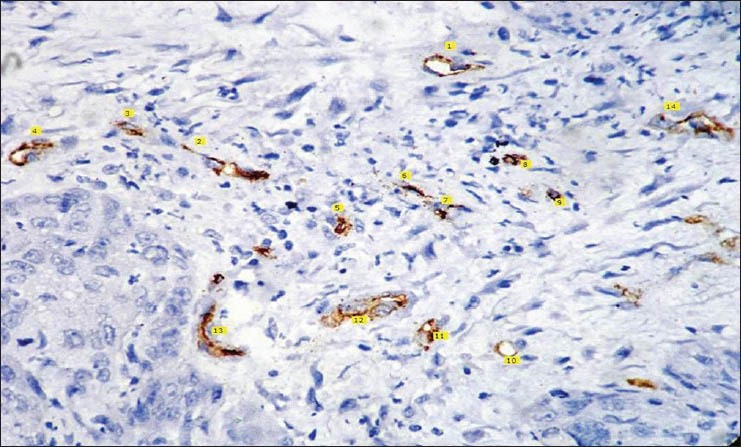
Histopathological image shows CD 34 staining for poorly differentiated squamous cell carcinoma (IHC stain, ×400)
Figure 8.
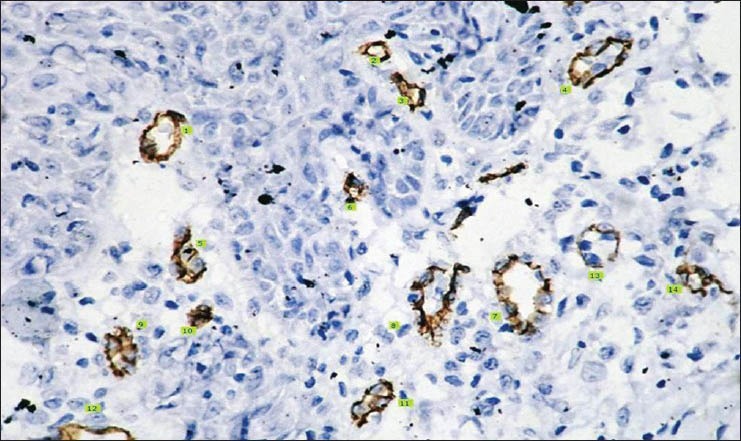
Histopathological image shows CD 34 staining for moderately differentiated squamous cell carcinoma (IHC stain, ×400)
Figure 9.
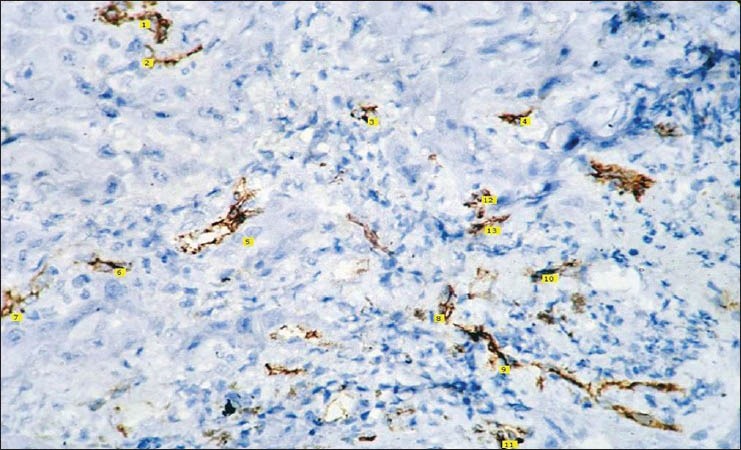
Histopathological image shows CD 34 staining for well differentiated squamous cell carcinoma (IHC stain, ×400)
Table 3 and Figure 10 show the comparison of MVD count with respect to VEGF intensity staining. Out of a total of 33 cases, four cases show negative staining with VEGF and a mean MVD count of 11.42 ± 2.50 SD, twenty cases show a staining intensity of 1+ with VEGF and a mean MVD count of 9.35 ± 5.43 SD, eight cases show a staining intensity of 2+ and a mean MVD count of 10.17 ± 5.20 and only one case shows a staining intensity of 3+ with VEGF and a mean MVD count of 9.33 ± 0.00 SD. By using ANOVA test P value is 0.897, that is, >0.05; therefore, there is no significant correlation between mean CD 34 counts with respect to VEGF intensity staining.
Table 3.
Comparison of MVD count with respect to VEGF intensity staining
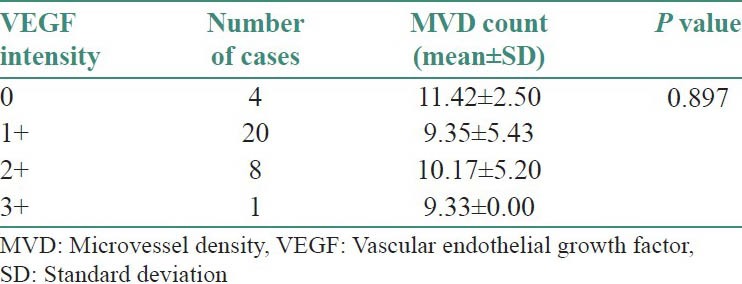
Figure 10.
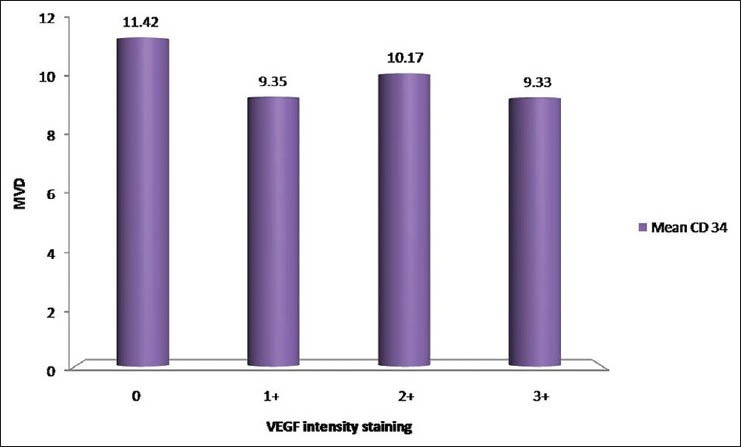
Comparison of MVD count with respect to VEGF intensity staining
Table 4 and Figure 11 show the comparison of MVD scores with respect to VEGF staining in various histopathological grades. Out of 15 cases of poorly differentiated OSCC, four cases show negative staining with VEGF and MVD count of 11.42 ± 2.50 SD, six cases show a staining intensity of 1+ with VEGF and mean MVD count 8.78 ± 6.24 SD, four cases show a staining intensity of 2+ and mean MVD count of 9.25 ± 2.50 SD and only one case showed a staining intensity of 3+ and a mean MVD count of 9.33. Six cases of moderately differentiated OSCC show a staining intensity of 1+ and mean MVD count of 5.63 ± 3.03 SD. Out of 12 cases of well differentiated OSCC, eight cases show a staining intensity of 1+ and a mean MVD count of 12.68 ± 4.64 SD and four cases show a staining intensity of 2+ and a mean MVD of 11.08 ± 7.39 SD.
Table 4.
Comparison of MVD scores with respect to VEGF staining intensity in various tumor grades
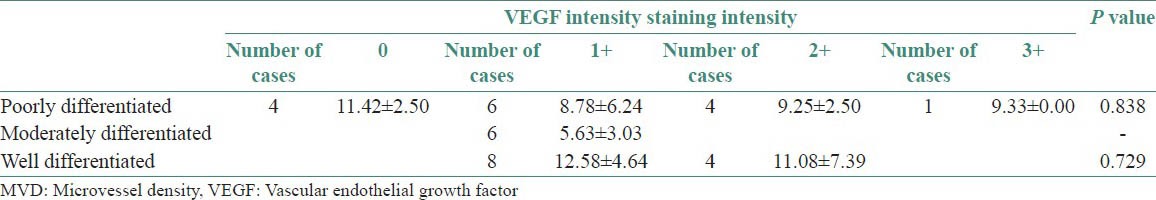
Figure 11.
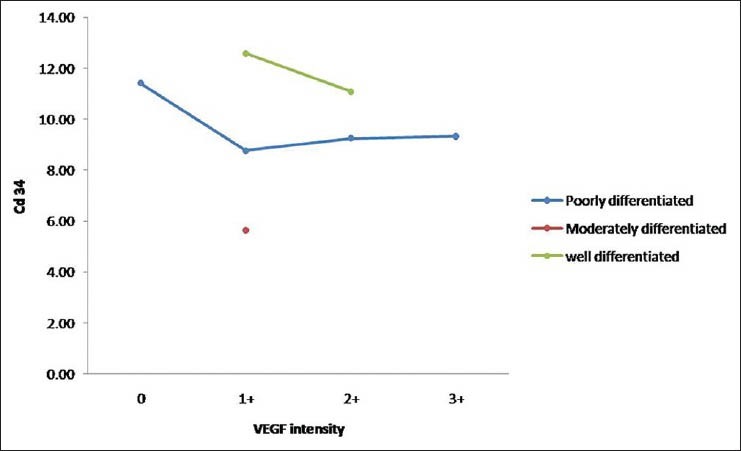
Comparison of MVD scores with respect to VEGF staining intensity in various tumor grades
By using ANOVA test, P > 0.05; therefore, there is no significant correlation between mean MVD counts with respect to VEGF intensity staining for poorly differentiated grade
By using two-tailed independent t-test, P > 0.05; therefore, there is no significant difference between mean MVD counts with respect to VEGF intensity staining for well differentiated grade.
DISCUSSION
It is a well-accepted paradigm that tumors recruit new blood vessels from the existing circulation (angiogenesis) and this participates in tumor invasion and metastasis.
Studies in the literature provide evidence for expression of VEGF by the tumor for neoangiogenesis, which is not only required for the tumor growth but also its metastasis.
On the basis of literary evidence a hypothesis was formulated that the tumors of the oral cavity express VEGF for their growth and that as expression of VEGF increases, MVD also increases.
To accept or reject the formulated hypothesis, a retrospective study was performed in which a total of 33 cases; 12 cases of well differentiated OSCC, six cases of moderately differentiated OSCC and 15 cases of poorly differentiated OSCC; were included.
VEGF expression and MVD was assessed using standard immunohistochemical procedure using anti-VEGF antibody and anti-CD 34 antibody staining, respectively.
The following observations were drawn from the present study:
Most of the OSCC tissues expressed positivity for VEGF (87.88%, that is, 29 out of 33)
The staining intensity of VEGF was independent of the grade of tumor (P = 0.094, that is, >0.05)
MVD was also independent of the grade of the tumor
No significant correlation was seen between VEGF and MVD counts either individually or with respect to the grade.
Staining intensity of VEGF was compared between the different grades of OSCC and was found to be independent of the grade of tumor [Table 1 and Figure 1]. Various studies have been done in the past with different molecules that are involved in the pathogenesis of the growth and metastasis of tumors. Pusztai et al., in 1993[5] and Lee et al., in 2006[6] reviewed trends in growth factor research and classified the growth factors and their receptors and presented a model of cell proliferation regulation by growth factors. They proposed a variety of molecules which were released by tumor cells, including Transforming growth factor (TGF) α, TGFβ, epidermal growth factor (EGF), platelet-derived growth factor (PDGF) and the whole family of Heparin binding growth factors (HBGFs), but assigned a prominent role to VEGF. The above results matched the findings by Maeda et al., in 1998,[1] Carlile et al., in 2001,[7] Johnstone and Logan in 2006,[8] and Miyahara et al., in 2007[9] who did not find any correlation between the VEGF staining intensity with the grade of OSCC. Astekar et al., in 2012,[10] however, found in their study that VEGF expression decreased from well differentiated to moderately differentiated to poorly differentiated OSCC. They also found MVD to correlate with VEGF expression. Our results also did not match those of Sedivy et al., in 2003[11] who also found a significant correlation between VEGF expression and tumor grade.
Shintani et al., in 2004,[12] made an interesting finding. They observed that in addition to VEGF which is known to play an important role in tumor angiogenesis, there are additional members of the VEGF family, that is, VEGF B, C and D which have been discovered. The VEGF family members currently includes five members in addition to the VEGF A/vascular permeability factor (VPF) namely the placenta growth factor (PLGF), VEGF B/VEGF-related factor (VRF), VEGF C/VEGF-related protein (VRP), VEGF D/c-fos-induced growth factor (FIGF) and VEGF E. The founding member, VEGF A, plays essential roles in vasculogenesis and angiogenesis. Its crucial role in tumor angiogenesis and blood-borne metastasis has been documented in a variety of cancers. Although expression of VEGF A in cancer has been studied extensively, the roles of other VEGF family members, that is, VEGF B, VEGF C, and VEGF D, in tumor angiogenesis and metastasis are poorly understood.
Shintani et al.,[12] also observed that VEGF A and B which are known to stimulate the formation of blood vessels in tumors were expressed in most tumor samples examined. Tumor VEGF A and B expression and MVD were found to be strongly correlated. In contrast, VEGF C and D expression are not related to MVD.
Intratumoral blood vessels are known to play an important role in cancer growth by supplying oxygen and nutrients; excreting metabolic products and is also associated with metastasis. Reports have shown that the immunostaining results using different vascular markers vary depending on the degree of differentiation of the vascular endothelial cells and degree of maturation of the vessels. Therefore, when only one antibody is used, some blood vessels or endothelial cells may remain undetected. This raises doubt over, whether previous reports indicated the true vascular distribution and density. Moreover, it remains unclear whether normal vessels and tumoral vessels show the same immunoreactivity with various antibodies.[13]
According to Astekar et al., (2012),[10] differences between various studies could be due to different antibodies used (CD 31, CD 34 and factor VIII) by authors to define endothelium and different methodologies used in assessment of various parameters besides interobserver variation. Tae et al., in 2000,[14] cited lack of a standardized direct method to measure angiogenesis as a factor. Also none of the existing methods can differentiate between resting and active angiogenic vessels. A commonly used indirect method consists of measuring the density of the microvasculature in histological sections of the tumor and considering this to represent the angiogenic status of the tumor. This approach has led to conflicting results regarding the value of MVD as a prognostic indicator in solid tumors. Also as tumor grows, the total number of microvessels is increased in parallel with tumor volume. MVD is maintained similarly during head and neck tumorigenesis.
Astekar et al.,[10] also cited that differences between immunohistochemical protocols, like selection of the paraffin block, level of section within the tissue block, that is, superficial or deep and variability in the selection of hot spot identification may contribute to the variation of the results amongst different researchers. Also quantifying MVD involves selection of neovascular hotspot areas which may not always be representative of tumor.
Thus, we needed new endothelial cell markers that would detect only active neoangiogenic vessels. The search for specific markers of angiogenic vessels has identified certain molecules, such as the α5β3 and α5β5 integrins, as possible candidates.[14]
These findings thus contribute to the available knowledge of VEGF expression in OSCC. They also establish a direct relation between VEGF and tumor growth which is dependent on neoangiogenesis. VEGF is the initial factor that is responsible for angiogenesis but according to Shieh et al., 2004,[15] it has been proposed that tumor cells can also acquire nutrition by nonangiogenesis or angiogenesis independent pathways. In OSCC, this could be the reason for the observation that oral cancer is less angiogenesis-dependent. So in order to continue growing, it is the inherent property of the tumor to be able to maintain its blood supply.
Hanahan and Folkman in 1996 reviewed the various horizons of angiogenesis research. According to them, angiogenesis inhibitors form an important component of therapeutic strategies aimed at invasive metastatic tumors. Secondly, as methods for early detection of certain cancers improve, it may become possible to interfere with initial tumor development by blocking the angiogenic switch that precedes the progression to invasive cancer.[16]
Integrins are a large family of heterodimeric transmembrane receptors that mediate the interaction of cells with the extracellular matrix (ECM) and are believed to be involved in tumor cell survival and metastasis and in tumor angiogenesis. The α5β6 is an epithelial-specific integrin that is a receptor for the ECM proteins fibronectin, vitronectin, tenascin and the latency: associated peptide (LAP) of TGF-α. The integrin α5β6 is not detectable on normal keratinocytes in vivo, but expression is increased significantly in OSCC and in vitro studies have proved it to actually promote tumor progression.[17,18]
Fabricius et al., in 2011 used immunohistochemistry of fresh-frozen human tumor tissues to analyze the presence of integrins α5β3, α5β5 and α5β1, together with integrin ligands, vitronectin, osteopontin, fibronectin and fibrinogen, in human OSCCs. They found increased staining in tumors compared with the controls, and staining was demonstrated for α5β3 in endothelia. α5β5 staining was increased in thetumor samples, but this was associated with increased expression in stroma rather than in endothelia. Modestly increased expression of α5β1 was observed in the tumor samples, and this was associated with tumor cells, endothelia and stroma. Confirmation of the presence of these integrins and their association with tumor cells, endothelia or stroma suggests their potential for these integrins in human oral tumors.[19]
CONCLUSION
The above aspect together with the fact that no significant correlation could be established between VEGF and MVD does put forth the following:
Tae et al.,[14] state that as a tumor grows, the total number of microvessels does increase in parallel to the tumor volume. Thus, MVD is maintained in tumorigenesis
Currently used markers cannot differentiate between resting and active endothelial cells. This emphasizes the need for a marker that specifically marks active neoangiogenic vessels
Markers are also needed to distinguish normal vessels from intratumoral and peritumoral vessels
Future research would also therefore benefit from a standardization of the research protocol. The determination of VEGF isoform profiles within oral tissues is also necessary to improve our understanding of the role of various isoforms of VEGF.
Future therapies targeted at the molecular mechanism of angiogenesis, especially those that interact with VEGF and its receptors or antibodies against VEGF and its receptors may prove useful in the management of oral cancers. Similarily, the increased expression of integrins within tumors, particularly expression associated with endothelial cells, supports the principle of selective integrin blockade as a novel anticancer strategy.[19]
However, a greater understanding of the role of VEGF in oral cancer is required before VEGF technology can be utilized to improve treatment.[8]
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Maeda T, Matsumura S, Hiranuma H, Jikko A, Furukawa S, Ishida T, et al. Expression of vascular endothelial growth factor in human oral squamous cell carcinoma: Its association with tumour progression and p53 gene status. J Clin Pathol. 1998;51:771–5. doi: 10.1136/jcp.51.10.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cortesina G, Martone T. Molecular metastasis in head and neck squamous cell carcinoma: Review of literature. Acta Otorhinolaryngol Ital. 2006;26:317–25. [PMC free article] [PubMed] [Google Scholar]
- 3.Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–8. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 4.Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- 5.Pusztai L, Lewis CE, Lorenzen J, Mcgee JO. Growth factors: Regulation of normal and neoplastic growth. J Pathol. 1993;169:191–201. doi: 10.1002/path.1711690204. [DOI] [PubMed] [Google Scholar]
- 6.Lee HS, Kim KW, Kim WJ. Expression of angiogenin, TGF-β, VEGF, APEX and TNF-α in oral squamous cell carcinoma. J Kor Oral Maxillofac Surg. 2006;32:8–18. [Google Scholar]
- 7.Carlile J, Harada K, Baillie R, Macluskey M, Chisholm DM, Ogden GR, et al. Vascular endothelial growth factor (VEGF) expression in oral tissues: possible relevance to angiogenesis, tumour progression and field cancerisation. J Oral Pathol Med. 2001;30:449–57. doi: 10.1034/j.1600-0714.2001.030008449.x. [DOI] [PubMed] [Google Scholar]
- 8.Johnstone S, Logan RM. The role of vascular endothelial growth factor in oral dysplasia and oral squamous cell carcinoma. Oral Oncol. 2006;42:337–42. doi: 10.1016/j.oraloncology.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 9.Miyahara M, Tanuma J, Sugihara K, Semba I. Tumor Lymphangiogenesis correlates with lymph node metastasis and clinicopathologic parameters in oral squamous cell carcinoma. Cancer. 2007;110:1287–94. doi: 10.1002/cncr.22900. [DOI] [PubMed] [Google Scholar]
- 10.Astekar M, Joshi A, Ramesh G, Metgud R. Expression of vascular endothelial growth factor and microvessel density in oral tumorigenesis. J Oral Maxillofac Pathol. 2012;16:22–6. doi: 10.4103/0973-029X.92968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sedivy R, Beck-Mannagetta J, Haverkampf C, Battistutti W, Honigschnabil S. Expression of vascular endothelial growth factor C correlates with the lymphatic micro-vessel density and nodal status in oral squamous cell carcinoma. J Oral Pathol Med. 2003;32:455–60. doi: 10.1034/j.1600-0714.2003.00168.x. [DOI] [PubMed] [Google Scholar]
- 12.Shintani S, Li C, Ishikawa T, Mihara M, Nakashiro K, Hamakawa H. Expression of vascular endothelial growth factor A, B, C and D in oral squamous cell carcinoma. Oral Oncol. 2004;40:13–20. doi: 10.1016/s1368-8375(03)00127-1. [DOI] [PubMed] [Google Scholar]
- 13.Nagatsuka H, Hibi K, Gunduz M, Tsujigawa H, Tamamura R, Sugahara T, et al. Various Immunostaining patterns of CD 31, CD 34 and endoglin and their relationship with lymph node metastasis in oral squamous cell carcinomas. J Oral Pathol Med. 2005;34:70–6. doi: 10.1111/j.1600-0714.2004.00227.x. [DOI] [PubMed] [Google Scholar]
- 14.Tae K, El-Naggar AK, Yoo E, Feng L, Lee JJ, Hong WK, et al. Expression of vascular endothelial growth factor and microvessel density in head and neck tumorigenesis. Clin Cancer Res. 2000;6:2821–8. [PubMed] [Google Scholar]
- 15.Shieh YS, Lee HS, Shiah SG, Chu YW, Wu CW, Chang LC. Role of angiogenic and non-angiogenic mechanisms in oral squamous cell carcinoma: correlation with histologic differentiation and tumor progression. J Oral Pathol Med. 2004;33:601–6. doi: 10.1111/j.1600-0714.2004.00252.x. [DOI] [PubMed] [Google Scholar]
- 16.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–64. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 17.Bamdyopadhyay A, Raghavan S. Defining the role of integrin alphavbeta6 in cancer. Curr Drug Targets. 2009;10:645–52. doi: 10.2174/138945009788680374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas GJ, Hart IR, Speight PM, Marshall JF. Binding of TGF-beta1 latency-associated peptide (LAP) to alpha (v) beta6 integrin modulates behaviour of squamous carcinoma cells. Br J Cancer. 2002;87:859–67. doi: 10.1038/sj.bjc.6600545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fabricius EM, Wildner GP, Kruse-Boitschenko U, Hoffmeister B, Goodman SL, Raguse JD. Immunohistochemical analysis of integrins αvβ3, αvβ5 and α5β1, and their ligands, fibrinogen, fibronectin, osteopontin and vitronectin, in frozen sections of human oral head and neck squamous cell carcinomas. Exp Ther Med. 2011;2:9–19. doi: 10.3892/etm.2010.171. [DOI] [PMC free article] [PubMed] [Google Scholar]


