Abstract
Myoepithelial carcinoma is characterized by nearly exclusive myoepithelial differentiation and evidence of malignancy. It may arise de novo or in preexisting benign tumors including pleomorphic adenoma and benign myoepithelioma. A 39-year-old lady presented with painless progressive swelling on the right cheek and right side of palate. On surgery, there was a mass in right maxillary sinus which was surgically excised and diagnosed on histopathology as pleomorphic adenoma. Subsequently, there were two recurrences. The first recurrence was in the right maxilla after 2 years that was removed surgically and diagnosed as pleomorphic adenoma. One year later, she came with rapidly progressive swelling in bilateral cheeks and face. Intraoperatively, there was a large tumor in both maxillary sinuses with extensive local infiltration. Histologically, it was diagnosed as myoepithelial carcinoma. Carcinoma ex pleomorphic adenoma is usually a high grade malignancy. It occurs most commonly in parotid gland followed by submandibular glands, minor salivary glands and occasionally in sublingual gland. To the best of our knowledge, this is the first case of myoepithelial carcinoma arising in a recurrent pleomorphic adenoma in the maxillary sinus.
Keywords: Carcinoma ex-pleomorphic adenoma, myoepithelial carcinoma, maxillary sinus
INTRODUCTION
Carcinoma occurring in a pleomorphic adenoma, also known as carcinoma ex pleomorphic adenoma comprises 2-4% of all salivary gland tumors. It occurs most commonly in parotid gland followed by submandibular glands, minor salivary glands and occasionally in sublingual gland.[1] Histologically, it is usually a high grade malignancy.[2] Carcinoma ex pleomorphic adenoma has been rarely reported in the maxillary sinus.[3,4] Myoepithelial carcinoma is the malignant counterpart of myoepithelioma, characterized nearly by exclusive myoepithelial differentiation and evidence of malignancy, usually in the form of infiltration of surrounding tissues. These tumors can recur and may also lead to nodal and/or distant metastases. Myoepithelial carcinoma can arise de novo or occasionally in a setting of preexisting benign tumors including pleomorphic adenoma and benign myoepithelioma.[1,5] We present a case of myoepithelial carcinoma arising in a recurrent pleomorphic adenoma in the maxillary sinus.
CASE REPORT
A 39-year-old lady presented with painless progressive swelling on the right cheek and right side of palate for 2 months. On examination there was a 2 × 2 cm firm, non-tender swelling. She had undergone a surgery elsewhere 2 years back for a palatal swelling, the details of which were not available. Computed tomography (CT) revealed a 29 × 28 mm enhancing mass in the right side of hard palate with large defect in the palatine process, extending into right maxillary sinus and nasal cavity, anteriorly up to the vestibule and inferiorly into the oral cavity. Right inferior partial maxillectomy was done with excision of the tumor which was sent for histopathological examination. Intraoperatively there was an encapsulated 6 × 4 × 3 cm size mucosa covered mass filling right maxillary sinus.
Two years later, she presented with recurrent swelling in right infraorbital region for 4 months associated with mild pain. On examination, the swelling was small, firm and non-tender. CT revealed a tumor over anterior wall of right maxillary sinus extending to soft tissues anterior to right maxilla. The tumor was excised via Caldwell-Luc approach and sent for histopathological evaluation.
Subsequently, 1 year later, she presented with rapidly progressive swelling of bilateral cheeks and lateral surface of nose for a month, associated with breathing difficulty. On examination there was a 6 × 5 cm red tender swelling on the face with flattening contour of nose infraorbitally, extending to upper lip with obliteration of bilateral nasal cavities. On CT, there was an extensive heterogeneously enhancing soft tissue density lesion in both maxillary sinuses with destruction of the walls and extension into both ethmoid sinuses, nasal cavity, maxillary alveoli, soft tissue of upper lip, bilateral infratemporal region and both orbits. There was no intracranial extension. The mass was excised surgically. Intraoperatively, there was extensive friable tumor adherent to the skin, involving bilateral maxillary cavities with extension into the right orbit, right nasal cavity with erosion of septum and floor of mouth, both pterygopalatine fossa and bilateral pterygoid muscles. The tumor was sent for histopathological examination.
The initial excision specimen was partial right maxillectomy, with a tumor at the superior aspect. The tumor measured 4.5 × 3 × 2.5 cm on gross examination, with a grey white firm cut surface. Microscopy [Figure 1a] showed pleomorphic adenoma composed of sheets of myoepithelial cells and ductular structures lined by epithelial cells set in chondromyxoid stroma with extensive hyalinization, foci of myxoid change and osseous metaplasia. There was no evidence of malignancy.
Figure 1.
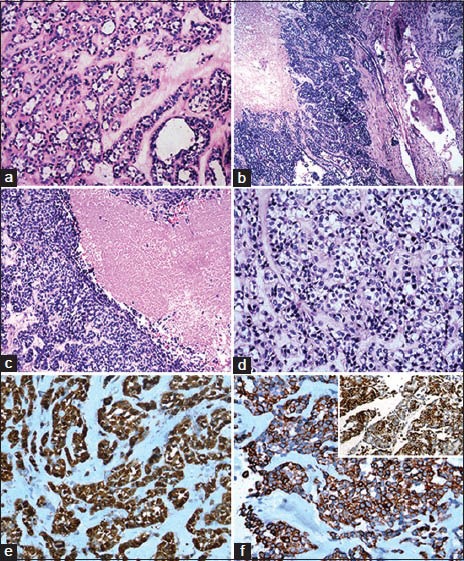
(a) Pleomorphic adenoma: sheets of myoepithelial cells and ductular structures lined by epithelial cells set in chondromyxoid stroma (H&E stain, ×100). Myoepithelial carcinoma: Cellular tumor with infiltration of adjacent bone (b) and areas of geographic necrosis (c) (H&E stain, ×100). (d) Cells arranged in lobules, nests, trabeculae and cords with hyperchromatic, pleomorphic, mitotically active nuclei (H&E stain, ×200); (e) Immunoprofile of myoepithelial carcinoma displaying positivity for S-100 (IHC stain, ×100), (f) Cytokeratin (IHC stain, ×100) and (g) Focally SMA (inset, IHC stain, ×400)
The excised recurrent tumor grossly consisted of multiple soft tissue fragments, 0.5-1.8 cm in maximum dimension. Microscopy showed pleomorphic adenoma, without atypia or any evidence of malignancy.
The third specimen was initially sent to pathology intraoperatively for frozen section, wherein a provisional diagnosis of cellular neoplasm with atypical features suggestive of malignancy was given. Subsequently, the entire specimen was received for histopathological evaluation.
Tumor specimens from right and left maxillary sinuses were received separately. The specimen from right maxillary sinus was an irregular soft tissue measuring 5 × 2.5 × 2 cm, with a partly solid and partly cystic cut surface. Excised tumor from left maxillary sinus was received as multiple irregular fragments of soft tissue, largest measuring 6 × 5.5 × 2.5 cm, with greyish yellow cut surface containing hemorrhagic areas.
Microscopy [Figure 1b–d] showed a cellular tumor composed of lobules, nests, trabeculae and cords of polygonal cells with eccentrically placed hyperchromatic nuclei, mild to moderate pleomorphism, inconspicuous nuceoli, mitotic activity (up to 3/10 high power field (HPF)) and moderate amounts of clear cytoplasm, surrounded by fibromyxoid stoma with hyalinization. There were large areas of geographic necrosis, foci of cystic change and hemorrhage. Lymphovascular invasion was present. There was infiltration of the adjacent bone. Immunohistochemically, the neoplastic cells were positive for S100 [Figure 1e], pancytokeratin [Figure 1f] and focally [Figure 1g] for smooth muscle actin (SMA). A diagnosis of myoepithelial carcinoma arising in a recurrent pleomorphic adenoma was given.
DISCUSSION
Carcinoma ex pleomorphic adenoma, that is malignant transformation occurring in a pleomorphic adenoma, constitutes nearly 3.6% of all salivary gland malignancies.[5] Clinically it presents as rapid enlargement over a period of 3-6 months, in a long standing mass. There may be associated pain, facial nerve palsy and fixation to skin or underlying tissues with restricted mobility. Most commonly, it occurs in the parotid gland. Other sites include submandibular gland, minor salivary glands and rarely nasopharynx.[1,5] Carcinoma ex pleomorphic adenoma occurring in maxillary sinus has been rarely reported.[3,4] Grossly, there may be presence of necrosis, hemorrhage or cystic degeneration. The diagnosis of this entity requires either histological evidence of coexisting pleomorphic adenoma or previous diagnosis of pleomorphic adenoma at that site.[5] The extent of malignancy can be classified as in situ or intracapsular, minimally invasive (≤1.5 mm and frankly invasive (>1.5 mm invasion beyond capsule).[5,6] Usually, the malignant component is high grade such as ductal adenocarcinoma, undifferentiated carcinoma, etc., Other types such as adenocarcinoma not otherwise specified, adenosquamous carcinoma, adenoid cystic carcinoma, epithelial-myoepithelial carcinoma and myoepithelial carcinomas can also occur.[5,7] Carcinoma ex pleomorphic adenoma are aggressive tumors and may lead to regional metastases and/or distant metastases, most commonly to lung followed by bone, abdomen and brain.[5] At times, it may present as a diagnostic challenge for the pathologist as the pleomorphic adenoma component may be small and hyalinized. Hence, careful examination is necessary for diagnosis. Also, it may be difficult to classify the malignant component as one of the more commonly occurring salivary gland malignancy.[2] The degree of invasion carries prognostic significance.[2,5,6,8] Other prognostic factors include tumor stage, grade, extent, proliferative index,[2] perineural invasion and marked cytological atypia.[9,10] According to some authors, vascular invasion, high mitotic activity and histological subtype may also play a role in predicting outcome of patients. Katabi et al., found that myoepithelial carcinoma was more common in widely invasive carcinoma ex pleomorphic adenomas than in intracapsular and minimally invasive tumors. Myoepithelial carcionoma subtype may increase the risk of recurrence in these tumors.[8]
Myoepithelial carcinomas are rare malignant tumors of salivary glands, characterized by exclusive myoepithelial differentiation and infiltration into surrounding tissues.[9,10] These tumors are histologically aggressive. They may recur or even metastasize. Myoepithelial carcinomas occur most frequently in the parotid gland, followed by submandibular gland and uncommonly in minor salivary glands[5,9] including palate, tongue, floor of mouth, maxillary sinus, larynx,[9] buccal mucosa, nasal cavity and lower alveolus.[9,11] They may present clinically as a painless swelling, increasing in size over months to years.[9,10,11] Sometimes, there may be history of rapid enlargement in a long standing mass.[9,11] Uncommonly, they may occur in a preexisting benign tumor such as pleomorphic adenoma or benign myoepithelioma.[10,11] Myoepithelial carcinomas are extremely rare in maxillary sinus and only few cases have been reported.[9,12] Myoepithelial carcinoma arising in a background of pleomorphic adenoma has not been previously reported in the maxillary sinus, to the best of our knowledge.
Grossly, myoepithelial carcinoma is of variable size, grey-white and firm, with cut surface showing areas of necrosis and cystic change.[1,5] Tumors arising in the parotid are usually partially or completely encapsulated, while at other sites they may be unencapsulated.[11] Histologically, they may show various patterns including multinodularity, sheets, trabeculae, nests or reticular pattern of myoepithelial cells separated by fibrovascular septa. There may be multiple nodules with hypercellular areas in the periphery and myxoid or necrotic areas in the center resembling comedo necrosis. The cellular morphology can be epithelioid, spindle cell type, stellate, plasmacytoid/hyaline, clear cell type or a mixture of two or more of these.[1,5,9] The epithelioid variant is the most common[9] and is characterized by polygonal cells with eosinophilic cytoplasm and central nuclei. Plasmacytoid variant shows eccentric nuclei, abundant glassy eosinophilic cytoplasm. In spindle cell variant, there are elongated bipolar cells with myoid or fibroblastic features, central nuclei, arranged in fascicles. In the clear cell variant, tumor cells show abundant pale eosinophilic to clear cytoplasm and central nuclei.[9,10,13] Occasionally, the cells may show signet ring cell-like morphology.[5] The histology may be low grade with mild pleomorphism, inconspicuous nucleoli and fine chromatin, or high grade with nuclear enlargement, pleomorphism, hyperchromasia, prominent nucleoli, irregular nuclear membrane and occasionally tumor giant cells. Pleomorphism and mitotic activity is variable and cellular atypia may not be present in all cases.[1,9] Mitotic activity more than 7/10 mitoses per high power fields (HPF) or Ki-67 proliferation index >10% has been considered as the diagnostic criteria for myoepithelial carcinomas by Nagao et al.[10] Infiltration into the adjacent tissue is the most reliable criteria for diagnosing malignancy in myoepithelial neoplasms. Usually there is invasion into the adjacent salivary gland and soft tissue. There may be infiltration into the adjacent bone in tumors arising in maxillary sinus or palate. The intervening tumor matrix may show myxoid change or hyalinization. Hypovascularity may be conspicuous. Metaplastic change, including squamoid, chondroid or sebaceous metaplasia can also occur.[9] In our case, there were polygonal cells with eccentric nuclei and clear cytoplasm, arranged in lobules, nests, cords and trabeculae. There was cytological atypia with mild to moderate pleomorphism and hyperchromasia, increased mitotic activity (up to 3/10 HPF), geographic areas of necrosis, foci of cystic change and hemorrhage. The intervening stroma was fibromyxoid with foci of hyalinization. There was lymphovascular invasion and infiltration of the adjacent bone.
The differential diagnoses of myoepithelial carcinoma encompasses a wide variety of tumors including myoepithelioma, epithelial myoepithelial carcinoma, clear cell adenocarcinoma, synovial sarcoma, malignant peripheral nerve sheath tumor, leiomyoma, plasmacytoma, malignant melanoma and many more owing to its morphological heterogeneity.[1,9] Myoepithelial carcinomas may demonstrate intacytoplasmic glycogen on periodic acid-Schiff (PAS) staining which is sensitive to diastase, particularly in the clear cell subtype.[9] Immunohistochemically, myoepithelial carcinomas are positive for cytokeratin including Cam 5.2, AE1:AE3 and 34β12, vimentin and S-100. Smooth muscle markers including calponin, smooth muscle actin (SMA), muscle-specific actin (MSA), smooth muscle myosin (SMM), caldesmon, glial fibrillary acidic protein (GFAP), CK14, CD10, p63 and Bcl2 may be expressed in a proportion of cases and calponin appears to be most sensitive.[1,10,14] Diagnosis requires coexpression of cytokeratin and one of the myoepithelial markers.[5] Carcinoembryonic antigen (CEA; marker for luminal differentiation), epithelial membrane anitgen (EMA), CK7, desmin and CD57 are usually negative.[5,9,10,11] p53 overexpression may indicate a worse outcome.[10] Myoepithelial carcinoma is composed of modified myoepithelial cells which may not show complete immunohistochemical expression profile of non-neoplastic myoepithelial cells. In our case, the tumor cells showed immunohistochemical expression of S100, pancytokeratin and focal SMA.
According to some authors, myoepithelial carcinomas arising de novo may be more aggressive tumors as compared to those arising in recurrent pleomorphic adenomas.[15] Myoepithelial carcinoma arising in pleomophic adenoma may be an under reported category, probably due to difficulty in diagnosing the myoepithelial component especially in the absence of marked cytological atypia, mitoses or necrosis; and the inconspicuous nature of the coexisting pleomorphic adenoma.[8,9] Evidence of a preexisting pleomorphic adenoma at the same site may be crucial in reaching accurate diagnosis, which is essential for optimal management of the patient considering the risk of recurrence and metastases.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Wenig BM, Heffer CS, editors. 2nd ed. Philadelphia: WB Saunders; 2008. Atlas of Head and Neck Pathology; pp. 582–702. [Google Scholar]
- 2.Lewis EJ, Olsen KD, Sebo TJ. Carcinoma ex pleomorphic adenoma: Pathological analysis of 73 cases. Hum Pathol. 2001;32:596–604. doi: 10.1053/hupa.2001.25000. [DOI] [PubMed] [Google Scholar]
- 3.Shin SY, Park JG, Ahn ST. A case of carcinoma ex pleomorphic adenoma in the maxillary sinus. J Korean Soc Plast Reconstr Surg. 2001;28:421–3. [Google Scholar]
- 4.Chen HH, Lee LY, Chin SC, Chen IH, Liao CT, Huang SF. Carcinoma ex pleomorphic adenoma of soft palate with cavernous sinus invasion. World J Surg Oncol. 2010;8:24–8. doi: 10.1186/1477-7819-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnes L, Eveson JW, Reichart P, Sidransky D, editors. Lyon, France: IARC Press; 2005. Pathology and Genetics of Head and Neck Tumours. World Health Organisation Classification of Tumours. [Google Scholar]
- 6.Tortoledo ME, Luna MA, Batsakis JG. Carcinomas ex pleomorphic adenoma and malignant mixed tumors histomorphologic indexes. Arch otolaryngol. 1984;110:172–6. doi: 10.1001/archotol.1984.00800290036008. [DOI] [PubMed] [Google Scholar]
- 7.Altemani A, Martins MT, Freitas L, Soares F, Araujo NS, Araujo VC. Carcinoma ex pleomorphic adenoma: Immunoprofile of the cells involved in carcinomatous progression. Histopathol. 2005;46:635–41. doi: 10.1111/j.1365-2559.2005.02157.x. [DOI] [PubMed] [Google Scholar]
- 8.Katabi N, Gomez D, Klimstra DS, Carlson DL, Lee N, Ghossein R. Prognostic factors of recurrence in salivary carcinoma ex pleomorphic adenoma, with emphasis on the carcinoma histologic subtype: A clinicopathologic study of 43 cases. Hum Pathol. 2010;41:927–34. doi: 10.1016/j.humpath.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 9.Savera AT, Sloman A, Huvos AG, Klimstra DS. Myoepithelial carcinoma of the salivary glands: A clinicopathologic study of 25 patients. Am J Surg Pathol. 2000;24:761–74. doi: 10.1097/00000478-200006000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Nagao T, Sugano I, Ishida Y, Tajima Y, Matsuzaki O, Konno A, et al. Salivary gland malignant myoepithelioma: A clinicopathologic and immunohistochemical study of ten cases. Cancer. 1998;83:1292–9. doi: 10.1002/(sici)1097-0142(19981001)83:7<1292::aid-cncr4>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 11.Kane SV, Bagwan IN. Myoepithelial carcinoma of the salivary glands: A clinicopathologic study of 51 cases in a tertiary cancer center. Arch Otolaryngol Head Neck Surg. 2010;136:702–12. doi: 10.1001/archoto.2010.104. [DOI] [PubMed] [Google Scholar]
- 12.Graadt van Roggen JF, Baatenburg-de Jong RJ, Verschuur HP, Balhuizen JC, Slootwweg PJ, van Krieken JH. Myoepithelial carcinoma (malignant myoepithelioma): First report of an occurrence in the maxillary sinus. Histopathology. 1998;32:239–41. doi: 10.1046/j.1365-2559.1998.00382.x. [DOI] [PubMed] [Google Scholar]
- 13.Alos L, Cardesa A, Bombi JA, Mallofre C, Cuchi A, Traserra J. Myoepithelial tumors of salivary glands: A clinicopathologic, immunohistochemical, ultrastructural, and flow cytometric study. Semin Diagn Pathol. 1996;13:138–47. [PubMed] [Google Scholar]
- 14.Yu G, Ma D, Sun K, Li T, Zhang Y. Myoepithelial carcinoma of the salivary glands: Behavior and management. Chin Med J (Engl) 2003;116:163–5. [PubMed] [Google Scholar]
- 15.Di Palma S, Guzzo M. Malignant myoepithelioma of salivary glands: Clinicopathological features of ten cases. Virchows Arch A Patholo Anal Histopathol. 1993;423:389–96. doi: 10.1007/BF01607152. [DOI] [PubMed] [Google Scholar]


