Abstract
Introduction:
The microwave oven has been used quite often for tissue processing, but there are very few studies describing its use in decalcification of bone or teeth. In this study we have attempted to decalcify bone and teeth using a microwave oven and compare the process and results with conventional decalcification methods.
Aims and objectives:
The objectives of the study were to determine and compare routine decalcification with microwave decalcification of bone and teeth using 5% nitric acid, 5% formic acid, and 14% ethylenediaminetetraacetic acid (EDTA) with respect to speed of decalcification, preservation of tissue structure and staining efficacy.
Materials and methods:
In our study the total sample size used for both routine and microwave decalcification was 30 premolar teeth and 30 pieces of condyles. The three solutions were dilute nitric acid (5%), formic acid (5%), and EDTA (14%). Each set consisting of the same type of premolars and condyles in each of the three decalcifying solutions were used in both manual method and microwave method.
Results:
The results in the present study confirmed the fact that the microwave method using nitric acid was indeed the fastest decalcifying method needing just about 2 days for condyle and 4 days for premolars, compared with routine decalcification. The results also showed that the overall histological picture was good with EDTA and formic acid irrespective of the methods used. In the routine method, nitric acid gave poor cellular detail when compared with microwave method.
Conclusion:
With our study we conclude that microwave oven decalcification is faster than routine decalcification irrespective of the decalcifying agents used. The tissue preservation and staining efficacy was good in microwave nitric acid decalcification compared to routine nitric acid decalcification. Both formic acid and EDTA show good tissue preservation and staining efficacy irrespective of the method used.
Keywords: Bone, condyle, decalcification, microwave oven, teeth
INTRODUCTION
Preservation of hard tissues close to the living state is essential for understanding of cellular and subcellular structures and functions. The cutting of thin sections by ordinary methods is impossible in the case of tissues such as teeth, bone, teratomas containing bony tissue, lesions that have become partly calcified, odontomes and bony lesions. Such tissues must be treated to remove calcium phosphate by a process known as “decalcification”, thereby making the tissue soft enough to be cut by the microtome.[1]
Decalcification of hard tissue is one of the most technique-0sensitive procedures in the histopathology laboratory. It is of special significance in oral pathology as decalcification of bone and teeth is a routinely required procedure.[2]
Decalcification is carried out by chemical agents, either with acids to form soluble calcium salts or chelating agents that bind to calcium ions.[3]
In the manual method of decalcification, hard tissues are placed in a decalcifying agent at room temperature with changes of the solution at regular intervals till the end point is reached. Microwave decalcification is a novel technique compared to the manual method. In this method, hard tissues are placed in the decalcifying agent in a microwave oven for intermittent periods with regular changes of the solution till the end point is reached. Microwave irradiation has been shown to speed up the process of decalcification significantly–from days to hours.[4]
Balaton and Loget (1989) have reported that the decalcification of bone is accelerated about 10 times compared with that at ambient temperature.[5]
The aim of the present study was to determine and compare routine decalcification with microwave decalcification of bone and teeth using nitric acid, formic acid and EDTA with respect to speed of decalcification, preservation of tissue structure and staining efficacy.
MATERIALS AND METHODS
Sample collection
Premolars extracted for orthodontic reasons and sections of mandibular condyle from specimens of resected mandibles from the archives of the Department of Oral and Maxillofacial Pathology were used in the study.
Thirty premolar teeth and 30 pieces of condyles were decalcified by both routine and microwave method using three decalcifying solutions (five condyle pieces and five teeth in each). The three solutions were dilute nitric acid (5%), formic acid (5%) and ethylene diamine tetra acetic acid (14%). These solutions were chosen as they are commonly used and easily available.
Microwave setup
A domestic microwave oven (LG Intellowave, Model 1911HE) with a fixed rotary plate, maximum power output of 700 W and input voltage 230 V-50 HZ was used. A glass beaker containing 100 ml of distilled water was preheated for 5 s to warm up the magnetron. This was replaced by100 ml fresh distilled water and irradiated to maintain the temperature at around 41-43°C. This took 14 s. The glass beaker was placed at different points in the oven while irradiating it to determine the best position of the specimen during microwave decalcification, since the microwave oven used had a constant timing but not a constant temperature [Figure 1a and b].
Figure 1.

(a) Microwave oven with beaker containing decal solution and specimen. (b) Housing for microwave oven with outlet for fumes
All the specimens were fixed in 10% neutral buffered formalin fixative and then washed in water for about 30 min before decalcification.
Decalcification
All the specimens were weighed and labeled for standardization of procedure. Each sample was then suspended in a Coplin jar with the help of a thread in approximately 100 ml of decalcifying agent for decalcification. The exact time at the start of decalcification was noted. The decalcifying solutions were changed and pH and temperature of the solutions were recorded on a daily basis.
In the manual decalcification procedure, the solution was changed once in 3 days and the pH and temperature were recorded on a daily basis. In the microwave technique, the specimens were irradiated for eight cycles of 8 s each (at 1-h intervals) per day for formic and nitric acids, and eight cycles of 10 s each at 1-h intervals per day for EDTA so that the temperature of all three decalcifying solutions was maintained at around 41-43°C. The decalcifying solution was changed every day and the end-point was ascertained on a daily basis using the calcium oxalate method (Clayden 1952).[3]
Tissue processing and staining
After ensuring complete decalcification, the tissues were washed using distilled water for 30 min, following which the specimens were subjected to manual tissue processing. The condyle pieces were taken whole and teeth were each cut into two halves, both longitudinally and transversely. After processing, the tissues were embedded in paraffin and were sectioned to a thickness of 7-8 μm using the soft tissue microtome. The sections were then stained by Harris’ hematoxylin and eosin Y.
The stained sections of decalcified bone and teeth were assessed for the quality of staining and preservation of tissue details as objectively as possible. The following parameters were recorded for each specimen:
For condyle pieces,
Missing osteocytes from the lacunae
For teeth,
Shrinkage of pulp away from the dentinal wall
Damage to odontoblastic layer
For both,
Yellow discoloration of specimen
Tearing and crumpling of section
Patchy staining.
The data obtained from the above observations were entered and analyzed using Statistical Package for Social Sciences (SPSS) version 10.5 IBM. Descriptive statistics for all variables were presented.
RESULTS
The decalcification of condyles in nitric acid, formic acid and EDTA took 22, 33 and 57 days, respectively for complete decalcification by the manual method; whereas, the microwave oven technique took 2, 6 and 9 days, respectively [Graph 1a].
Graph 1.
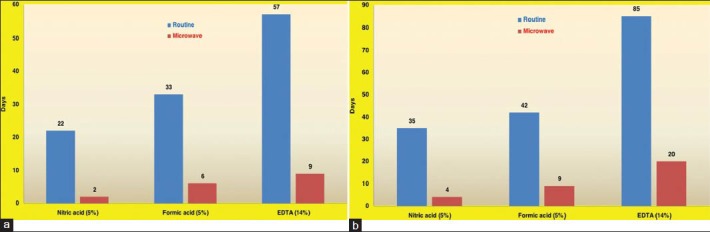
(a) Comparison of duration of condyle decalcification by both methods (b) Comparison of duration of tooth decalcification by both methods
The decalcification of premolars in nitric acid, formic acid and EDTA took 35, 42 and 85 days, respectively for complete decalcification whereas the microwave oven technique took 4, 9 and 20 days, respectively [Graph 1b].
The stained sections of decalcified bone and teeth were assessed for the preservation of tissue details and quality of staining. The presence of osteocytes within their lacunae [Graph 2a] was not statistically significant; but the percentages show there is better preservation of tissue in the microwave oven method compared to routine method with all three decalcifying agents [Figure 2].
Graph 2.
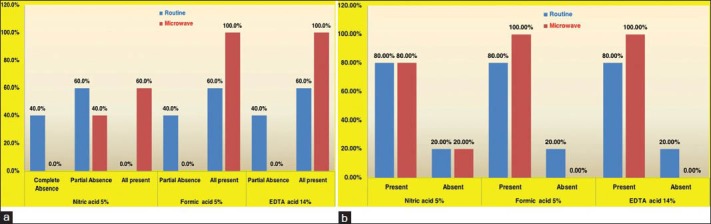
(a) Distribution of osteocytes visible in the lacunae in bone sections (b) Distribution of pulp shrinkage in tooth sections
Figure 2.
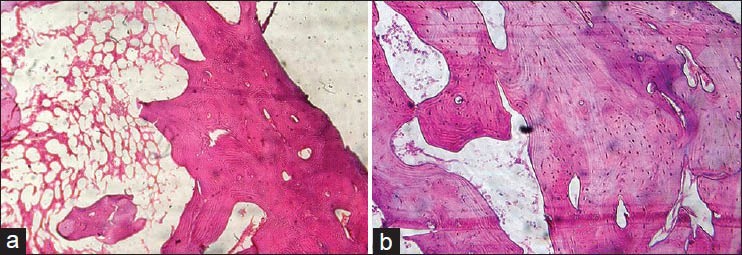
(a) Histologic section of bone decalcified by manual method using 5% nitric acid (H&E stain, ×100). (b) Histologic section of bone decalcified by microwave method using 5% nitric acid (H&E stain, ×100)
Shrinkage of the pulp away from dentinal wall [Graph 2b] was observed to be 80% for all three decalcifying agents in the routine method. In the microwave method, this feature was seen in 80%, 100% and 100% in nitric acid, formic acid and EDTA, respectively. These results were not statistically significant.
The presence of damaged odontoblastic layer [Graph 3a] was minimal (20%) in the samples treated with nitric acid by microwave method when compared to routine method (80%). Though it was also not found to be statistically significant, the percentages show tissue preservation to be better in the microwave method as compared to routine method. All the teeth samples treated with formic acid and EDTA showed no damage to the odontoblastic layer [Figure 3]
Graph 3.
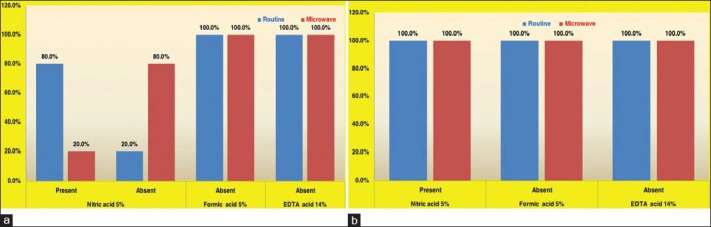
(a) Distribution of damaged odontoblastic layer in tooth sections (b) Distribution of yellow discoloration in bone and tooth sections
Figure 3.
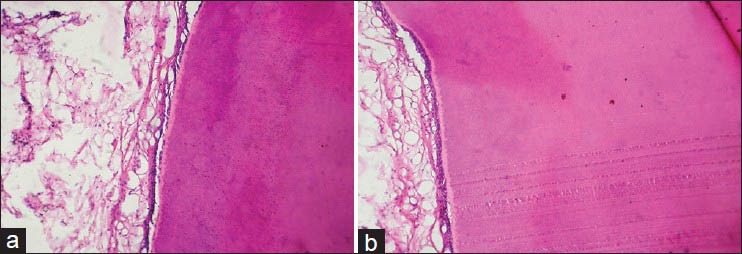
(a) Histologic section of tooth decalcified by manual method using EDTA (H&E stain, ×100) (b) Histologic section of tooth decalcified by microwave method using EDTA (H&E stain, ×100)
Yellow discoloration of the condyle and teeth specimens [Graph 3b] was present in 5% nitric and absent in formic acid and EDTA irrespective of the method used.
The presence of patchy staining [Graph 4] was 100% in nitric acid decalcification by manual method compared to 40% in microwave method. This difference was statistically significant and shows staining efficacy to be better in the microwave method compared to routine method using nitric acid. But in formic acid and EDTA, all the samples showed patchy staining in both the methods.
Graph 4.
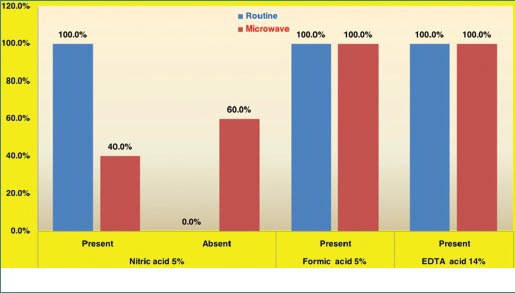
Distribution of patchy staining of bone and tooth sections
DISCUSSION
Bone decalcification is a time-consuming process. It takes weeks and preservation of the tissue structure depends on the quality and velocity of the demineralization process. A new method using microwave oven was seen to accelerate the decalcification.[6] The choice of decalcifying agent and method is largely dictated by the urgency of the procedure.[7]
The potential application of microwave energy in histotechnology was first recognized by Mayers (1970). This form of nonionizing radiation produces alternating electromagnetic fields that result in the rotation of dipolar molecules such as water and the polar side chains of proteins through 180° C at the rate of 2.45 billion cycles/second. The molecular kinetics so induced result in the generation of energy flux which continue until radiation ceases.[8]
The idea of using microwaves to decrease the time for decalcification of temporal bones was originally introduced by Hellstrom and Nilsson (1992) for rat cochleas. More recently, microwaves have been demonstrated to be useful in reducing the time needed for decalcification in EDTA of dense, primate temporal bones (Madden and Henson, 1997).[9]
The energy produced by microwaves generated in a domestic oven interacts with dipolar molecules by imparting kinetic energy and altering the electric fields. This energy induces a dielectric field leading to a rapid oscillation of dipolar molecules at about 180°C, generating heat that is rapidly distributed homogeneously within the tissue.[6]
Pitol et al, (2007) showed there was a 30-fold increase in decalcification speed compared to the traditional method when the material was irradiated in a microwave oven.[6] However, Balaton and Loget (1989) reported that the decalcification of bone is accelerated about 10 times in the microwave oven compared with that at ambient temperature.[5]
Pitol et al., used a domestic microwave oven for decalcification of rat bone using EDTA 8.5% solution and showed a reduction of experiment time from 45 days in the conventional method to 48 h in the microwave-aided method.[6] In our study 14% EDTA solution took 57 days in the conventional method and 9 days using microwaves for complete decalcification of the condyle.
Our decalcification of condyles in routine nitric acid took 22 days; whereas, the microwave oven technique took 2 days. Similar results were obtained by Balaton and Loget (1989).[5]
Our decalcification of condyle in routine 5% formic acid took 33 days; whereas, the microwave oven technique took 6 days. Similar results were obtained by Roncaroli et al, (1991).[4] They used a kitchen microwave oven for bone biopsies in 5% formic acid.
Impressions of histological sections are subjective and affected by many variables, for example, fixation, processing, cutting technique, staining timings, etc.[3] It is important for histological analysis that the sections be obtained with the least possible alterations during processing.[6] Damaged odontoblastic layer, shrinkage of pulp away from dentinal wall, and presence of osteocytes within the lacunae may be affected by fixation as well as by the choice of decalcifying agent. Strong acids are faster in their action than EDTA and formic acid, but need to be monitored closely as they carry an increased risk of tissue damage due to hydrolysis of proteins, which can result in maceration or the dissolution of the soft tissue components, with possible complete loss of histological detail.[3]
In our study the presence of osteocytes within the lacunae was observed in all the specimens decalcified using microwave method with all the three decalcifying agents, whereas they were completely absent in nitric acid and partially absent in formic acid and EDTA specimens decalcified by routine method.
Shrinkage of pulp away from dentinal wall in teeth may be affected by fixation and processing techniques as well as by decalcifying agents. In our study, shrinkage of pulp away from dentinal wall in teeth was seen in both the methods using all the decalcifying agents, with statistically insignificant results.
Strong acids such as nitric acid can decalcify rapidly, but cause serious deterioration of stainability reviewed in Stevens et al, (1990) and Callis and Sterchi (1998). Using chelating agents such as EDTA for decalcification might circumvent this problem. Decalcification in EDTA has little or no effect on tissues other than on the bone mineral itself. It only binds with calcium ions and gradually depletes the crystal size of the outer layer of the hydroxyapatite crystal. However, the major disadvantage is that decalcification by EDTA proceeds only slowly, with incubation times up to several weeks depending on the extent of mineralization.[10]
In our study, damaged odontoblastic layer in teeth was seen in most of the specimens in the routine nitric acid method; whereas, the microwave method showed significant absence of damage to the odontoblastic layer in teeth. In case of formic acid and EDTA, both methods showed absence of damage to the odontoblastic layer in teeth. The study in this criterion demonstrates that by microwave method one can preserve the tissue details almost precisely.
Moore (1994) noted that the yellow staining of tissue which results from prolonged decalcification with nitric acid solutions may detract from the macroscopic appearance, but does not affect the histological examination since the color leaches from the specimen during processing.[11] Culling states that this yellow discoloration may interfere with subsequent staining.[12] Stanley (1983) hypothesized that the specimen decalcified in nitric acid underwent a spontaneous yellow discoloration due to formation of nitrous oxide, which was responsible for the damage to the tissues.[1] In our study, we observed the yellowish discoloration in all the specimens decalcified by nitric acid by both methods and the discoloration was totally absent in the specimens decalcified by formic acid and EDTA in both the methods.
Regarding patchy staining, the microwave nitric acid method shows a significant reduction in presence of patchy staining when compared to the routine method. The other observation made in this study is that the overall staining of the tissue was similar in both the techniques except in the routine nitric acid method which showed inadequate nuclear staining.
The overall histological impression in the microwave method compared to routine method was significantly better. Cellular structures could be well-appreciated in all the sections of EDTA and formic acid. Nitric acid gave poor cellular detail, but was rapid in its action.
Limitations of the present study
A larger sample size would have given more conclusive results
The times taken for decalcification are likely to be different if used on different weights of bone and other teeth than premolars, as the decalcification time is dependent on the size and structural density of the hard tissue
Since we have used a domestic microwave oven, our recording of the temperatures may have been only approximate.
CONCLUSION
The present study provided an insight into routine and microwave decalcification of bone and teeth and led to the following conclusions:
The microwave method decalcifies both bone and teeth much faster than the routine method and in both the techniques nitric acid shows faster decalcifying ability followed by formic acid, and then EDTA.
Tissue preservation and staining efficacy is poor using nitric acid in the routine method compared with the microwave method; whereas, it is good using both formic acid and EDTA in both routine and microwave methods.
Thus, it is evident that microwave decalcification is faster than routine decalcification using all three decalcifying agents. There is no statistically significant difference between all the three decalcifying agents where preservation of tissue structure and staining efficacy are concerned irrespective of the methods used, except for patchy staining in the condyle and teeth using nitric acid, which shows a statistically significant difference between the routine and microwave methods.
ACKNOWLEDGMENT
I would like to thank Dr. Kavita Rao, Professor and Head, Department of Oral and Maxillofacial Pathology, VS Dental College and Hospital, Bangalore, for her support and encouragement during the course of the study; as well as my son, husband, and parents for standing by me through all my endeavors - Dr. R Sangeetha.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Lynch SR. 4th ed. London: W. B. Saunders; 1983. Lynch's Medical Laboratory Technology; pp. 937–944. [Google Scholar]
- 2.Bhaskar SN. 10th ed. New Delhi: CBS Publishers and Distributors; Orban's Oral Histology and Embryology; pp. 349–354. [Google Scholar]
- 3.Callis GM, Bancroft JD. 6th ed. Edinburgh: Churchill Livingstone; 2008. Theory and Practice of Histological Techniques; pp. 338–360. [Google Scholar]
- 4.Roncaroli F, Mussa B, Bussolati G. Microwave oven for improved tissue fixation and decalcification. Pathologica. 1991;83:307–10. [PubMed] [Google Scholar]
- 5.Vongsavan N, Mathews B, Harrison GK. Decalcification of teeth in a microwave oven. Histochem J. 1990;22:377–80. doi: 10.1007/BF01003173. [DOI] [PubMed] [Google Scholar]
- 6.Pitol DL, Caetano FH, Lunardi LO. Microwave induced fast decalcification of rat bone for electron microscopic analysis: An ultrastructural and cytochemical study. Braz Dent J. 2007;18:153–7. doi: 10.1590/s0103-64402007000200013. [DOI] [PubMed] [Google Scholar]
- 7.De-Deus G, Reis C, Fidel S, Fidel RA, Paciornik S. Longitudinal and quantitative evaluation of dentin demineralization when subjected to EDTA, EDTAC, and citric acid: A co-site digital optical microscopy study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:391–7. doi: 10.1016/j.tripleo.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 8.Mathai AM, Naik R, Pai MR, Rai S, Baliga P. Microwave histoprocessing versus conventional histoprocessing. Ind J Pathol Microbiol. 2008;51:12–6. doi: 10.4103/0377-4929.40383. [DOI] [PubMed] [Google Scholar]
- 9.Keithley EM, Truong T, Chandranait B, Billings PB. Using the microwave oven for decalcification of human temporal bones. Newsletter of the NIDCD National Temporal Bone, Hea Balance Pathol Res Registry. 2001;9:1–5. [Google Scholar]
- 10.Alers JC, Kritjenberg PJ, Vissers KJ, van Dekken H. Effect of bone decalcification procedures on DNA in situ hybridization. EDTA is highly preferable to a routinely used acid decalcifier. J Histochem Cytochem. 1999;47:703–10. doi: 10.1177/002215549904700512. [DOI] [PubMed] [Google Scholar]
- 11.Moore RJ. Bone. In: Woods AE, Ellis R, editors. Laboratory histopathology. New York: Churchill Livingstone; 1994. pp. 72–10. [Google Scholar]
- 12.Culling CF, Allison RT, Barr WT. 4th ed. London: Butterworths; 1984. Cellular Pathology Technique; pp. 408–30. [Google Scholar]


