Abstract
Examination of microscopic sections of animal tissues reveals facts which are not always related to its normal histology or pathology. Processing of tissue specimens consists of lengthy procedures from the stage of surgical removal to the stained and mounted microscopic sections. Defects are common in tissue sections as a result of faulty procedures. These defects are referred to as artifacts. They lead to misinterpretation of histopathological diagnosis but at times they throw limelight into diagnosis. This paper attempts to put together all the facts regarding the various artifacts that are encountered in histopathology.
Keywords: Artifact, biopsy, diagnosis, histopathology
INTRODUCTION
It is a fact that artifact is not a fact but misinterpreted as a fact. It is derived from the two words art and factum. Artifacts are a common phenomenon encountered in a variety of diagnostic procedures in medicine. It is defined as being any structure or feature that has been produced by the processing of a tissue.[1] Artifacts may be produced at any stage beginning from the time of biopsy to the final stage of mounting. These artifacts result in alteration of normal morphologic or cytologic features,[2] thus interfering or obscuring the interpretation of histopathological diagnosis. According to the stage at which they are formed artifacts can be classified into different categories as artifacts produced during:
Surgical biopsy procedure
Fixation
Tissue processing
Embedding
Microtomy
Mounting
Staining
Cover-slipping.
BIOPSY PROCEDURE
A few of the artifacts encountered in microscopic sections are caused by factors related to surgical procedures. Intralesional injection produces epithelial vacuolation, connective tissue separation and extravasation of red blood cells.[3] This is best avoided by administering anesthetics to the area adjacent to the biopsy site. Hemorrhage is a common complication occurring during surgery. An inexperienced histopathologist may interpret this for a pathologic change [Figure 1a]. Pressure marks on the surface of the biopsied specimen can be produced when held by forceps with excessive force prior to fixation, referred to as crush or compression artifacts.[4,5] These are usually seen at the periphery of the lesion. Split artifacts are produced by penetration of forceps into the tissue, leaving gaps and compression zones around tissues[4] [Figure 1b]. Crush/split artifacts can be avoided by use of blunt forceps rather than toothed forceps. In punch biopsy, fragmentation artifacts are common which may be attributed to the use of scissors at the base of tissue for releasing it. According to Meghna and Ahmed Mujib, punch biopsy procedure produced fewer artifacts when compared to biopsy by scalpel.[6]
Figure 1.
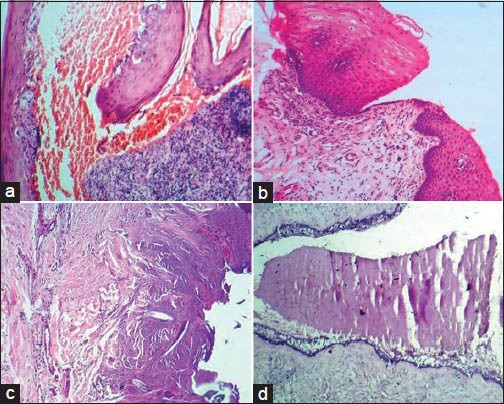
(a) Hemorrhage artifact. (b) Histopathological section showing split artifact due to usage of sharp forceps during biopsy procedure. (c) Coagulation of proteins within the tissues as a result of biopsy using laser. (d) Venetian blind artifact produced by vibration of tissue block/knife (H&E stain, ×100)
Coagulation of proteins results from dehydration of the tissues during biopsy which may be caused either by cauterization or by chemicals used in sterilization of surgical instruments. Electrocautery in parotid surgery causes oncocytoid changes in the acinar cells.[7] Laser or electrosurgery produces tissue distortion by inducing carbonization, nuclear elongation and vacuolar degeneration[4] [Figure 1c].
Another commonly observed artifact in microscopic tissue sections is entrapped suture material. Presence of suture material is of less significance in pathology but in turn can damage microtome knives leading to tears in sections.[8] Removal of suture material prior to fixation is required. Gel foam or surgical sponge is used to control bleeding in various surgical procedures. The presence of gel foam on a histological section produces a characteristic appearance of distorted spaces on the surface which may be filled with blood surrounded by slightly basophilic gelatin walls.[8]
FIXATION
Fixation is a process which attempts to preserve the tissues in a life like condition by preventing autolysis and putrefaction. The volume of fixative should be 20 times that of the specimen with thickness not exceeding 6 mm.[9] Autolytic changes may occur due to fresh tissues sticking together, adherence of specimen to the inner surface of the fixative container, inadequate amount of fixative, thick tissue specimens, or insufficient time spent in fixative.[1] Inaccurate fixation produces shrinkage or crenation with hypertonic saline and swelling/bursting of cells with hypotonic saline. Usage of a normal phosphate buffered saline (PBS) based fixative corrects such problems. Alcohol fixatives tend to make tissue sections brittle resulting in microtome sectioning artifacts with chattering and a “venetian blind” appearance [Figure 1d].
Intraepithelial cleft formation and acantholysis occurs as a result of formation of calcium carbonate residue due to formalin evaporation from unsealed bottles.[1]
Acid formalin hematin, a dark brown microcrystalline pigment is produced by the reaction of formic acid (unbuffered formalin) and heme molecule from hemoglobin in an acidic pH. It is usually found adjacent to erythrocytes in tissue sections and may simulate microorganisms. This can be confirmed by polarized light microscopy and prevented by using neutral buffered formalin or fixation in phenol formalin.[9] This pigment can be removed using saturated alcoholic picric acid solution. Deposition of chrome oxide pigments within tissues occurs with the use of chrome fixatives.
Fall out of sections from slides during staining procedures can occur as an after effect of inadequate fixation. Over fixation causes bleaching artifacts. Unfixed areas within a tissue move and localize in some place other than the original location producing “streaming artifact”. This change is seen associated with glycogen in gluteraldehyde fixation, where there is considerable loss and diffusion of glycogen in tissues.[10] Generally fixation at room temperature is sufficient to maintain excellent morphological detail, but a rise in temperature can increase the rate of fixation (microwave oven with an optimum temperature of 45-55°C).[11] Under heating produce poor sectioning quality and overheating causes vacuolation, over-stained cytoplasm and pyknotic nuclei.
GROSSING AND PROCESSING
Floaters or cross-contamination artifacts are small pieces of tissue that appear on a slide which do not belong to that particular area and have floated in during grossing, processing or floatation of cut-sections. They may also arise from sloppy procedures on the cutting bench such as dirty towels, instruments or gloves that have remnants of tissue that is carried over to the next case. Therefore, it is essential to do only one specimen at a time and clean thoroughly before opening the container of the next case. Thin and narrow tissue specimens tend to curl during processing. This will cause difficulty in orienting the tissue while embedding leading to formation of tangential sections [Figure 2a].
Figure 2.
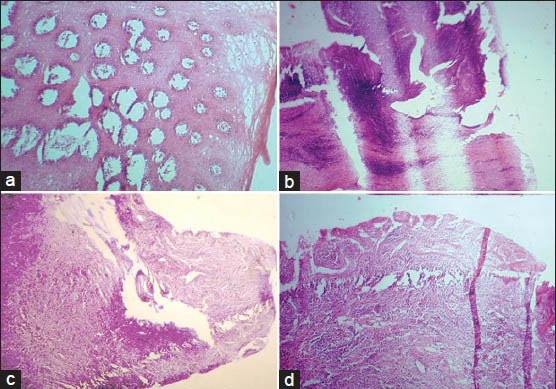
(a) Tangential section of epithelium caused by improper orientation. (b) Thick and thin section formed due to loosely attached microtome knife. (c) Knife scoring appeared in the section due to a small nick in the knife edge. (d) Folds and wrinkles within the histological section produced by a blunt microtome knife (H&E stain, ×100)
According to Panja et al., tissue processing by microwave method produced the least amount of tissue shrinkage. The usage of noxious chemicals like formalin and xylene is eliminated by this technique.[11,12] If the automatic tissue processor is improperly adjusted or there is a power failure, the basket containing cassettes may remain elevated resulting in dehydration of tissue specimens by exposure to air. Excessive dehydration will give the tissue a dry homogenous appearance. This might also cause cracking and excessive staining of tissue sections.[1,13] Biopsy foam pads used in embedding cassettes may produce grid-like/triangular-shaped artifacts resembling vascular channels.[14]
Incomplete dehydration leads to water entrapment within the tissues and cause inadequate staining or opacity within the section. This may be prevented by frequent changing of processing solutions and covering their containers to avoid moisture contamination[1] Inadequate infiltration of tissue with paraffin results in wrinkles that run in all directions.[1] This occurs due to faulty fixation, dehydration, clearing and insufficient time in molten wax. Properly fixed small tissues when processed with long schedules, becomes excessively shrunken, dry, brittle and difficult to cut. They appear as overstained, fragmented or crushed sections. Prevention is by using short schedule of processing.[13]
EMBEDDING
Entrapment of air around the tissue is a common finding during embedding. This causes the tissue to fall out or vibrate during the cutting procedure leading to venetian blind artifact which appears as zones of compressed tissue separated by open spaces.[8] Embedding of multiple tissues having variable consistencies in the same block can produce artifacts.[1] Hydrophilic processing fluids may be retained within the embedded block of tissue and result in wrinkled tissue sections. If the hardness of the embedding medium is greater than the infiltrated tissue, wrinkles or cracks appear in the tissue sections. Use of soft wax or hard embedding medium, rapid cooling of wax, contamination with clearing agent, denaturation of wax and insufficient dehydration results in tear artifacts.[13]
MICROTOMY/SECTIONING ARTIFACTS
Thick and thin sections and chatter/venetian blind artifact are formed as a result of loosely attached microtome knife or tissue block, steep angle of the cutting knife, hard tissue or wax, and presence of calcification in tissues[15] [Figure 2b]. Scratch lines appear in the sections due to small nicks in the knife edge, large knife clearance angle, hard material embedded in the wax or hard material in the tissue[15] [Figure 2c]. Crumbling of sections occur on cutting if the knife is blunt or the wax is too soft or due to contamination of wax with clearing agent or water or due to slow cooling of wax[15] [Figure 2d]. Loss of bevel on the knife edge produces compression of the block which in turn leads to formation of creases in the cut sections.[15] Displacement of tissue components especially bone during microtomy is a common finding in association with the use of dull knife, soft embedding medium, rough sectioning, incorrect blotting and poor adhesion of sections to the glass slide [Figure 3a].
Figure 3.
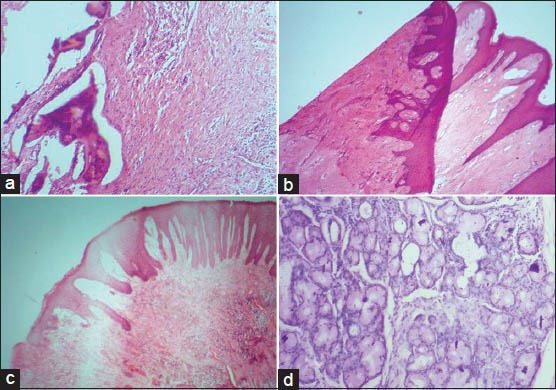
(a) Displacement of bone during microtomy in association with the use of dull knife. (b) Microscopic section showing folding. (c) Residual wax within the stained section. (d) Stain deposits within salivary gland tissue (H&E stain, ×100)
FLOATATION AND MOUNTING
Artifacts that appear in this stage include contamination by microorganisms (fungi), air borne fibers, hair, cellulose fibers, floaters or bubbles beneath the sections. Contamination by exfoliated squamous cells is another common artifact caused by fingers or sneezes/coughs.[13] Special care has to be taken during processing and floatation in order to avoid folding of microscopic tissue sections [Figure 3b]. As the tissue sections are flattened in the water bath, bubbles of air may become trapped beneath them. Collapsed bubble artifact occur due to collapsing of air bubbles entrapped beneath the sections leaving cracked areas when dry, which fail to adhere to the glass slide properly and show altered staining.[16]
STAINING
Failure to remove wax from sections completely result in impairment of staining known as residual wax artifact [Figure 3c]. Stain deposits may appear in sections if the dye solutions are old or unfiltered [Figure 3d]. Eosin flakes, seen above the focal plane of the tissue section, are precipitated dye derived from an unfiltered stock solution.[1] Drying up of sections between the last xylene and cover slipping result in entrapment of minute bubbles over the nuclei leading to dark nuclei lacking visible detail (corn flake artifact).[8] The presence of water in the sections masks microscopic detail and causes leaching of stains [Figure 4a and b]. Washing eosin-stained sections in tap water with an acidic pH leads to leaching of the stain into mounting media. This is more common where there is high humidity and is due to atmospheric moisture being absorbed by alcohols and particularly xylene substitutes. If there is presence of moisture still in the section after cover slipping due to moisture in alcohols and clearing agents, albeit in small amounts, eosin will bleed from the section.[13]
Figure 4.
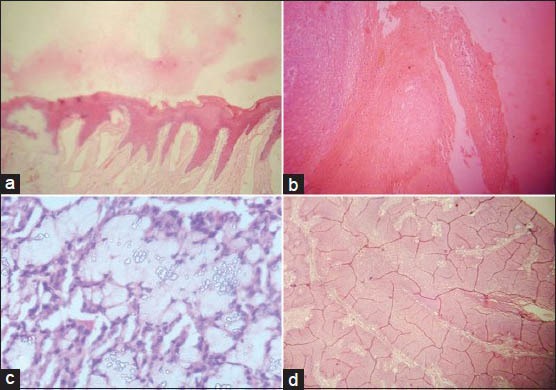
(a and b) Section showing eosin leaching. (c) Thin mounting media resulting in the formation of air bubbles. (d) Section with cracking of DPX (H&E stain, ×100)
COVER SLIPPING
Bubbles may form under the cover slips if the mounting media is too thin [Figure 4c]. Incorrectly prepared resin based mountants tend to decay over time causing crystallization and cracking of mounting media [Figure 4d]. Bleaching of stain is an unwanted outcome of prolonged exposure of the sections to light. Hence, stained sections should be stored in dark storing cabinets. Presence of fingerprints can be avoided by using slide holders. If mounting bench is kept neat and tidy, unwanted elements like debris, fibers or even fungi may be prevented from contaminating the tissue sections.[1]
ARTIFACTS IN DIAGNOSIS
Artifacts are well-known for their diagnostic misinterpretation but not always. Few artifacts have been proven as diagnostic clues to histopathology.
Cholesterol clefts in radicular cysts or periapical granuloma are produced as a result of dissolution of lipids during processing that leave behind needle like spaces [Figure 5a]. Lacunar cells, the diagnostic clue to nodular sclerosis, a variant of Hodgkin's lymphoma is an artifact induced by formalin fixation and absent with other fixatives. These cells are formed by retraction of cytoplasm towards the nuclear membrane thus giving the appearance of cells enclosed within lacunae.[17] Max Joseph Space (Caspary Joseph Space) associated with lichen planus is an artifactual space in the subepithelial region caused during processing and is attributed to basal cell degeneration[18] [Figure 5b]. Formalin induced fluorescence can detect melanin pigment in amelanotic melanoma where melanin is not demonstrable in routine hematoxylin and eosin (H and E) section.[19]
Figure 5.
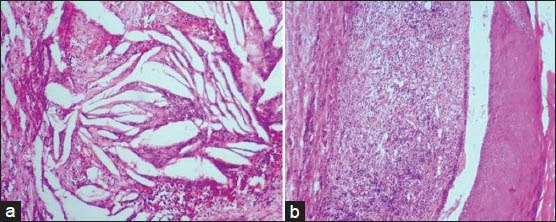
(a) Clefts formed as a result of dissolution of cholesterol crystals. (b) Max Joseph space formed as a result of basilar degeneration (H&E stain, ×100)
CONCLUSION
Artifacts are encountered in most microscopic sections and play a role in the interpretation of histopathological diagnosis. Most of these artifacts may not be intentional and might go unnoticed causing pitfalls in diagnosis. Proper handling of specimens and avoidance of faulty techniques will reduce artifacts, thus help to establish appropriate diagnosis.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.McInnes E. Artefacts in histopathology. Comp Clin Path. 2005;13:100–8. [Google Scholar]
- 2.Margarone JE, Natiella JR, Vaughan CD. Artifacts in oral biopsy specimens. J Oral maxillofac Surg. 1985;43:163–72. doi: 10.1016/0278-2391(85)90154-5. [DOI] [PubMed] [Google Scholar]
- 3.Kimire S, Hirai A, Shimizu H. Epidermal vacuolation: An artefact due to injection of local anesthetics. Arch Dermatol Res. 1981;270:413–9. doi: 10.1007/BF00403785. [DOI] [PubMed] [Google Scholar]
- 4.Logan RM, Goss AN. Biopsy of the oral mucosa and use of histopathology services. Aust Dent J. 2010;55(1 Suppl):9–13. doi: 10.1111/j.1834-7819.2010.01194.x. [DOI] [PubMed] [Google Scholar]
- 5.Jephcott A. Surgical management of the oral tissues: 2 surgical techniques. Dent Update. 2007;34:654–7. doi: 10.12968/denu.2007.34.10.654. [DOI] [PubMed] [Google Scholar]
- 6.Meghna SM, Ahmedmujib BR. Surgical artefacts in oral biopsy specimens: Punch biopsy compared to conventional scalpel biopsy. J Oral Maxillofac Pathol. 2007;11:11–4. [Google Scholar]
- 7.Shick PC, Brannon RB. Oncocytoid artifact of the parotid gland: A newly reported artifact. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;86:720–2. doi: 10.1016/s1079-2104(98)90210-4. [DOI] [PubMed] [Google Scholar]
- 8.Rolls GO, Farmer NJ, Hall JB. 1st ed. Melbourne (Australia): Leica Biosystems Pty LTD; 2012. Artefacts in histological and cytological preparations; p. 106. [Google Scholar]
- 9.Thompson SW, Luna LG. 1st ed. Illinois: Springfield; 1978. An atlas of artifacts encountered in the preparation of microscopic tissue sections. [Google Scholar]
- 10.Yoshiki S, Thakahashi R, Tanaka S. Preservation of glycogen in decalcified hard tissues. Histochemie. 1970;21:249–56. doi: 10.1007/BF00304216. [DOI] [PubMed] [Google Scholar]
- 11.Mathai AM, Naik R, Pai MR, Rai S, Baliga P. Microwave histoprocessing versus conventional histoprocessing. Indian J Pathol Microbiol. 2008;51:12–6. doi: 10.4103/0377-4929.40383. [DOI] [PubMed] [Google Scholar]
- 12.Panja P, Sriram G, Saraswathy TR, Sivapadasundharam B. Comparison of three different methods of tissue processing. J Oral Maxillofac Pathol. 2007;11:15–7. [Google Scholar]
- 13.Woods AE, Ellis RC, editors. Edinburg: Churchill Livingstone; 1994. Laboratory histopathology. A complete reference. [Google Scholar]
- 14.Farrell DJ, Thompson J, Morley AR. Tissue artefacts caused by sponges. J Clin Pathol. 1992;45:923–4. doi: 10.1136/jcp.45.10.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Culling CF. 2nd ed. London: Butterworth and Co (Publishers) Ltd; 1963. Hand book of histopathological techniques; p. 123. [Google Scholar]
- 16.Niemann TH, Tranovich JG, De Young BR. Biopsy bag artifact. Am J Clin Pathol. 1998;110:224–6. doi: 10.1093/ajcp/110.2.224. [DOI] [PubMed] [Google Scholar]
- 17.Rosai J. Lymph nodes. In: Rosai J, editor. Ackerman's Surgical Pathology. 8th ed. St Louis: Mosby; 1996. pp. 1661–773. [Google Scholar]
- 18.King, Friday D M.D, Hunter, John AA, Holubar, Karl The Caspary-Robinson space. Am J Dermatopathol. 1984;6:161–2. doi: 10.1097/00000372-198404000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Pattari SK, Dey P. Facts about artefacts in diagnostic pathology. Indian J Pathol Micrbiol. 2002;45:133–6. [PubMed] [Google Scholar]


