Abstract
We have attempted to identify the covalent structure of the M protein molecule of group A streptococci that is responsible for inducing type-specific, protective immunity. M protein was extracted from type 24 streptococci, purified, and cleaved with cyanogen bromide. Seven cyanogen bromide peptides were purified and further characterized. Together, the peptides account for the entire amino acid content of the M protein molecule. Each of the purified peptides possessed the type-specific determinant that inhibits opsonic antibodies for group A streptococci. The primary structures of the amino-terminal regions of each of the purified peptides was studied by automated Edman degradation. The partial sequences of two of the peptides were found to be identical to each other and to that of the uncleaved M protein molecule through at least the first 27 residues. The amino-terminal sequences of the remaining five peptides were identical to each other through the twentieth residue but completely different from the amino-terminal region of the other two peptides. However, the type-specific immunoreactivity and the incomplete analysis of the primary structure of the seven peptides suggest that the antiphagocytic determinant resides in a repeating amino acid sequence in the M protein molecule.
Full text
PDF
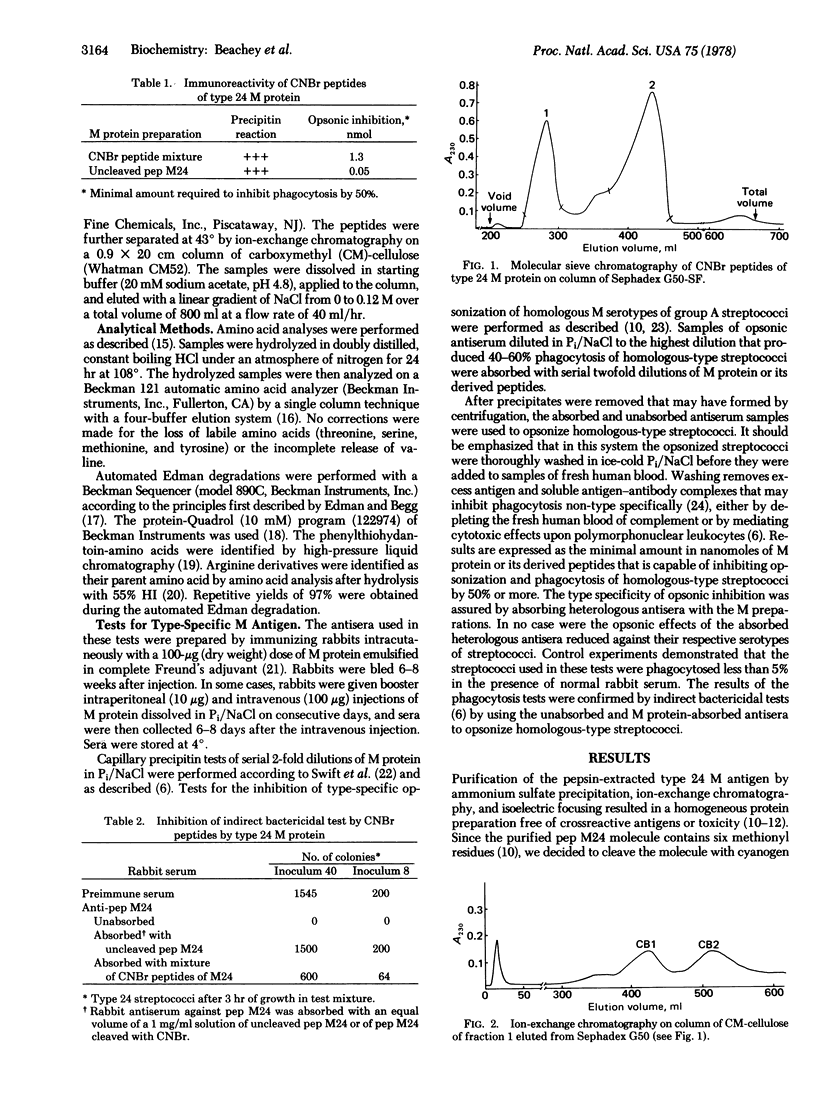
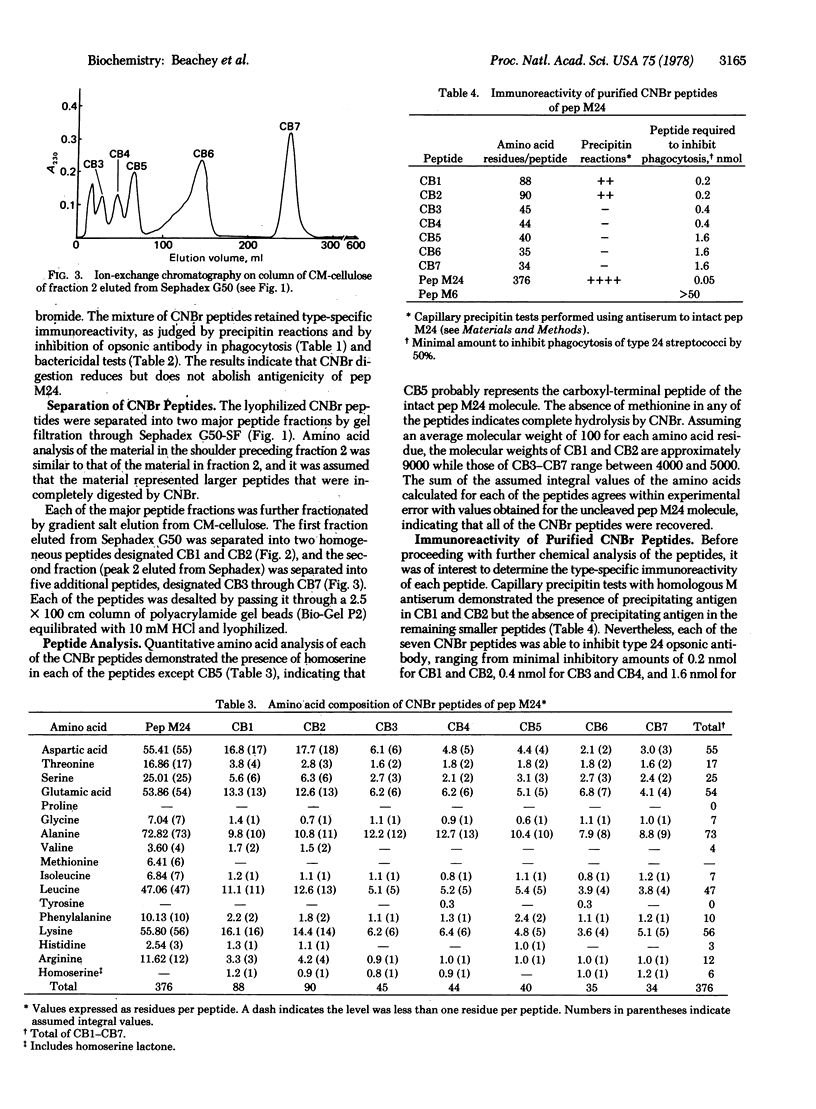


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beachey E. H., Alberti H., Stollerman G. H. Delayed hypersensitivity to purified streptococcal m protein in guinea pigs and in man. J Immunol. 1969 Jan;102(1):42–52. [PubMed] [Google Scholar]
- Beachey E. H., Campbell G. L., Ofek I. Peptic digestion of streptococcal M protein. II. Extraction of M antigen from group A streptococci with pepsin. Infect Immun. 1974 May;9(5):891–896. doi: 10.1128/iai.9.5.891-896.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beachey E. H., Chiang E. Y., Seyer J. M., Kang A. H., Chiang T. M., Stollerman G. H. Separation of the type specific M protein from toxic cross reactive antigens of group A streptococci. Trans Assoc Am Physicians. 1977;90:390–400. [PubMed] [Google Scholar]
- Beachey E. H., Cunningham M. Type-specific inhibition of preopsonization versus immunoprecipitation by Streptococcal M proteins. Infect Immun. 1973 Jul;8(1):19–24. doi: 10.1128/iai.8.1.19-24.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beachey E. H., Ofek I., Bismo A. L. Studies of antibodies to non-type-specific antigens associated with streptococcal M protein in the sera of patients with rheumatic fever. J Immunol. 1973 Nov;111(5):1361–1366. [PubMed] [Google Scholar]
- Beachey E. H., Stollerman G. H., Chiang E. Y., Chiang T. M., Seyer J. M., Kang A. H. Purification and properties of M protein extracted from group A streptococci with pepsin: covalent structure of the amino terminal region of type 24 M antigen. J Exp Med. 1977 Jun 1;145(6):1469–1483. doi: 10.1084/jem.145.6.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beachey E. H., Stollerman G. H. Mediation of cytotoxic effects of streptococcal M protein by nontype-specific antibody in human sera. J Clin Invest. 1973 Oct;52(10):2563–2570. doi: 10.1172/JCI107448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beachey E. H., Stollerman G. H. Toxic effects of streptococcal M protein on platelets and polymorphonuclear leukocytes in human blood. J Exp Med. 1971 Aug 1;134(2):351–365. doi: 10.1084/jem.134.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker C. G. Enhancing effect of type specific antistreptococcal antibodies on emergence of streptococci rich in M-protein. Proc Soc Exp Biol Med. 1967 Jan;124(1):331–335. doi: 10.3181/00379727-124-31736. [DOI] [PubMed] [Google Scholar]
- Cunningham M. W., Beachey E. H. Peptic digestion of streptococcal M protein. I. Effect of digestion at suboptimal pH upon the biological and immunochemical properties of purified M protein extracts. Infect Immun. 1974 Feb;9(2):244–248. doi: 10.1128/iai.9.2.244-248.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham M., Beachey E. H. Immunochemical properties of streptococcal M protein purified by isoelectric focusing. J Immunol. 1975 Oct;115(4):1002–1006. [PubMed] [Google Scholar]
- Dixit S. N., Seyer J. M., Oronsky A. O., Corbett C., Kang A. H., Gross J. Covalent structure of collagen: amino acid sequence of alpha1-CB6A of chick skin collagen. Biochemistry. 1975 May 6;14(9):1933–1938. doi: 10.1021/bi00680a020. [DOI] [PubMed] [Google Scholar]
- Edman P., Begg G. A protein sequenator. Eur J Biochem. 1967 Mar;1(1):80–91. doi: 10.1007/978-3-662-25813-2_14. [DOI] [PubMed] [Google Scholar]
- Fischetti V. A., Gotschlich E. C., Siviglia G., Zabriskie J. B. Streptococcal M protein extracted by nonionic detergent. I. Properties of the antiphagocytic and type-specific molecules. J Exp Med. 1976 Jul 1;144(1):32–53. doi: 10.1084/jem.144.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox E. N., Wittner M. K., Dorfman A. Antigenicity of the M proteins of group A hemolytic streptococci. 3. Antibody responses and cutaneous hypersensitivity in humans. J Exp Med. 1966 Dec 1;124(6):1135–1151. doi: 10.1084/jem.124.6.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox E. N., Wittner M. K. New observations on the structure and antigenicity of the M proteins of the group A streptococcus. Immunochemistry. 1969 Jan;6(1):11–24. doi: 10.1016/0019-2791(69)90174-8. [DOI] [PubMed] [Google Scholar]
- Fox E. N., Wittner M. K. The multiple molecular structure of the M proteins of group A streptococci. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1118–1125. doi: 10.1073/pnas.54.4.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havlícek J., Alouf J. E., Raynaud M. Hétérogénéité antigénique de la protéine M de Streptococcus pyogenes type 24. Ann Inst Pasteur (Paris) 1969 Dec;117(6):745–755. [PubMed] [Google Scholar]
- Kang A. H. Studies on the location of intermolecular cross-links in collagen. Isolation of a CNBr peptide containing -hydroxylysinonorleucine. Biochemistry. 1972 May 9;11(10):1828–1835. doi: 10.1021/bi00760a015. [DOI] [PubMed] [Google Scholar]
- LANCEFIELD R. C., PERLMANN G. E. Preparation and properties of type-specific M antigen isolated from a group A, type 1 hemolytic streptococcus. J Exp Med. 1952 Jul;96(1):71–82. doi: 10.1084/jem.96.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smithies O., Gibson D., Fanning E. M., Goodfliesh R. M., Gilman J. G., Ballantyne D. L. Quantitative procedures for use with the Edman-Begg sequenator. Partial sequences of two unusual immunoglobulin light chains, Rzf and Sac. Biochemistry. 1971 Dec 21;10(26):4912–4921. doi: 10.1021/bi00802a013. [DOI] [PubMed] [Google Scholar]
- Stollerman G. H. Prospects for a vaccine against group A streptococci: the problem of the immunology of M proteins. Arthritis Rheum. 1967 Jun;10(3):245–255. doi: 10.1002/art.1780100311. [DOI] [PubMed] [Google Scholar]
- Vosti K. L., Johnson R. H., Dillon M. F. Further characterization of purified fractions of M protein from a strain of group A, type 12 Streptococcus. J Immunol. 1971 Jul;107(1):104–114. [PubMed] [Google Scholar]
- WAHL R., DRACH G., CAYEUX P. ALLERGIE CUTAN'EE RETARD'EE DU LAPIN 'A STREPTOCOCCUS PYOGENES (GROUPE A). ROLE DES PROT'EINES BASIQUES ET EN PARTICULIER DE LA PROT'EINE M. Ann Inst Pasteur (Paris) 1964 Jan;106:58–78. [PubMed] [Google Scholar]
- Zimmerman C. L., Pisano J. J., Appella E. Analysis of amino acid phenylthiohydantoins by high speed liquid chromatography. Biochem Biophys Res Commun. 1973 Dec 19;55(4):1220–1224. doi: 10.1016/s0006-291x(73)80024-5. [DOI] [PubMed] [Google Scholar]


