Abstract
Stevens–Johnson Syndrome (SJS) and toxic epidermal necrolysis (TEN) are closely related severe, acute mucocutaneous reactions usually caused by drugs. They are acute life-threatening conditions and cause widespread necrosis of the epithelium. There is persistence of a high risk of SJS or TEN in relation to human immunodeficiency virus (HIV) infection associated with exposure to nevirapine (NVP). In this article, we present nine cases of SJS and one case of TEN in HIV-seropositive individuals who developed cutaneous, oral, ocular and genital lesions while being treated with NVP.
Keywords: Drug reaction, HIV, nevirapine, Steven–Johnson, toxic epidermal necrolysis
INTRODUCTION
In 1922, Stevens and Johnson first described Stevens–Johnson syndrome (SJS) as an immune complex hypersensitivity reaction that can be caused by many factors such as infections, drugs and malignancies.[1] It is a progressive, fulminating, severe variant of erythema multiforme with other variant being toxic epidermal necrolysis (TEN). TEN is a rare and potentially fatal dermatological condition. Reported incidence rates of SJS are 1-6 cases/106 person-years, and for TEN 0.4-1.2 cases/106 person-years.[2] SJS refers to cases with less than 10% of body surface involvement and TEN to those with more than 30% involvement.[3] Burning sensation, edema, erythema of the lips and buccal mucosa are some of the first signs. Skin lesions are initially erythematous macules that rapidly develop central necrosis with vesicles, bullae and denudation on the face, trunk and extremities. Diffuse oral and genital ulcerations may be present. The most common sites include labial mucosa, buccal mucosa, tongue, floor of the mouth and the soft palate. Hemorrhagic crusting of the vermillion zone of the lips is common. There is severe involvement of the conjunctiva, nasopharynx and larynx.[4] TEN and SJS are acute life-threatening conditions with a significant impact on high mortality rate (5-35%).[5,6]
Human immunodeficiency virus (HIV) infected patients on exposure to nevirapine (NVP) have significantly increased risk of SJS or TEN. The underlying mechanism for cutaneous reactions is not clear, but the probable hypothesis could be drug-specific cytotoxic lymphocytes.[7]
CASE REPORT
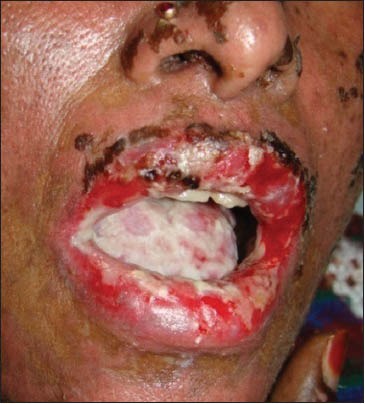
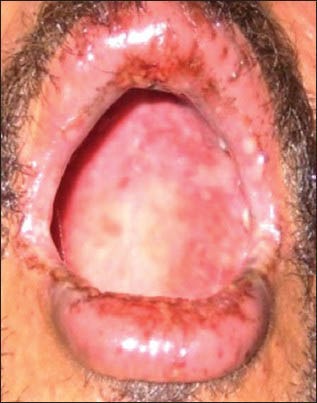
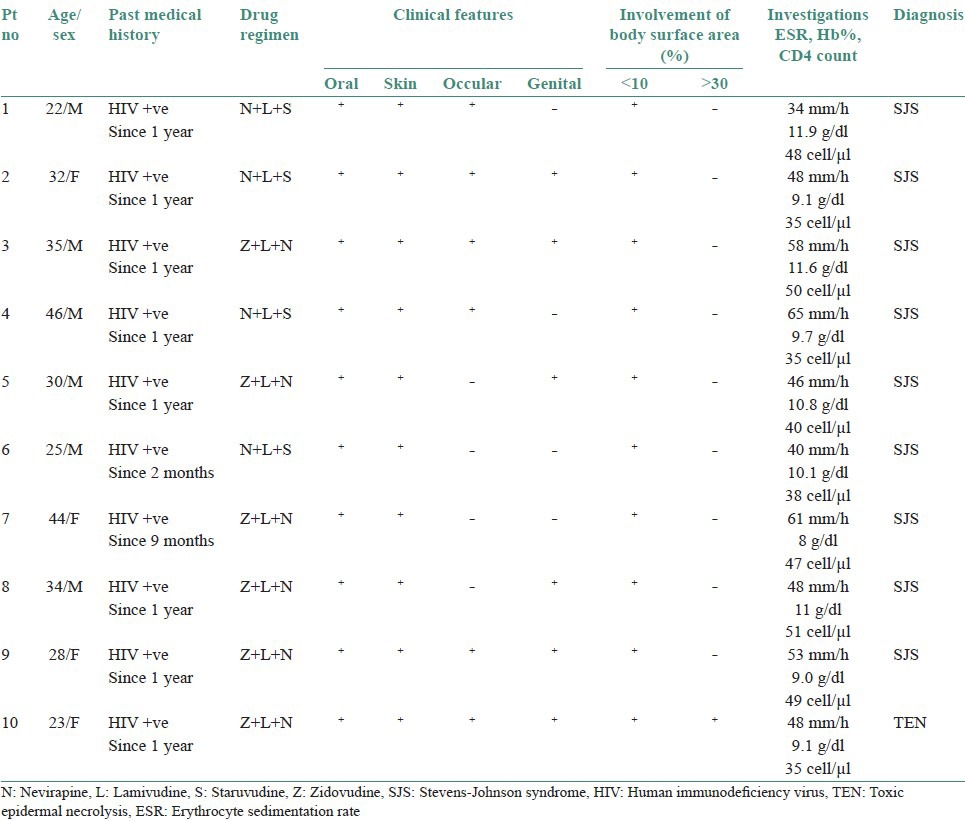
Ten cases of HIV-seropositive patients were reported to Voluntary Counseling Confidential Testing Center (VCCTC), with fever and mucocutaneous ulcerations with a mean duration of 10 days. All the patients were diagnosed as HIV positive one year back (approximately). Out of 10 patients, 6 were males and 4 were females with age range of 22-46 years. On examination, multiple erythematous papules, plaques were present all over the body in nine patients [Figure 1]. The entire skin covering the body surface was denuded and peeled off with minor manipulation and appeared blackish in color in one patient [Figure 2]. Intraorally, multiple oral ulcers of the buccal mucosa, tongue, palate, labial mucosa, soft palate and floor of the mouth were seen in patient with TEN [Figures 3 and 4]. Hemorrhagic crusting of the lips was also noted [Figure 5]. Ophthalmic examination revealed conjunctivitis and ulceration of genitalia was also noted in six patients. Four patients showed involvement of skin, oral, ocular and genital regions. There was no previous history of drug allergy. There was a gradual onset of the lesions between 7 and 14 days following the initiation of the highly active anti retroviral therapy (HAART). All the laboratory findings (Total white blood cell count, (TWBC), Total red blood cell count (TRBC), differential count (DC) and platelet count) were in normal limits, but erythrocyte sedimentation rate (ESR) and hemoglobin (Hb)% were altered. The CD4 count for all the patients was below 100 cells/μl. Based on the history and clinical presentation, a diagnosis of SJS and TEN was given. HAART was discontinued and 2 ml dexamethasone (4 mg/ml) was given to manage mucocutaneous rashes. Intravenous fluids and vitamin supplements were also administered. Summary data for nine patients with SJS and one patient with TEN induced by NVP has been listed in Table 1.
Figure 1.
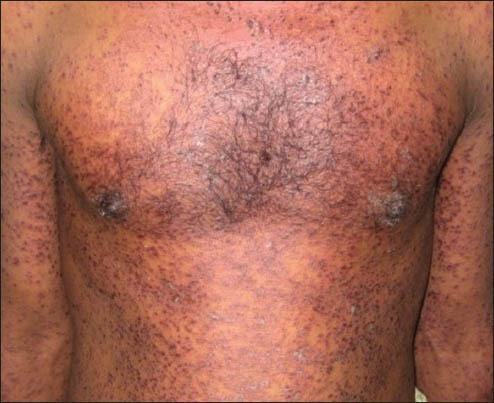
Multiple erythematous papules, plaques were present all over the body
Figure 2.
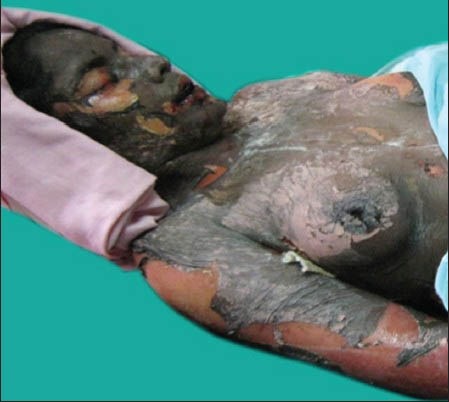
Entire body surface skin denuded and peeled off with minor manipulation and appeared blackish in color (TEN)
Figure 3.
Multiple oral ulcers present on the tongue
Figure 4.
Multiple oral ulcers present on the palate
Figure 5.
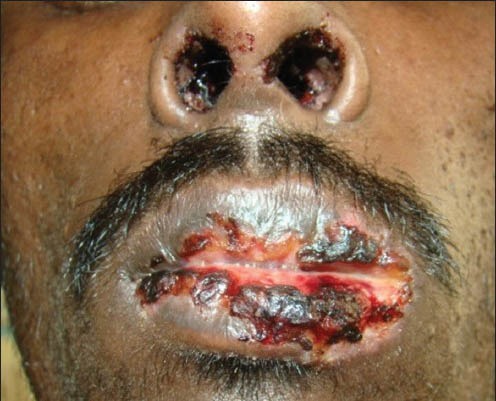
Hemorrhagic crusting of the vermillion zone of the lips noted
Table 1.
Summary of nine cases of SJS and one case of TEN induced by Nevirapine
DISCUSSION
SJS and TEN are cytotoxic hypersensitivity reactions resulting in systemic disease, characterized by sloughing of the skin and mucous membranes at the dermal-epidermal junction.[8] There is a growing evidence that SJS and TEN belong to a single group of diseases with common causes and mechanisms.[9] Both are acute life-threatening conditions.[8]
Recent reports have linked SJS to the use of drugs rather than other etiologic factors. Antibiotics (sulphonamides) are the most common cause of SJS, followed by analgesics, cough and cold medication, nonsteroidal antiinflammatory drug (NSAID), and psycho-epileptics. NVP has also been implicated in the pathogenesis of SJS. There may be a genetic predisposition in developing SJS. Individuals with antigens like human leukocyte antigen Bw44, HLA-B12, and HLADQB1 * 0601 appear to be more susceptible to developing SJS.[1] SJS in children is frequently attributed to infectious agents but may also be related to drug intake.[10] Granulysin is a cationic cytolytic protein released primarily by cytotoxic T cells (CTLs) and NK cells. It is the key molecule responsible for the disseminated keratinocyte death and tissue damage and results in the unique clinical presentation of SJS/TEN.[11]
NVP is a nonnucleoside reverse transcriptase inhibitor approved by the US Food and Drug Administration (FDA) used in HAART, combination regimens for the treatment of HIV infection. Major adverse reactions, including skin rashes and hepatotoxicity occur in approximately 3% of HIV-infected individuals who receive long-term course of NVP. The greatest risk occurred during the first few weeks of NVP treatment.[12] There is persistence of a high risk of SJS or TEN in relation to HIV infection associated with exposure to NVP. The mechanisms probably involve drug specific cytotoxic lymphocytes.[7] Upregulation of keratinocyte Fas L expression is the critical trigger for keratinocyte destruction during TEN.[13] According to the EuroSCAR, study was designed as an international multicenter study aiming at an ongoing surveillance of medication risks for SJS and TEN based on a case-control methodology. A few medications are associated with high risk of SJS or TEN. Prescribing any one of the following drugs requires thorough evaluation of expected benefits:
Nevirapine
Lamotrigine
Carbamazepine
Phenytoin
Phenobarbital
Cotrimoxazole and other antiinfective sulfonamides
Sulfasalazine
Allopurinol
Oxicam-NSAIDs
A delay of 4-28 days between beginning of drug use and onset of the adverse reaction is the most suggestive timing supporting drug causality in SJS or TEN.[9] In our 10 cases, the lesions developed between 1 and 2 weeks after the initiation of HAART, which consisted of NVP.
Burning sensation, edema and erythema of the lips and buccal mucosa are some of the initial signs. Diffuse oral ulcerations of the labial mucosa, buccal mucosa, tongue, floor of the mouth and the soft palate are present. Skin lesions are initially erythematous macules that rapidly develop central necrosis with vesicles, bullae and denudation on the face, trunk and extremities. Mucosal erosions can occur in at least two sites, including conjunctivae, mucous membranes of the nares, mouth, anorectal juctions, vulvovaginal and urethral meatus. Other clinical features include pneumonitis, productive cough, fever, headache and malaise.[14]
In all 10 cases, the mucocutaneous manifestations were similar and associated prodromal symptoms were fever, headache and malaise.
The clinical differential diagnosis of SJS includes Staphylococcal Scalded Skin Syndrome, Kawasaki disease, acute Graft-Versus-Host disease and bullous Systemic Lupus Eythematosus.[14]
Investigations include a complete blood count (CBC), which may be normal. Markedly raised values may indicate a superimposed infection. Liver function tests, electrolytes and other chemistries may be needed. Cultures of blood, urine and wounds are indicated when an infection is coexistent.[1] There are no specific diagnostic tests and the diagnosis is mainly clinically supported. Biopsy of peri-lesional tissue, with histological and immunofluorescence examination are essential if a specific diagnosis is required.[15]
All the 10 cases revealed classic clinical presentation of SJS/TEN. As the patients were in a severe debilitating condition we could not subject to histopathology and immunofluorescence. Blood picture showed normal values except for altered ESR, indicating superimposed infection.
Treatment of these two life-threatening conditions includes prompt recognition and withdrawal of suspected drugs and hospitalization. Infection control, isolation measures and management of cutaneous coverage improve the symptoms and facilitate injuries toward epithelization. Mucosal hygienic measures are of prime importance to increase the patient's comfort and to prevent complications.[16] Oral lesions were treated with topical lignocaine gel. In some patients, oral hygiene was maintained by the use of chlorhexidine mouth wash. Application of paraffin externally was recommended for crusting skin lesions.[1]
After recovery, patients should be advised to avoid not only the suspected drug(s), but also chemically related compounds.[17]
NVP was stopped in all the 10 cases and patients were treated symptomatically by administering intravenous 5% dextrose, dexamethasone 2 ml and high protein supplements were included in the diet. HAART (lamivudine, efavirenz, zidovudine/staruvudine) was initiated again and continued without rechallenge of NVP. All the patients were followed up regularly for 6 months and the response to the treatment was satisfactory.
CONCLUSION
SJS and TEN are two rare but severe blistering mucocutaneous diseases that share common clinical and histopathological features but vary in the extent of epidermal detachment. Both are frequently associated with drug use.
Antiretroviral therapy is not only becoming increasingly effective, but also increasingly complex. Close monitoring and follow-up for patients placed on NVP for HIV are paramount not only for therapeutic response but also for the development of severe complications such as SJS/TEN. As documented in our cases, prompt recognition and institution of appropriate therapies, including transfer to a burn centre, can positively impact survival of patients affected by SJS/TEN. As efforts continue in the development of medications with more favorable adverse effect profiles, treating physicians must remain aware of new and developing syndromes associated with antiretroviral use.
One must be careful and suspect SJS, if a patient is on HAART regimen containing NVP and presents with symptoms arising from irritation of the skin and mucous membranes.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Namayanja GK, Nankya JM, Byamugisha JK, Ssali FN, Kityo CM, Rwambuya SD, et al. Stevens-Johnson syndrome due to niverapine. Afr Health Sci. 2005;5:338–40. doi: 10.5555/afhs.2005.5.4.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sedghizadeh PP, Kumar SK, Gorur A, Mastin C, Boros AL. Toxic epidermal necrolysis with a rare long-term oral complication requiring surgical intervention. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:e29–33. doi: 10.1016/j.tripleo.2007.12.023. [DOI] [PubMed] [Google Scholar]
- 3.Lale Atahan I, Ozyar E, Sahin S, Yildiz F, Yalçin B, Karaduman A. Two cases of stevens Johnson syndrome: Toxic epidermal necrolysis possibly induced by amifostine during radiotherapy. Br J Dermatol. 2000;143:1072–3. doi: 10.1046/j.1365-2133.2000.03847.x. [DOI] [PubMed] [Google Scholar]
- 4.Neville, Damn, Allen, Bouquot . Oral and Maxillofacial Pathology. 3rd ed. India: Saunders; 2009. Dermatologic diseases; pp. 776–9. [Google Scholar]
- 5.Roujeau JC, Kelly JP, Naldi L, Rzany B, Stern RS, Anderson T, et al. Medication use and the risk of Stevens Johnson syndrome or toxic epidermal necrolysis. N Engl J Med. 1995;333:1600–7. doi: 10.1056/NEJM199512143332404. [DOI] [PubMed] [Google Scholar]
- 6.Pierre-Dominique G, Jean–Claude R. Treatment of severe drug reactions–Stevens Johnson syndrome, Toxic Epidermal Necrolysis and Hypersensitivity syndrome. Dermatol Online J. 2002;8:5. [PubMed] [Google Scholar]
- 7.Fagot JP, Mockenhaupt M, Bouwes-Bavinck JN, Naldi L, Viboud C, Roujeau JC EuroSCAR Study Group. Nevirapine and the risk of stevens Johnson syndrome or toxic epidermal necrolysis. AIDS. 2001;15:1843–8. doi: 10.1097/00002030-200109280-00014. [DOI] [PubMed] [Google Scholar]
- 8.Mistry RD, Schwab SH, Treat JR. Stevens Johnson Syndrome and Toxic Epidermal Necrolysis consequence of treatment of an emerging pathogen. Pediatr Emerg Care. 2009;25:519–22. doi: 10.1097/PEC.0b013e3181b0a49a. [DOI] [PubMed] [Google Scholar]
- 9.Mockenhaupt M, Viboud C, Dunant A, Naldi L, Halevy S, Bouwes Bavinck JN, et al. Stevens Johnson syndrome and toxic epidermal necrolysis: Assesment of medication risks with emphasis on recently marketed drugs. The EuroSCAR-Study. J Invest Dermatol. 2008;128:35–44. doi: 10.1038/sj.jid.5701033. [DOI] [PubMed] [Google Scholar]
- 10.Laffitte E, Nenadov Beck M, Hofer M, Hohl D, Panizzon RG. Severe Stevens-Johnson Syndrome induced by contrast medium iopentol (imagopaque) Br J Dermatol. 2004;150:367–8. doi: 10.1111/j.1365-2133.2003.05763.x. [DOI] [PubMed] [Google Scholar]
- 11.Chung WH, Hung SI, Yang JY, Su SC, Huang SP, Wei CY, et al. Granulysin is a key mediator for disseminated keratinocyte death in Stevens Johnson Syndrome and Toxic Epidermal Necrolysis. Nat Med. 2008;14:1343–50. doi: 10.1038/nm.1884. [DOI] [PubMed] [Google Scholar]
- 12.McKoy JM, Bennett CL, Scheetz MH, Differding V, Chandler KL, Scarsi KK, Yarnold PR, et al. Hepatotoxicity associated with long-versus short-course HIV-prophylactic nevirapine use: A systematic review and meta-analysis from the Research on Adverse Drug events and Reports (RADAR) project. Drug Saf. 2009;32:147–58. doi: 10.2165/00002018-200932020-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Viard I, Wehrli P, Bullani R, Schneider P, Holler N, Salomon D, Hunziker T, et al. Inhibition of toxic epidermal necrolysis by blockade of CD95 with human intra venous immunoglobulin. Science. 1998;282:490–3. doi: 10.1126/science.282.5388.490. [DOI] [PubMed] [Google Scholar]
- 14.Samimi SS, Siegfried E. Stevens Johnson Syndrome developing in a girl with Systemic Lupus Erythematosus on high dose corticosteroid therapy. Pediatr Dermatol. 2002;19:52–5. doi: 10.1046/j.1525-1470.2002.00002.x. [DOI] [PubMed] [Google Scholar]
- 15.Farthing P, Bagan JV, Scully C. Mucosal diseases series. Number IV Erythema Multiforme. Oral Dis. 2005;11:261–7. doi: 10.1111/j.1601-0825.2005.01141.x. [DOI] [PubMed] [Google Scholar]
- 16.Radenkova-Saeva J. A non fatal case of lyell's syndrome. J IMAB. 2008;1:18–20. [Google Scholar]
- 17.Balasundaram S, Ranganathan K, Umadevi K, Gunaseelan R, Kumaraswamy N, Solomon S, et al. Oral lesions associated with niverapine related Stevens Johnson syndrome: A report of four cases. J Oral Maxillofac Pathol. 2011;15:39–44. doi: 10.4103/0973-029X.80024. [DOI] [PMC free article] [PubMed] [Google Scholar]


