Abstract
Granuloma formation with multinucleated giant cells is seen in numerous diseases. A granuloma is a focus of chronic inflammation consisting of a microscopic aggregation of macrophages surrounded by a collar of lymphocytes and plasma cells. In this article, we present a case of granuloma formation with multiple Langhans giant cells along with an overview of the differential diagnoses, which include mycobacterium diseases, other bacterial infections, fungal infections, protozoal infections, and other granulomatous diseases.
Keywords: Granuloma, langhans giant cells, tuberculous lymphadenitis
INTRODUCTION
A granuloma is a focus of chronic inflammation consisting of a microscopic aggregation of macrophages that are transformed into epithelium like cells surrounded by a collar of mononuclear leukocytes, principally lymphocytes, and plasma cells.[1,2,3] Langhans giant cells are multinucleated giant cells present in numerous diseases. In this article, we present a case of a granuloma with Langhans giant cell along with an overview of the differential diagnoses.
CASE REPORT
A 55-year-old male patient reported with a complaint of swelling in the left side of his face for the past 3 months. The patient noted a gradual increase of the swelling to the present size over a period of 3 months. The swelling was painless with no discharge. There was no history of fever or associated symptoms like cough and expectoration. He had no dental infection or history of trauma. He had no history of oral habits involving tobacco or other products. Radiograph of the chest did not reveal any significant findings.
On extraoral inspection, there was a single swelling of size 2 × 2 cm in the left submandibular region extending posteriorly from the angle of the mouth. Borders of the swelling were well-defined, and the overlying skin appeared normal. On palpation, the swelling was firm, non-tender, and not indurated. The overlying skin was pinchable, with no appreciable warmth. The patient was advised hematological and histopathologic investigations. He reported after 2 weeks when the swelling become fluctuant with development of a pointing abscess.
Hematologic investigations revealed a negative HIV status and normal hemogram values except for an elevated erythrocyte sedimentation rate (ESR) level. On aspiration, a purulent fluid was collected with the swelling remaining firm. A biopsy for histopathological examination was suggested. On surgical exploration, the swelling revealed lesional tissue with yellowish areas suggestive of degeneration. The specimen was sent for histopathological evaluation.
Histologically, the lesional tissue was encapsulated and exhibited lymph node architecture with germinal centers [Figure 1]. The lesional tissue exhibited multiple granuloma formation and numerous giant cells. The granuloma was composed of central epitheloid and foamy macrophages bounded by lymphocytes and plasma cells [Figure 2]. Multinucleated giant cells were seen in different foci [Figure 3]. A few granulomas exhibited eosinophilic areas suggestive of central necrosis [Figure 4]. The giant cells were Langhans type displaying numerous nuclei arranged in horseshoe pattern and an eosinophilic cytoplasm [Figure 5a and b]. Some of the giant cells exhibited arrangement of the nuclei at both poles [Figure 6]. Nuclei were uniform and appeared normal and vesicular. The giant cells were not associated with hemorrhagic foci, which were fairly observed. The histopathology was suggestive of a chronic granulomatous disease.
Figure 1.
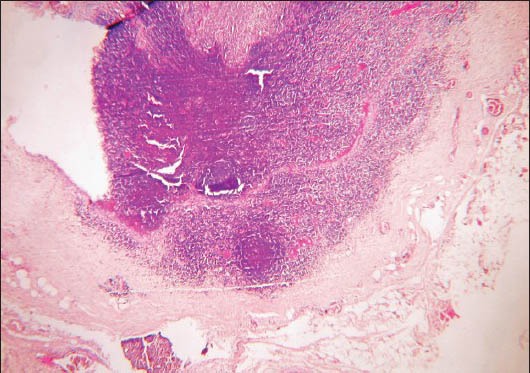
Photomicrograph showing encapsulated lesional tissue exhibiting lymph node architecture (H&E stain, ×40)
Figure 2.
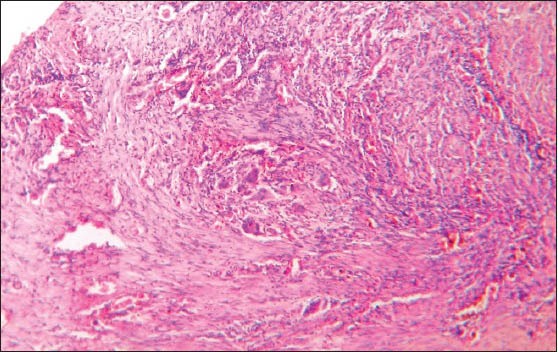
Photomicrograph showing granuloma formation characterized by central epitheloid, foamy macrophages and presence of giant cells (H&E stain, ×40)
Figure 3.
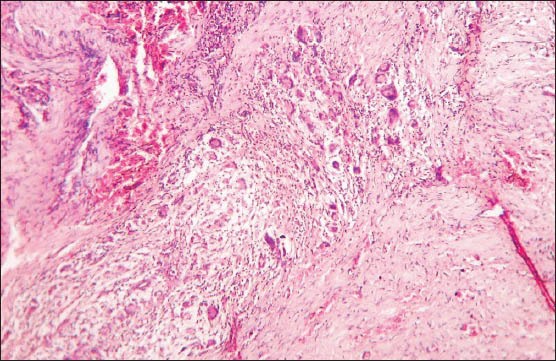
Photomicrograph showing multiple giant cells (H&E stain, ×100)
Figure 4.
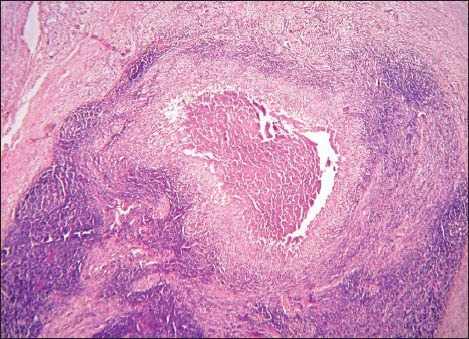
Photomicrograph showing granuloma exhibiting eosinophilic areas suggestive of central necrosis (H&E stain, ×40)
Figure 5.
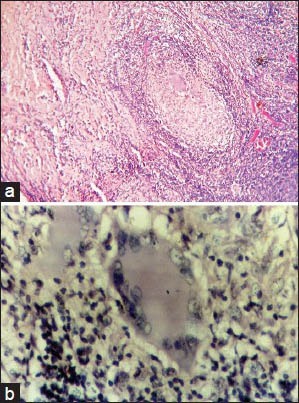
(a) Photomicrograph showing granuloma exhibiting Langhans (H&E stain, ×400) giant cells displaying numerous nuclei arranged in horseshoe pattern. (b) Photomicrograph showing granuloma exhibiting Langhans giant cells displaying numerous nuclei arranged in horseshoe pattern (H&E stain, ×400)
Figure 6.
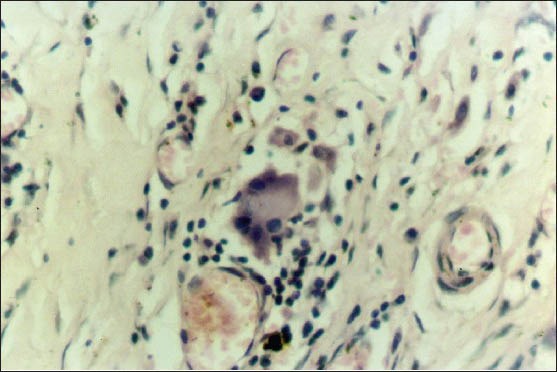
Photomicrograph showing granuloma exhibiting Langhans giant cells displaying bipolar arrangement of nuclei(H&E stain, ×400)
Based on the histopathology, the differential diagnoses considered were:
-
Mycobacterial diseases
- Tuberculosis
- Leprosy
- Bacterial infections
- Syphilis
- Actinomycosis
- Cat scratch disease
- Fungal infections
- Histoplasmosis
- Blastomycosis
- Paracoccidioidomycosis
- Protozoal infections
- Toxoplasmosis
- Miscellaneous diseases
- Sarcoidosis
- Orofacial granulomatosis
- Crohn's disease
- Melkersson-Rosenthal syndrome
- Wegener's granulomatosis.
The patient was advised for further investigations to confirm the diagnosis but could not be followed-up as he was transferred. A list of hematological, microbiologic, and immunologic investigations was advised to be done at his work place.
DISCUSSION
Granulomatous inflammation is a distinctive pattern of chronic inflammatory reaction characterized by focal accumulation of activated macrophages, which often develop an epitheloid appearance.[1,2,3,4,5]
A granuloma is a focus of chronic inflammation consisting of a microscopic aggregation of macrophages that are surrounded by a collar of lymphocytes and plasma cells.[1,2,5] A granuloma is a focal compact collection of inflammatory cells, mononuclear cells predominating, usually as a result of the persistence of a nondegradable product and of active cell-mediated hypersensitivity.[2] There is a complex interplay between invading organisms or prolonged antigenemia, macrophage activity, a Th1 cell response, B cell overactivity, and a vast array of biological mediators.[2]
In hematoxylin and eosin (H and E) sections, epitheloid cells have a pale pink cytoplasm with indistinct cell boundaries and appear to be merging with one another. These cells can fuse to form giant cells with diameter of 40-50 μm. These giant cells have a large mass of cytoplasm with 20 or more nuclei arranged haphazardly or in a horseshoe pattern peripherally-the Langhans type.[1,2,3,4] These giant cells can be formed by cell fusion and nuclear division without cytoplasmic separation.[5] In Langhans giant cells, the nuclei are either arranged in a horseshoe pattern at the periphery or clustered at the two poles of the giant cell.[4,6]
In granulomatous inflammation, macrophages engulf the foreign body/material and present some of it to the T-lymphocytes, thus activating them. These cells produce cytokines like interleukin (IL)-2 in response. IL-2 activates other T cells, and interferon (IFN) - gamma activates other macrophages, resulting in a continued response. Macrophages are also converted into epitheloid and multinucleated giant cells.[1,2,3,4,5,7]
The most common granulomatous diseases are mycobacterial diseases like tuberculosis with the other differential diagnoses being bacterial infections, fungal infections, protozoan infections and other diseases like sarcoidosis, oral granulomatosis, Crohn's disease and Wegener's granulomatosis.[1,2,3]
In granulomatous disease, it is necessary to identify the specific etiologic agent by further investigations such as special stains (e.g. tuberculosis), culture methods (e.g. fungal infections) and molecular techniques such as polymerase chain reaction (PCR) and serologic tests as in syphilis.[1,2,3]
Tuberculosis is caused by Mycobacterium tuberculosis (M. tuberculosis) and M. bovis, an acid and alcohol fast organism.[5,7,8] Histopathology is characterized by the presence of epitheloid granuloma with Langhans giant cells.[8] In response to the infection, the activated macrophages; cytokine interferon (IFN) and T cell activity produces a type IV reaction. This reaction combined with ischemia results in central caseation necrosis in the tuberculous granuloma.[5,9,10] Demonstration of the mycobacteria can be done with Ziehl-Neelsen staining or by immunofluorescence using auramine-rhodamine. Mycobacterial culture and detection of mycobacterial deoxyribonucleic acid (DNA) using PCR are other diagnostic tests.[8]
Leprosy is caused by Mycobacterium leprae, resulting in granulomatous disease.[5] Microscopically, a granulomatous inflammatory response is seen with granuloma formation and presence of Langhans giant cells.[9,11] Microorganisms can be demonstrated using Fite stain and a positive lepromin test.[6,9,12]
Syphilis is a sexually transmitted infection caused by Treponema pallidum, a spirochete, demonstrated by dark ground illumination and silver staining or immunoflourescence.[5] Other diagnostic tests include Venereal Disease Research Laboratory (VDRL), Treponema pallidum hemagglutination assay (TPHA), and fluorescent antibody tests.[5,6] Granulomas in syphilis are well-circumscribed and surrounded by lymphocytes and plasma cells.[11]
Actinomycosis is a chronic bacterial disease exhibiting clinical and microscopic features similar to fungal infections. It is caused by Actinomyces israelii, an anaerobic, gram positive organism. A granulomatous response with central abscess formation is seen in this disease.[9] Pus draining from the lesion contains yellow granules known as “sulfur granules” which are aggregates of the causative organism. Definitive diagnosis can be done by identification of the organism using Gomori's methanamine silver stain, microscopic evaluation of tissue sections and culture tests.[6,9]
Cat scratch disease is caused by Bartonella henselae, producing a granulomatous reaction with intraepithelial neutrophilic abscess.[5] The other histopathologic features are destruction of lymph node architecture, necrosis, and lymphocytic infiltration with granuloma formation.[8] The causative organism can be demonstrated using Warthin-Starry silver stain.[8,12]
Histoplasmosis is a fungal infection caused by Histoplasma capsulatum causing systemic and oral lesions. Microscopically, granuloma formation with multinucleated giant cells is seen. Leukocytoclastic vasculitis and dermal necrosis are other histologic features.[11] Identification of organism in biopsy specimen is required to establish a definitive diagnosis.[9]
Blastomycosis is caused by Blatomyces dermatitidis and is also called as Gilchrist's disease. Other than granuloma formation, neutrophil microabscesses, purulence, and peudoepitheliomatous hyperplasia are more commonly seen in blastomycosis.[9,11]
Paracoccidioidomycosis is caused by Paracoccidioides brasiliensis. Paracoccidioidomycosis is one of the most common deep mycoses in many regions of Latin America, particularly in Brazil. Microscopically, it shows granulomatous inflammatory reaction with giant cells, macrophages, lymphocytes, plasma cells, polymorphonuclear neutrophilic leukocytes, and eosinophils.[13] Purulence is less common in Paracoccidioidomycosis.[9,11]
Toxoplasmosis is a protozoan disease caused by Toxoplasma gondii. Serologic tests demonstrating an elevated titer of antibodies are useful in the diagnosis of this infection.[5,8]
Sarcoidosis, also known as Boeck's sarcoid is a chronic disease of unknown etiology, resulting in granuloma formation in the lungs, lymph node, and salivary glands.[6] With molecular techniques, mycobacterial deoxy ribo nucleic (DNA) and ribonucleic acid (RNA) have been identified in sarcoid tissues.[9] Sarcoid granulomas are noncaseating, contain Langhans and other giant cells and are surrounded by lymphocytes.[8] Other histologic features include stellate inclusions called asteroid bodies and concentric lamellar calcifications called Schaumann bodies.[9,14] Labial gland biopsy and elevated levels of angiotensin converting enzyme (ACE) are used in diagnosing the disease.[8] Elevated serum calcium levels and adenosine deaminase levels for macrophage activity in granulomas are other diagnostic tests. Kveim skin test can also be done along with gallium scintiscanning.[9] Polaroscopy reveals little crystalline refractile things (LCRT) within the granuloma.[11] Recently a latent infection of Propionibacterium acnes has been postulated to be the cause of sarcoidosis.[15]
Wegener's granulomatosis is a potentially lethal granulomatous inflammation affecting nasal tissue and has a characteristic “strawberry gum” proliferative gingivitis.[8] Histopathologic features include vasculitis with destruction of small arteries, dense inflammatory infiltrate and presence of giant cells. Staining for antineutrophil cytoplasmic antibodies (ANCA) is positive in this disease.[8,9]
Crohn's disease is of unknown etiology, affecting the ileocaecal region, causing thickening and ulceration. Histologically, there are noncaseating granulomas seen along with multinucleated Langhans giant cells.[8,9,11]
Orofacial granulomatosis is characterized by swellings in gingiva and lips due to granulomatous reactions. These lesions could be seen as a result of reaction to food additives like cinnamon or tartrazine. Histologically, there are scattered granulomas with large multinucleated giant cells.[8,12]
CONCLUSION
Granuloma formation with multinucleated giant cells is seen in numerous diseases. Sometimes, the oral lesion can lead to the diagnosis of underlying systemic disease. The importance of histopathologic evaluation is paramount in the definitive diagnosis of these lesions. The above differential diagnoses should be investigated thoroughly in a chronic granulomatous disease.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Chattopadhyay A. The granulomatous response and oral cavity. Indian J Dent Res. 1994;5:15–8. [PubMed] [Google Scholar]
- 2.James DG. A clinicopathological classification of granulomatous disorders. Postgrad Med J. 2000;76:457–65. doi: 10.1136/pmj.76.898.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar V, Abbas AK, Fausto N. 7th ed. Saunders: Pennsylvania; 2006. Pathologic Basis of Disease. [Google Scholar]
- 4.Soler P, Bernaudin JF. Physiology of granulomas. Rev Pneumol Clin. 1993;49:257–61. [PubMed] [Google Scholar]
- 5.Macfarlane RS, Reid R, Collander R. 5th ed. London: Churchill Livingstone; 2000. Pathology illustrated. [Google Scholar]
- 6.Harsh M. 5th ed. Anshan: Jaypee India; 2005. Textbook of pathology. [Google Scholar]
- 7.Hernandez-Pando R, Bornstein QL, Aguilar Leon D, Orozco EH, Madrigal VK, Martinez Cordero E. Inflammatory cytokine production by immunological and foreign body multinucleated giant cells. Immunology. 2000;100:352–8. doi: 10.1046/j.1365-2567.2000.00025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cawson RA, Odell EW. 7th ed. London: Churchill Livingstone; 2002. Cawson's essentials of oral pathology and oral medicine. [Google Scholar]
- 9.Regezi JA, Sciubba JJ, Jordon RC. 4th ed. Missouri: Saunders; 2003. Oral pathology, clinical pathologic correlations. [Google Scholar]
- 10.Algood HM, Lin PL, Flynn JL. Tumor necrosis factor and chemokine interactions in the formation and maintenance of granulomas in tuberculosis. Clin Infect Dis. 2005;41:S189–93. doi: 10.1086/429994. [DOI] [PubMed] [Google Scholar]
- 11.Maize JC, Walter HC, Burgdorf MD, Hurt MA, LeBoit PE, Metcalf JS, et al. 1st ed. Pennsylvania: Churchill Livingstone; 1998. Cutaneous pathology. [Google Scholar]
- 12.Greenberg MS, Glick M. 10th ed. Canada: BC Decker; 2003. Burket's oral medicine, diagnosis and treatment. [Google Scholar]
- 13.Kaminagakura E, Bonan PR, Jorge J, Almeida OP, Scully C. Characterization of inflammatory cells in oral paracoccidioidomycosis. Oral Dis. 2007;13:434–9. doi: 10.1111/j.1601-0825.2006.01319.x. [DOI] [PubMed] [Google Scholar]
- 14.Ma Y, Gal A, Koss MN. The pathology of pulmonary sarcoidosis: Update. Semin Diag Pathol. 2007;24:150–61. doi: 10.1053/j.semdp.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 15.Inoue Y, Suga M. Granulomatous diseases and pathogenic microorganism. Kekkaku. 2008;83:115–30. [PubMed] [Google Scholar]


