Abstract
Oral cancer is one of the ten leading cancers of the world. In India, it is one of the common cancers and is an important public health problem. Tobacco plays significant role in etiology of oral squamous carcinoma. Tobacco which is chewed or smoked contains many alkaloids which are known carcinogens. Oral submucous fibrosis (OSMF) is a disease of the Indian subcontinent, which through immigration has a worldwide distribution. Betel nut chewing plays significant role in etiology of OSMF. The nut alkaloids have been shown experimentally to result in stimulation of collagen synthesis by fibroblasts in vitro, which can induce precancerous conditions.
Materials and Methods:
The present study was undertaken to detect nitrate and nitrite factor in saliva of cases with oral carcinoma, OSMF and normal individuals without any habits and to determine whether increased salivary nitrate and nitrite level is significant in oral carcinoma and submucous fibrosis using biochemical parameters.
Conclusion:
We conclude that the major inducer of oral squamous cell carcinoma (OSCC) is exposure to tobacco. Recent studies have demonstrated that oxidative and nitrosative stress contributes to the development of oral carcinogenesis through deoxyribonucleic acid (DNA) damage. Salivary composition of OSCC patients is substantially altered with respect to free radical-involved mechanisms.
Keywords: Betel nut, nitrite, oral squamous cell carcinoma, oral submucous fibrosis, salivary nitrate
INTRODUCTION
Oral cancer is one of the ten leading cancers of the world. In India, it is one of the common cancers and is an important public health problem. In India every year, about 7% of all cancer deaths in males and 4% in females have been reported to be due to oral carcinoma. Tobacco plays significant role in etiology of oral squamous carcinoma. Tobacco which is chewed or smoked in some form or other contains many alkaloids and a compound N-nitrosonornicotine (NNN) which are known carcinogens. The possibility that a carcinogen is formed from nicotine was suggested in 1962 by Druckrey I and Prcussmann. They pointed out this alkaloid is a precursor of NNN.[1] Since then, interest in nitrosation of tobacco alkaloids has increased and several studies to study the formation and analysis of N-nitrosamines in tobacco products have been carried out.
Oral submucous fibrosis (OSMF) is a disease of the Indian subcontinent, which through immigration has a worldwide distribution. The prevalence rate varies from 0.2 to 0.5%. Betel nut chewing plays significant role in etiology of OSMF. The habit of chewing betel quid with or without tobacco is common among Indians. The nut alkaloids have been shown experimentally to result in stimulation of collagen synthesis by fibroblasts in vitro. In vitro experiments with arecoline and nitrite have shown that “areca nut” alkaloid can give rise to at least four N-nitrosamines, which can induce precancerous conditions.
In the present study salivary estimation of nitrate and nitrite were carried out in the patients of OSMF, oral carcinoma and in healthy persons with normal oral mucosa, devoid of any habits.
MATERIALS AND METHODS
A total of 52 cases were included in the present study for comparative evaluation from outpatient department of Oral Diagnosis and Department of Oral Pathology. Twenty-eight cases of oral carcinoma based on clinical and histological diagnosis and 24 cases of severe OSMF were selected for the present study. All the cases had betel nut chewing history or having tobacco habit in some form or the other. Since most of the cases were having difficulty in opening the mouth it was not possible to take biopsies for histopathological diagnosis. Hence, the selection of cases was done on the basis of clinical diagnosis only. Twenty-five normal persons having clinically normal oral mucosa and devoid of any habits were selected as control group.
Collection of saliva samples
Ten milliliter of whole unstimulated saliva samples were collected from all patients in the morning in a clean sterilized test tube. Saliva was immediately centrifuged for 30 min at 3,000 rpm to remove particulate matter. If sample contained suspended solids, it was filtered through a 0.45 nm pore diameter membrane filter.
Different methods used for estimation of salivary nitrate and nitrite levels are:
Preparation of chemical reagents
Stock nitrite solution
1.23320 g sodium nitrite was dissolved in 1,000 ml to get 1 ml of 250 μg. During analysis the stock solution was diluted from 1 ml to 500 ml with distilled water to get 1 ml of 0.5 μg.
Sulfanilamide reagent
Five grams of sulfanilamide powder was dissolved in 50 ml of concentrated HCL and 300 ml water. This solution was further diluted to 500 ml with distilled water.
N-(1-Naphthyl) ethylenediamine -dihydrochloride solution
Five hundred milligram of NED dihydrochloride was dissolved in 500 ml water. The solution was stored in a dark bottle.
Preparation of chemical reagents (for nitrate estimation)
Stock nitrate solution
72.8 mg anhydrous potassium nitrate was dissolved and diluted to 1,000 ml with distilled water 1 ml of 100 μg.
Phenol disulfonic acid
Twenty-five grams of white phenol was dissolved in 150 ml of concentrated H2SO4 was added and stirred.
Potassium hydroxide 12N
A total of 673 g KOH was dissolved and diluted in distilled water to get 1,000 ml solution.
Standard silver sulfate
4.40 g Ag2SO4 crystals were dissolved and diluted in 1,000 ml distilled water to get 1 ml of 1 mg Cl.
Detection of salivary nitrate factor
After collection of saliva from patient it was centrifuged for 30 min. If sample contained suspended solids, it was filtered through a 0-45 nm pore diameter. membrane filter. To 50 ml clear sample, that is, 5 ml saliva diluted to 50 ml with distilled water and neutralized to pH 7, 1 ml sulfanilamide solution was added. The reagent was allowed to react for 2-8 min. After the above step, 1 ml NED dihydrochloride solution was added and mixed immediately (the absorbance was measured after 10 min, but before 10 min the optical density was noted on calorimeter). Nitrite level was estimated by referring the standard graph.
Sample processing for nitrite estimation
Following steps were followed for pretreatment of sample:
Color removal: if the sample has a color in excess of 10 units, 3 ml aluminium hydroxide to 150 ml sample was added, stirred well and allowed to settle for a few minutes
Nitrite removal: sulfonic acid was added to the sample to suppress nitrite interference
Chloride removal: the chloride content of the sample was determined and precipitated out as AgCl by adding appropriate amount of Ag2SO4 solution. The clarified sample is neutralized to pH 7. Suitable aliquot of the sample was taken and evaporated to dryness on water bath. The residue was dissolved using glass rod with 2 ml phenol disulfonic acid reagent. The solution was diluted and transferred to Nessler tubes. Ten milliliter potassium hydroxide (12N) was added in development (yellow color). The intensity of color developed was measured at 410 nm with a light path of 1 cm using calorimeter. The concentration of nitrate in sample was found out by referring the standard graph.
Preparation of calibration curve for nitrate
Aliquots of standard nitrate solution in the range of 0.1-10 mg NO3-N/L were taken using 50 ml as a final volume. The solution was evaporated to dryness on water bath. The residue was dissolved using glass rod with 2 ml phenol disulfonic acid reagent. It was diluted with distilled water and transferred to Nessler tube. Then 8 ml of 12N KOH was added and made to 100 ml. The color developed was measured at 410 nm with a light path of 10 m. Calibration curve was drawn on cm-cm graph paper using optical density against concentration of nitrate. This graph was used for calculating nitrate in the samples [Figure 1].
Figure 1.
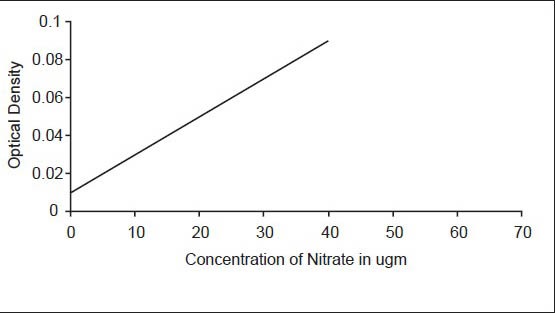
Salivary nitrate level
Preparation of calibration curve for nitrite
For plotting the calibration curve, aliquots of standard nitrite solution in the range of 1 μg NO2-N to 10 μg NO2-N were taken using 1 cm light path and considering 50 ml as a final volume. One milliliter sulfonamide solution was allowed to react for 2-8 min, followed by 1 ml of NED di-hydrochloride solution and mixed immediately. It was kept for 10 min for maximum color development. Absorbance was recorded after 10 min at 543 nm. Calibration curve was drawn on cm-cm graph paper using optical density against concentration of nitrite. This graph was used for calculating nitrite in the samples [Figure 2].
Figure 2.
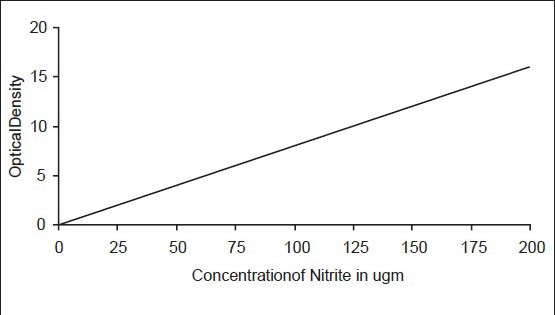
Salivary nitrite level
RESULTS
The age of the patients in oral carcinoma ranged from 30 to 70 years and showed a slight predilection towards males. Tables 1 and 2 shows the anatomical distribution of these lesions in the oral cavity. The salivary nitrate level in control group ranged from 75 to 250 μg/L which is in accordance with other studies. The salivary nitrite level in control group ranged from 30 to 100 μg/L. Salivary nitrate and nitrite levels did not differ significantly between men and women. Elderly persons showed increase in the levels of both salivary nitrate and nitrite. The salivary nitrate and nitrite level was not significant in OSMF patients when statistically compared to control group, except in patients with tobacco habits in some form or other and in OSMF patients who showed carcinomatous changes.
Table 1.
Anatomical distribution of oral squamous cell carcinoma patients
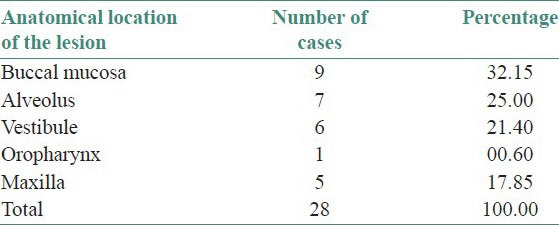
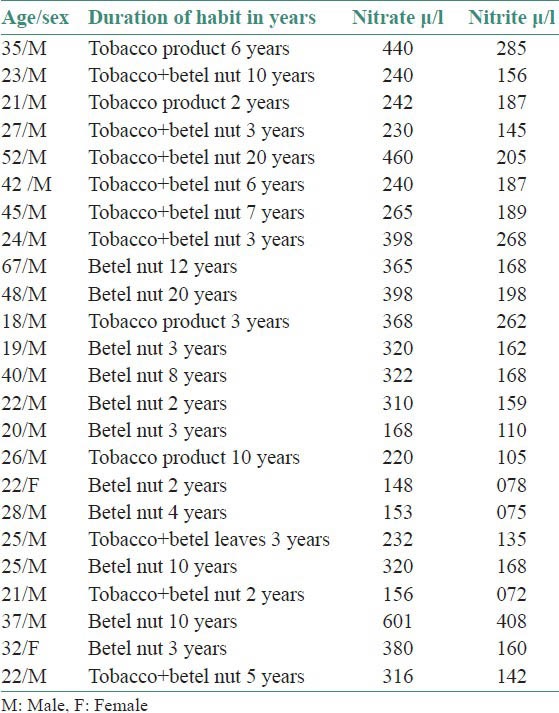
Table 2.
Salivary nitrate/nitrite level in oral submucous fibrosis patients
The salivary nitrate and nitrite level of oral carcinoma patients when compared with control group showed a significant increase. The rate of reduction of salivary nitrate to nitrite of oral carcinoma patients was compared with OSMF patients. The rate of reduction of oral carcinoma patients from salivary nitrate to nitrite was significant compared to OSMF patients and control group. It was interesting to note that the sites where the tobacco quid was kept for longer length of time were constantly exposed to nitrosoamine and other nitroso compounds. Sites such as buccal mucosa, alveolus and vestibule showed highest percentage of oral carcinoma lesions.
DISCUSSION
Oral carcinoma is one of the ten leading cancers in the world and tobacco plays a significant role in etiology of oral squamous cell carcinoma. The possibility that a carcinogen is formed from nicotine was suggested in (1962) when Druckrey I and Preussmann pointed out this alkaloid as a precursor of NNN. Salivary nitrates in the oral cavity are turned into nitrites (NO2-), which are of special importance as carcinogenesis promoters because they react with amines and amides to form the carcinogenic nitrosamines. The salivary nitrate level in control group ranged from 75 to 250 μg/L and salivary nitrite level ranged from 30 to 100 μg/L. The range of salivary nitrate/nitrite level reported by Green and Laura for persons without any habits is 200-600 μg (for nitrate average, 300 μg) and 30-210 μg (for nitrite average, 80 μg).[5]
According to Tenovuo, human whole saliva may also contain inhibitory agents against the nitrosation reactions. Individual variations in the amounts of these inhibitory agents may be casually linked to individual's susceptibility to oral carcinoma. Hence, there are variations in nitrate and nitrite level.[6] Hart and Walter reported that, since the salivary glands are active in concentrating nitrate in a similar manner to iodide, there is unlikely to be a reservoir in the serum. This implies that the salivary systems promoting the reduction of nitrate to nitrite can vary considerably in activity from one individual to another.[7] Wolff and Wasserman observed that in the quantities normally occurring in food or feed, nitrates became toxic only under conditions in which they were, or might be reduced to nitrites. Otherwise at reasonable concentrations, nitrate ions were rapidly excreted in the urine. Nitrate are not toxic, but may under some circumstances be the starting point for a chain of reaction that result in the conversion to toxic substances. The microbial environment causes reduction of nitrate to nitrite. Nitrites are more toxic than nitrates. Consumption levels that may cause long-term hazards in man have not been established.[8]
In the human oral cavity, nitrate secreted as a salivary component is reduced to nitrite and NO by certain bacteria, and salivary nitrite may be transformed to NO, NO2 and N2O3, which can lead to tyrosine nitration.[9,10] The concentration of nitrate in saliva is dependent on the amount of nitrate ingested.[11] The nitrate in saliva is reduced to nitrite and NO successively by bacteria such as Streptococcus salivarius, S. mitis and S. bovis. Thus, it seems that oral bacteria are one of the main sources of reduced nitrite in saliva.[12,13] The concentration of nitrite in saliva is dependent on the concentration of nitrate. The nitrite and NO formed in the human oral cavity can be oxidized by molecular oxygen and by salivary peroxidase.[8,14]
Nitrate and nitrite in oral carcinoma
In the present study, the salivary nitrate and nitrite level of OSMF patients [Table 2] and salivary nitrate/nitrite level of oral cancer patients [Table 3] were corelated statistically with normal persons having normal oral mucosa and without any habits. Oral carcinoma patients in the present study showed a significant increase in salivary nitrate and nitrite level compared to control group. The study of Sipahimalani et al., showed that the saliva of habitual tobacco chewers contained nitrosamines that were probably leached from the tobacco and/or formed in situ by the nitrosation of tobacco alkaloids as well as that of secondary amines. It also indicated that the mucosa of the oral cavity of tobacco chewers might be constantly exposed to nitrosamines and other nitroso compounds.[4]
Table 3.
Salivary nitrate/nitrite level in oral carcinoma patients
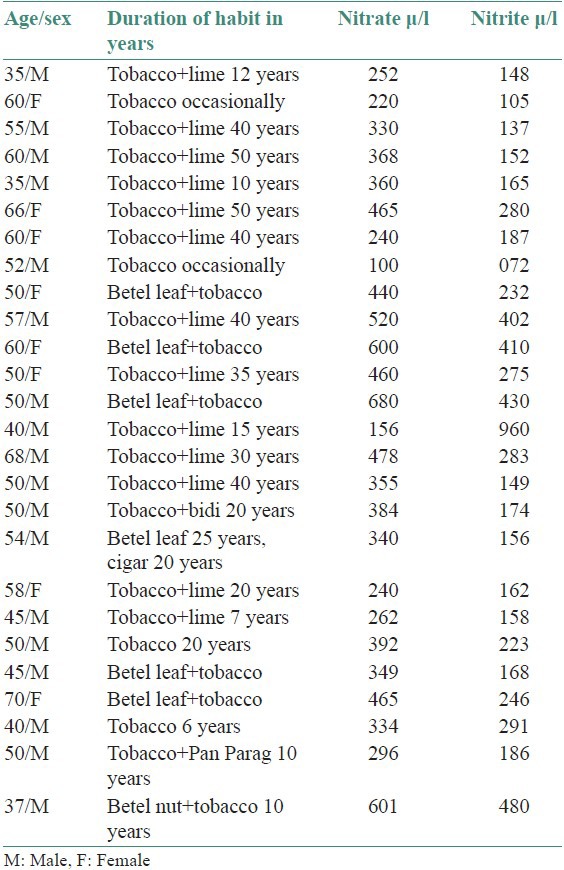
According to Tenovuo this observation might be linked to the reported increased prevalence of oropharyngeal carcinoma in tobacco chewers.[6] In the present study carcinogenic action of chewed tobacco is evident at those sites where the tobacco quid is kept for longer duration of time. In the present study, salivary nitrate/nitrite levels did not differ significantly between men and women. Most of the oral carcinoma patients in present study are elderly, in the age group of 35-70 years, the average age being 45 years. The rate of salivary nitrate to nitrite reduction in the present study is significant in oral carcinoma patients than normal persons and OSMF patients. Tenovuo reported that increasing age was generally associated with an increase in the levels of both salivary nitrate and nitrite and these levels did not differ significantly between men and women.[6]
In the present study, the OSMF patient who gave initial 8 year history of areca nut chewing and later for 2 years he started chewing tobacco products in combination with areca nut developed oral carcinoma. We can conclude that the major inducer of OSCC is exposure to tobacco. Recent studies have demonstrated that oxidative and nitrosative stress contributes to the development of oral carcinogenesis through DNA damage. Salivary composition of OSCC patients is substantially altered with respect to free radical-involved mechanisms.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Khandekar SP, Bagdey PS, Tiwari RR. Oral cancer and some epidemiological factors: A hospital based study. Indian J Community Med. 2006;31:157–9. [Google Scholar]
- 2.Shinn MB. Colorimetric method for determination of Nitrite. Industrial and Engineering chemistry. Analytical Edition. 1941;13:33–35. [Google Scholar]
- 3.Tsai-Yi F, Tannenbaum SR. Automatic colorimetric determination of N-nitroso compounds. J Agr Food Chem. 1971;19:1267–9. [Google Scholar]
- 4.Sipahimalani AT, Chadha MS, Bhide SV, Pratap AI, Nair J. Detection of N-Nitrosamines in the saliva of Habitual chewers of tobacco. Food Chem Toxicol. 1984;22:261–4. doi: 10.1016/0278-6915(84)90003-6. [DOI] [PubMed] [Google Scholar]
- 5.Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite and [N] Nitrate in biological fluids. Anal Biochem. 1982;126:131–8. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 6.Tenovuo J. The biochemistry of nitrates, nitrites, nitrosamines and other potential carcinogens in human saliva. J Oral Pathol. 1986;15:303–7. doi: 10.1111/j.1600-0714.1986.tb00630.x. [DOI] [PubMed] [Google Scholar]
- 7.Hart RJ, Walter CL. The formation of Nitrite and N-nitroso compounds in Saliva in vitro and in vivo. Food Chem Toxicol. 1953;21:749–53. doi: 10.1016/0278-6915(83)90208-9. [DOI] [PubMed] [Google Scholar]
- 8.Wolff IA, Wasserman AE. Nitrates, nitrites and nitrosamines. Science. 1972;177:15–19. doi: 10.1126/science.177.4043.15. [DOI] [PubMed] [Google Scholar]
- 9.Deng DJ. Progress of gastric cancer etiology: n-nitrosamides. World J Gastroenterol. 2000;6:613–8. doi: 10.3748/wjg.v6.i4.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tannenbaum SR, Sinskey AJ, Weisman M, Bishop W. Nitrite in human saliva. Its possible relationship to Nitrosamine formation. J Natl Cancer Inst. 1974;53:79–84. [PubMed] [Google Scholar]
- 11.Hawksworth GM, Hill MJ. Bacteria and the N-Nitrosation of secondary amines. J Cancer. 1971;25:520–6. doi: 10.1038/bjc.1971.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Wyk CW, Olivier A, de Miranda CM, van der Bijl P, Grobler-Rabie AF. Observations on the effect of areca nut extracts on oral fibroblast proliferation. J Oral Pathol Med. 1994;23:145–8. doi: 10.1111/j.1600-0714.1994.tb01103.x. [DOI] [PubMed] [Google Scholar]
- 13.Walters CL, Carr FP, Dyke CS, Saxby MJ, Smith PL, Walker R. Nitrate sources and nitrosamine formation in vitro and in vivo. Food Cosmet Toxicol. 1979;17:473–9. doi: 10.1016/0015-6264(79)90006-3. [DOI] [PubMed] [Google Scholar]
- 14.Walters CL, Smith PL. The effect of water borne nitrate on salivary nitrate. Food Cosmet Toxicol. 1981;19:297–302. doi: 10.1016/0015-6264(81)90388-6. [DOI] [PubMed] [Google Scholar]


