Abstract
A novel oxime grafting scheme was utilized to conjugate an ICAM-1 ligand (LABL), a cellular antigen ovalbumin (OVA), or both peptides simultaneously to hyaluronic acid (HA). Samples of HA only and the various peptide grafted HA were found to bind to dendritic cells (DCs). HA with grafted LABL and OVA showed the greatest binding to DCs. Dendritic cells treated with HA, HA with grafted LABL, or HA with grafted LABL and OVA, significantly suppressed T cell and DC conjugate formation, T cell proliferation and reduced proinflammatory cytokine production compared to untreated cells. These results suggest that HA serves as an effective backbone for multivalent ligand presentation for inhibiting T cell response to antigen presentation. In addition, multivalent display of both antigen and an ICAM-1inhibitor (LABL) may enhance binding to DCs and could potentially disrupt cellular signaling leading to autoimmunity.
Keywords: peptides, hyaluronic acid, targeted delivery, dendritic cells, T cells
INTRODUCTION
An ideal therapeutic intervention for autoimmune diseases would eliminate the cells that are actively involved in the pathogenesis of the autoimmune disease while maintaining protective immune responses. Strategies have been explored to selectively block major histocompatibility complex class II (MHCII) molecules associated with each autoantigen. In this approach, targeting only the subpopulation of offending antigen presenting cells (APCs) or antigen primed T cells could minimize global immunosuppression. For example, antigen-specific inhibition of the immunological synapse (IS) by simultaneously blocking antigen recognition and costimulation has been explored.1, 2
Receptors participating in adhesion or costimulation have become attractive targets for immune suppression. Additionally, a number of antigen peptide analogs have been shown to bind to “unloaded” MHCII molecules resulting in inhibition of antigen presentation to antigen specific T cells and also a reduction in T cell proliferation.3 Cell-cell communication at the IS includes both of these signals and others, orchestrated in thousands of receptor-ligand interactions. Receptor valency, geometry, and binding duration within the IS are well known factors affecting T cell activation.4–8
In these studies, ovalbumin antigen (ISQAVHAAHAEINEAGR) and a peptide capable of targeting and blocking ICAM-1 (ITDGEATDSG) were conjugated to hyaluronic acid (HA) using a variation of traditional aminooxy chemistry.9–11 The resulting graft polymer constructs displayed multiple copies of antigen, ICAM-1 blocking peptide (LABL), or a combination of both antigen and LABL. These graft polymers were then dosed in cell culture. The HA graft polymers were studied as potential inhibitors of the IS using a primary cell co-culture system consisting of DCs and T cells that present and recognize OVA, respectively. HA graft polymers with OVA antigen, LABL, or a 1:1 ratio of these peptides were explored to determine DC binding and subsequent DC-T cell conjugation, T cell proliferation, and cytokine production.12
RESULTS
Characterization of HA graft polymers
The conjugation of peptides to HA was achieved via a modified oxime chemistry scheme as previously reported.11 An aminooxy group was added to the N-terminus of the synthesized peptides to facilitate reaction to the HA backbone. After reaction, unreacted free peptide was removed through extensive dialysis. Reacted LABL and OVA peptides were quantified after the conjugation reaction via HPLC. Both LABL and OVA peptides were grafted to HA with similar ratios for graft polymers with only one peptide. The graft polymer with both peptides had an approximate 1:1 ratio of LABL and OVA, but approximately the same total peptide content as the other graft polymers as illustrated by total peptide number. The types of samples prepared and the peptide content of each polymer can be found in Table 1.
Table 1.
Conjugation amounts and resulting graft density for HA graft polymers.
| Sample | Concentration LABL (µMol) |
Concentration OVA (µMol) |
Total Mol % |
Theoretical # Peptide per HA polymer |
|---|---|---|---|---|
 |
21.69 | - | 41 | 32 |
 |
- | 24.9 | 47 | 37 |
 |
11.74 | 12.07 | 45 | 35 |
Additionally, the stability of the HA backbone as well as the HA graft polymers in RPMI media was assessed. These experiments were performed at conditions equal to that of the cell culture to ensure that the HA or the graft polymers were not degrading during the time of the experiment. The gel permeation chromatography data showed that even after 48 hours incubation in RPMI media at 37 °C both the HA and the graft polymer chromatograms remained unchanged, suggesting stability at these conditions during the experimental time frame. A representative chromatogram for the HA-LABL graft conjugate is shown in Supplemental Figure 1.
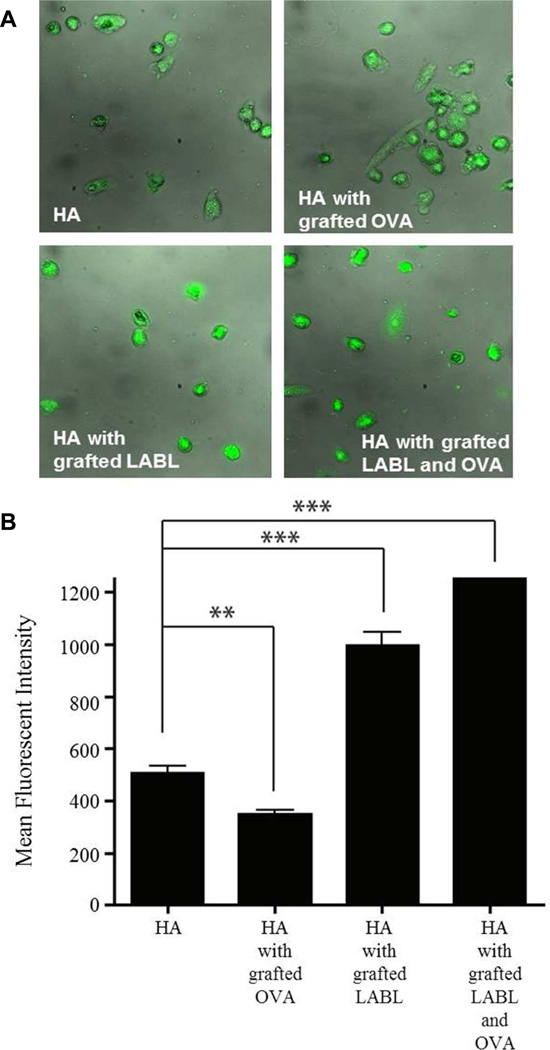
Figure 1.
Binding of HA graft polymers to dendritic cells loaded with OVA and matured with TNF-α. (A) Micrographs of DCs incubated with HA graft polymers for 15 min. (B) Fluorescent intensities of DCs as quantified from fluorescence micrographs. (C) Fluorescent intensities of DCs were confirmed using fluorescence spectroscopy. Data are presented as mean ± S.D. (n=3). *** indicates p<0.001 and ** indicates p<0.05 and * indicates p<0.01.
Binding of HA graft polymers to DCs
The binding of HA, HA with grafted LABL, HA with grafted OVA, or HA with grafted LABL and OVA to DCs was investigated in vitro by fluorescence microscopy and fluorescence spectroscopy. DCs were matured with TNF-α and loaded with OVA for 24 hours prior to addition of HA graft polymers labeled with the fluorophore FITC. Fluorescence microscopy revealed that DCs incubated for 15 min with HA with grafted LABL or HA with grafted LABL and OVA exhibited ~2 and 2.5 fold higher fluorescent intensities than DCs incubated with HA, respectively. DCs incubated with HA with grafted LABL and OVA were significantly more fluorescent than those treated with HA with grafted LABL (Figure 1A and B). The result was confirmed by fluorescence spectroscopy. Incubating DCs with HA with grafted LABL or HA with grafted LABL and OVA resulted in a significant increase in fluorescence intensity of DCs when compared to HA (Figure 1C). HA alone also showed substantial binding to DCs, as expected, since HA is a ligand for many different cell surface molecules such as CD44 present on cells, such as fibroblasts, smooth muscle cells, epithelial cells and immune cells such as DCs, neutrophils, macrophages, and lymphocytes.13, 14
It has been previously reported that free synthetic peptides can also bind directly to unloaded MHCII molecules and be recognized by T cells specific for that antigen.15–18 The higher fluorescence induced by HA with grafted LABL and OVA suggested that a binding event involving the OVA peptide may have augmented the fluorescence. The HA with grafted OVA alone did not bind DC efficiently. Furthermore, the reduction in fluorescence suggested the grafting of OVA may have hindered the binding of HA to nonspecific cell surface molecules. It was unclear whether OVA may provide a small enhancement in the binding of grafted HA polymers when co-grafted with LABL, even though some have suggested that “unloaded” MHC may bind free antigen with low affinity. 16–18
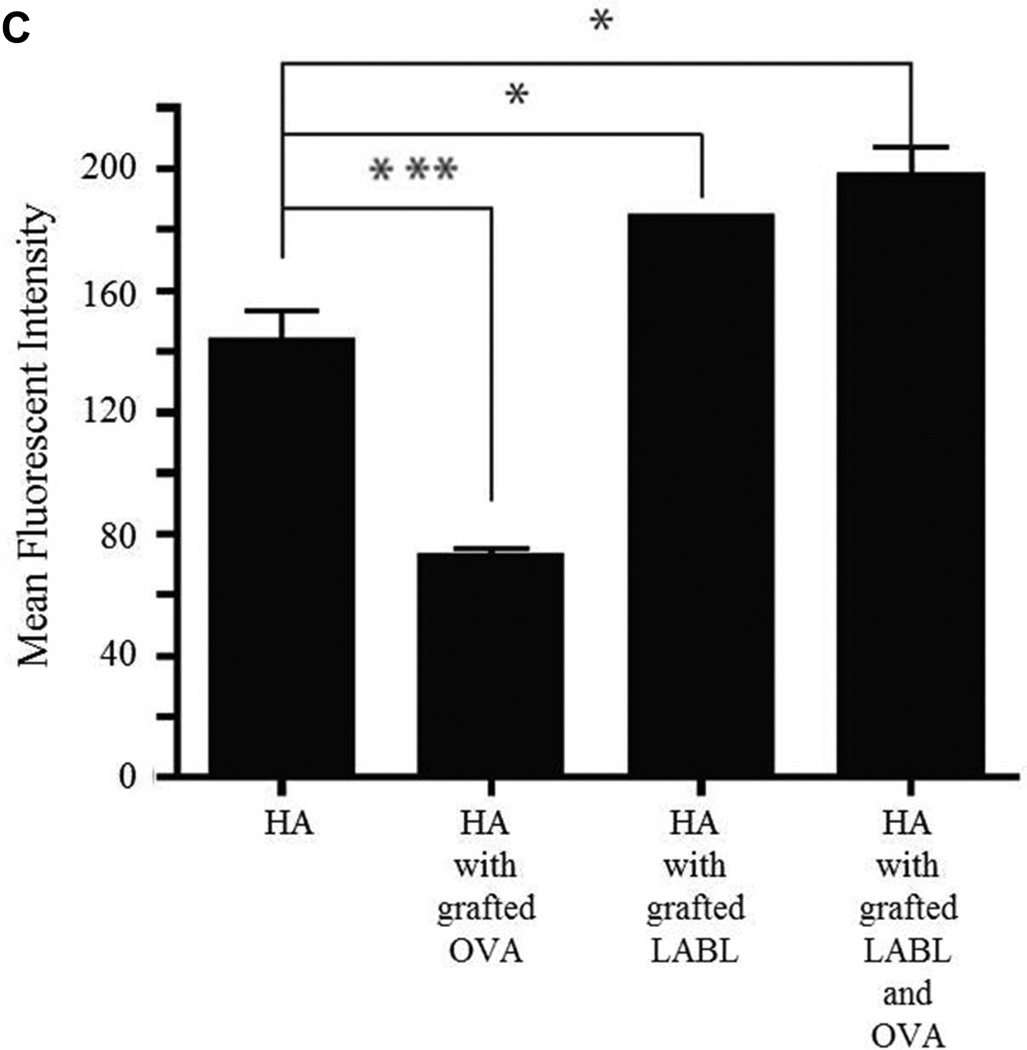
T cell proliferation was reduced by the HA graft polymers
The degree of T cell proliferation was determined after co-culture with DCs loaded with OVA and matured with TNF-α. After priming and maturation, DCs were pretreated with HA, HA with grafted LABL or HA with grafted LABL and OVA for 30 min. Then, these treated DCs were co-cultured with OVA specific T cells. After 24 hours (day 1) and 168 hours (day 7), OVA-specific T cell proliferation was measured by dilution of fluorescent dye (CFSE) using a FACScan flow cytometer. The percent of T cell proliferation was analyzed by calculating the percent of cells with diluted CFSE using FlowJo software. Both the HA with grafted LABL and OVA and the HA with grafted LABL incubated with DCs led to a statistically significant decrease in the number of T cells that had divided compared to the untreated group (Figure 2). There was no significant difference in the level of T cell proliferation between DCs treated with HA compared to untreated DCs. T cells alone without DCs were used as a negative control and yielded a T cell proliferation of only ~0.6%.
Figure 2.
Proliferation of T cells co-cultured with DCs for 1 day and 7 days. T cell proliferation was determined by using flow cytometry. Data are presented as mean ± S.D. (n=3). ** indicates p<0.01 and * indicates p<0.05.
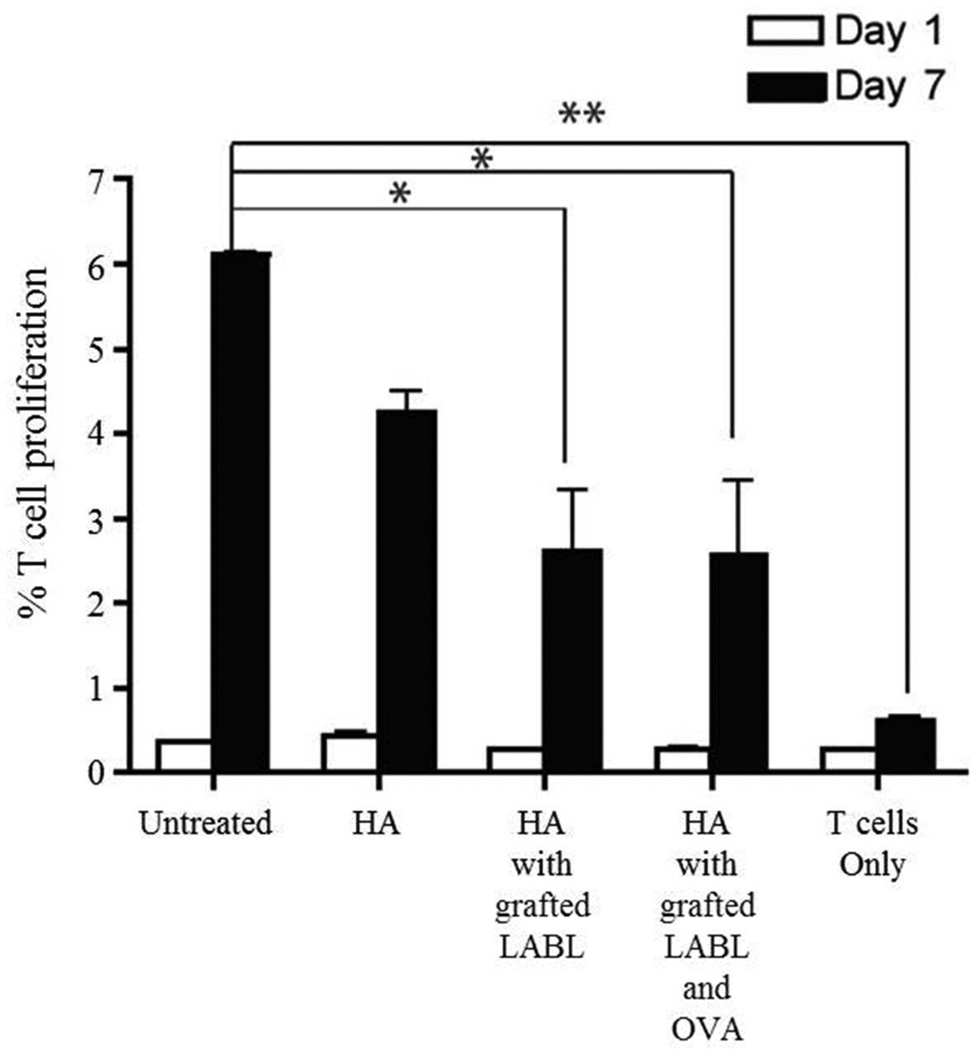
HA and HA graft polymers reduced cytokine production of DCs and OT-II T cells
Blocking of T cell proliferation by HA and HA graft polymers suggested that HA and HA graft polymers may also change cytokine production. To determine the effect of HA and HA graft polymers on cytokine production, DCs were again matured with TNF-α and loaded with OVA in a manner similar to the T cell proliferation study. These DCs were treated with HA, HA with grafted LABL or HA with grafted LABL and OVA for 30 minutes, washed with PBS, and co-cultured with primary OT II T cells for 7 days. TNF-α, IFN-γ, IL-4, IL-10 and IL-17 were then quantified by ELISA. After 7 days, all co-cultures of DCs and T cells had undetectable levels of IFN-γ, IL-4, and IL-10.
When compared to untreated DCs, the levels of TNF-α and IL-17 were significantly reduced in the presence of HA, HA with grafted LABL and HA with grafted LABL and OVA (Figure 3). The amount of TNF-α in the medium of cells exposed to HA, HA with grafted LABL or HA with grafted LABL and OVA was reduced from 10.9 (untreated) to 3.7, 4.1 and 8.2 ng/mL, respectively (Figure 3A). When T cells were cultured by themselves, there was little to no increase seen in TNF-α level. This result suggested that the production of TNF-α by DCs, which can induce inflammation, was inhibited in the presence of HA and HA graft polymers.19 Since TNF-α promotes inflammatory responses, TNF-α can lead to the pathogenesis of various inflammatory and autoimmune diseases.20 Blockade of TNF-α has shown therapeutic efficacy in a number of T cell-dependent autoimmune diseases such as rheumatoid arthritis, juvenile idiopathic arthritis, Crohn’s disease, inflammatory bowel disease and psoriasis.20, 21
Figure 3.
(A) The production of TNF-α in T cell/DC co-cultures where DCs were pretreated with HA, HA with grafted LABL, or HA with grafted LABL and OVA. HA and HA graft polymers decreased the secretion of TNF-α compared to untreated DCs (*** indicates p<0.001 and ** indicates p<0.01). (B) IL-17 in the same co-cultures of T cells and DCs pretreated with HA, HA with grafted LABL, or HA with grafted LABL and OVA was undetectable. Data presented as mean ± S.D. (n=3).
IL-17 has been associated with the pathogenesis of a wide range of inflammatory and autoimmune diseases including asthma, psoriasis, rheumatoid arthritis, inflammatory bowel disease, systemic sclerosis and systemic lupus erythematosus.22, 23 IL-17 produced from untreated cells was 13.9 ± 0.7 ng/mL, whereas the amount of this cytokine secreted from cells in the presence of HA and HA graft polymers was below the limit of detection, < 2pg/mL (Figure 3B). The decrease in cytokine production suggested that HA and HA graft polymers may have inhibited the differentiation of Th17 CD4 cells. The binding of HA and HA graft polymers to DC inhibited T cell and DC immunological synapse formation, which may inhibit T cell proliferation and any ensuing cytokine production (Figure 3). While some portion of T cells died after 7 days of incubation, the T cell proliferation assay measures dilution of the fluorescent dye thus normalizing all data across the study. Therefore, despite some expected T cell death, co-culture of T cells and DCs is sufficient to induce T cell proliferation and to activate TNF-α expression, as well as achieve statistically significant cytokine levels.
HA with grafted LABL, and HA with grafted LABL and OVA, showed increased binding to DCs, a reduction in T cell and DC conjugate formation, T cell proliferation, and suppression of TNF-α and IL-17 production. Additionally, these HA graft polymers did not show increased levels of IFN-γ, IL-4 and IL-10. These data suggest HA graft polymers displaying a cell adhesion antagonist, or a cell adhesion antagonist and antigen may be useful for targeting dendritic cells involved in inflammatory and autoimmune diseases as multivalent, antigen-specific immune modulators.
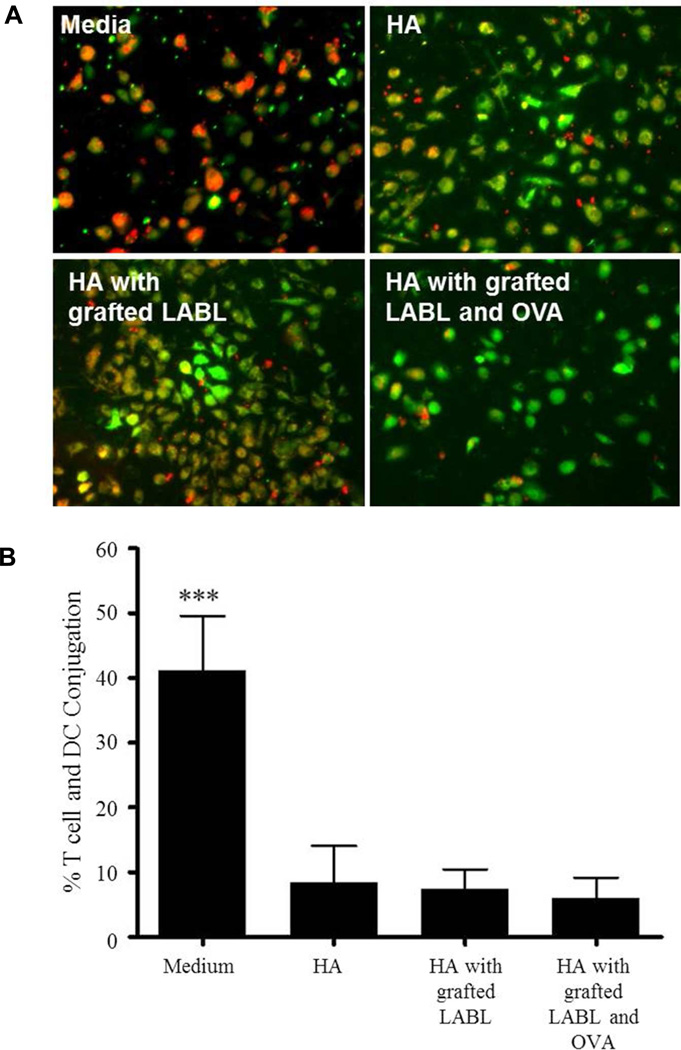
HA and HA grafted peptides significantly inhibited DC-T cell conjugation
DC - T cell conjugation is needed to induce T cell proliferation and differentiation. To evaluate the effect of the HA and HA with grafted peptides on DC - T cell conjugate formation, OVA loaded-DCs labeled with CFSE were treated with HA, HA with grafted LABL and HA with grafted LABL and OVA for 30 min and co-incubated with CMTMR-loaded T cells prepared from OT-II mice. In the presence of HA, HA with grafted LABL and HA with grafted LABL and OVA, conjugation of DCs and T cells was reduced significantly compared to untreated groups (Figure 4A). The percentage of T cells – DC interactions in media (~40%) as compared to treatments with HA (~9%), HA with grafted LABL (~8%), and HA with grafted LABL and OVA (~6%), correlating to a decrease of 79%, 82 and 85%, respectively, when compared to T cell with media treated DCs (Figure 4B).
Figure 4.
(A) Cell co-localization pictures showing T cells (green) and dendritic cells (red) and immunological synapse formation for (a) Media alone (b) HA alone (c) HA with grafted LABL (d) HA with grafted LABL and OVA. (B) Percent of T cell-DC conjugate formation analyzed by imaging analysis software. HA and HA with grafted peptides show a drastic decrease in conjugate formation between T cells and DCs. (*** indicates p<0.001)
METHODS
Cell culture and isolation
Bone marrow derived dendritic cells were generated from C57BL/6 wildtype mice as described.24 Briefly, 2 × 106 cells were isolated from bone marrow, plated on bacterial Petri dishes and cultured in 10 mL DC media (RPMI 1640, 10% heat inactivated fetal bovine serum, 100 µg/mL penicillin-streptomycin, 50 µM β-mercaptoethanol, 20 ng/mL murine granulocyte macrophage colony stimulating factor (GM-CSF) (R&D), and 2 nM L-glutamine. At 72 hours (Day 3), 10 mL of fresh DC media was added to each dish. On days 6 and 8, 10 mL of supernatant and cells were removed, cells recovered by centrifugation (90× g) and added back to the dish with fresh DC medium. On day 9 non-adherent cells were collected. On day 10, T cells were isolated from C57BL/6-TgN(OT-II.2a)-Rag1 spleens by passing spleens through a wire mesh. T cells were purified using a negative 178 selection, mouse T cell enrichment kit according to manufacturer’s directions (EasySep).
Peptide Synthesis
Peptides were synthesized using 9-fluorenylmethyloxycarbonyl-protected amino acid chemistry on polyethylene glycol-polystyrene resins, with an aminooxy carboxylic acid residue added to the N-terminus. The peptides synthesized were aminooxy-LABL (aminooxy-ITDGEATDSG, Ao-LABL), a cell-adhesion molecule antagonist, and aminooxy-ovalbumin peptide (OVA) (aminooxy–ISQAVHAAHAEINEAGR, Ao-OVA), a OVA antigen epitope. Peptides were deprotected, cleaved from resin, and isolated using precipitation in ether. Peptides were purified by preparatory high performance liquid chromatography (HPLC) followed by lyophilization. The identity of the synthesized peptide was confirmed by electrospray ionization mass spectrometry and purity of peptides was assessed using analytical HPLC.
Conjugation of LABL and OVA peptide to HA polymer
The aminooxy group of peptides was linked to hyaluronic acid. Peptides were mixed in a 1:2 molar ratio to the polymer reactive sites. Peptide-HAs were prepared by dissolving HA-FITC polymer (20 mg) in acetate buffer solution (20 mM, 5 mL) of pH 5.5 ± 0.1. Then, LABL peptide (27.8 mg) or OVA peptide (49.4 mg) was added and mixed gently. Reaction solutions were then stirred at 500 RPM using magnetic stir bars for 24 hrs at room temperature. HA with grafted LABL and OVA was prepared by adding LABL (13.8 mg) and OVA (24.8 mg) peptides simultaneously to HA-FITC (20 mg) in solution. Each solution of HA graft polymer was then transferred to dialysis bags (MWCO 3500 Da) and was dialyzed for 24 hrs with changing of water at least once to remove free peptides. The peptide-HAs were lyophilized.
Characterization of HA LABL and/or OVA graft polymer
The amount of LABL and OVA peptides conjugated on HA were quantified by determining the amount of unreacted free peptides in reaction medium using gradient reversed phase HPLC (SHIMADZU) using a C18 column. Additionally, the amount of peptide conjugated to HA for each of the graft conjugates was determined by digested in conjugated in acidic medium then running on HPLC as previously reported.11 The HPLC consisted of SCL-10A SHIMADZU system controller, LC-10AT VP SHIMADZU liquid chromatograph, SIL-10A XL SHIMADZU auto injector set at 30 µL injection volume, DGU-14A SHIMADZU degasser, sample cooler, and SPD-10A SHIMADZU UV-Vis detector (220 nm). The HPLC-UV system was controlled by a personal computer equipped with SHIMADZU class VP Software. All separations were carried out using a VydacR 179 HPLC Protein and Peptide C18 column. Gradient elution was carried out to determine the amount of LABL and OVA peptide at constant flow of 1 mL/min. Gradient elution was carried out to determine the amount of LABL peptide at constant flow of 1 mL/min, Mobile phase compositions were (A) acetonitrile-water (5:95) with 0.1% TFA and (B) 100% acetonitrile with 0.1% trifluoroacetic acid (TFA).
Characterization of HA and HA graft polymer conjugate stability in RPMI media
To assess the stability of HA and the manufactured graft polymer conjugates in cell culture, samples were prepared in RPMI media and then stored at 37 °C. At defined time points, 100 µL of sample was taken and analyzed using the Shimadzu HPLC system with a refractive index detector and Agilent PL-Aqua gel-OH SEC/GPC chromatography Columns. Isocratic elution was carried out with 0.1 M Ammonium Acetate with 0.25 M NaCl at pH 5 buffer at a constant flow of 0.6 mL/min for 45 min.
Binding of HA, HA with grafted LABL, HA with grafted OVA, and, HA with grafted LABL and OVA on DCs
The binding of HA, HA with grafted LABL, HA with grafted OVA, or HA with grafted LABL and OVA was monitored using fluorescence microscopy. The HA of all samples included a FITC label, unless otherwise mentioned. DCs (1 × 105 cells/mL) were added in an 8 well-plate (200 µL/well) and loaded with OVA (50 µg/mL) and matured with TNF-α (1,000 U/mL) for 24 hrs. DCs were washed with PBS and incubated with HA, HA with grafted LABL, HA with grafted OVA, or HA with grafted LABL and OVA (2 mg/mL, 300 µL) at 37 °C for 15 min. Cells were washed three times with PBS and fixed with 4% paraformaldehyde.
Fluorescence micrographs were acquired using the FITC filter set of a Nikon Eclipse 80i microscope equipped for epifluorescence. Micrographs were captured using an Orca ER camera (Hamamatsu, Inc., Bridgewater, NJ) and analyzed by Metamorph, version 6.2 (Universal Imaging Corp., West Chester, PA). All images were corrected for variations in excitation light intensity. Mean fluorescent intensity of cells incubated with HA graft polymers was determined by using ImageJ software.
The binding of HA graft polymers on DCs was also determined and analyzed by using a fluorescence spectrophotometer. DCs were loaded with OVA (50 µg/mL) and matured with TNF-α (1,000 U/mL) for 24 hr. Cells were washed three times with serum free RPMI1640 and incubated with HA, HA with grafted LABL, HA with grafted OVA, or HA with grafted LABL and OVA in serum free medium (2 mg/mL) for 15 min. After incubation, cells were washed three times with ice-cold PBS. HA graft polymers associated with the DCs were detected and compared using a fluorescence plate reader (Spectramax M5; ex: 450 nm, em: 500 nm).
Calculation of T cell and DC conjugate formation
DCs (4 × 105 cells/ml) were stained by incubating with 5 ml of CFSE in PBS (10 µM) for 10 min at 37 °C in PBS. The reaction of dye was quenched by the addition of 25 ml of complete culture medium and incubation at 4°C for 10 min. The remaining dye was washed away three times with complete culture medium. T cells were incubated with 10 µl of CMTMR orange fluorescent dye (5 µM) in 10 ml of PBS for 30 min at 37°C. The staining was quenched by incubating with complete culture medium for 30 min at 37°C followed by washing three times with complete culture medium. Dendritic cells (4 × 105 cells/ml) loaded with OVA (50 µg/ml) and activated with TNF-α (1,000 U/ml) were incubated with FITC labeled HA, HA with grafted LABL, and HA with grafted LABL and OVA (2 mg/ml) for 30 min at 37 °C. Untreated DCs were used as a positive control. Then, T cells (2 × 106 cells/ml) were incubated with DCs for 2 hr at 37 °C, washed three times with PBS and fixed with 4% paraformaldehyde. Cells were imaged using an Orca ER camera (Hamamatsu, Inc., Bridgewater, NJ) and analyzed by Metamorph, version 6.2 (Universal Imaging Corp., West Chester, PA). Number of T-cells attached to dendritic cells (red) and dendritic cells (green) were determined by analyzing red and green pixels, respectively by imaging analysis software. The percentage of T cell-conjugates was calculated.
To complete the T cell proliferation assay, primary T cells isolated from C57BL/6-TgN(OT-II.2a)-Rag1 mice were labeled with CFSE (5 µM) for 10 min at 37 °C, 5% CO2 to observe cell division by CFSE dilution. The dye reaction was quenched by the addition of 5 volumes of culture medium into T cells and incubated 10 min at 4°C. DCs (4 × 105 cells/mL) were loaded with OVA (50 µg/mL) and matured with TNF-α (1,000 U/mL) for 24 hrs in 24-well plate. DCs were treated with HA, HA with grafted LABL, HA with grafted OVA, or HA with grafted LABL and OVA (2 mg/mL) for 30 min, at 37 °C, 5% CO2 and washed three times with PBS. T cells (2 × 106 cells/mL) in serum free RPMI1640, IL-2 and 1% penicillin-streptomycin were incubated with DCs 7 days at 37 °C, 5% CO2. T cells collected after 24 hrs (1 day) and 168 hrs (7 days) were centrifuged at 16,089 g for 2 minutes and fixed with 4% paraformaldehyde. The CFSE dilution was measured by using FACScan flow cytometer. The percent of T cell proliferation was analyzed by calculating the percent of cells with diluted CFSE using FlowJo software.
Quantification of cytokines in cell culture supernatants by ELISA
DCs were loaded with OVA (50 µg/mL) and matured with TNF-α (1,000 U/mL) for 24 hrs in 24-well plate. DCs were treated with HA, HA with grafted LABL, HA with grafted OVA, or HA with grafted LABL and OVA (2 mg/mL) for 30 min, at 37 °C, 5% CO2 and washed three times with PBS. T cells (2 × 106 cells/mL) in serum free RPMI1640, IL-2 and 1% penicillin-streptomycin were incubated with DCs 7 days at 37 °C, 5% CO2. Supernatants of cell cultures were collected for cytokine detection. Secreted IFN-γ, TNF-α, IL-4, IL-10 and IL-17 were measured by an ELISA assay (Cytokine Core Lab, Baltimore, Maryland). ELISA was performed in Nunc Maxisorb ELISA strips freshly coated with capture antibody for 16 hours before the assay was performed. Detecting antibody and the streptavidin peroxidase conjugate were added into standard, all samples, and controls. Premixed substrate solution (Neogen) was then added. The plate was read on a Molecular Devices ELISA plate reader. Curve fitting was selected among linear, quadratic, and 4-point based on the best regression coefficient using the SoftPro software package.
Statistical analysis
Statistical evaluation of data was performed using an analysis of variance (one-way ANOVA). Newman–Keuls was used as a post-hoc test to assess the significance of differences. To compare the significance of the difference between the means of two groups, a t-test was performed; in all cases, a value of p < 0.05 was accepted as significant.
Supplementary Material
ACKNOWLEDGEMENTS
The Authors would like to acknowledge Dr.Kampol Woradit, Department of Engineering, Srinakharinwirot University, for his contributions to this manuscript as well as funding from the NIH 1R56AI091996-01A1.
REFERENCES
- 1.Wraith DC. Therapeutic peptide vaccines for treatment of autoimmune diseases. Immunol Lett. 2009;122(2):134–136. doi: 10.1016/j.imlet.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Larche M, Wraith DC. Peptide-based therapeutic vaccines for allergic and autoimmune diseases. Nat Med. 2005;11(4 Suppl):S69–S76. doi: 10.1038/nm1226. [DOI] [PubMed] [Google Scholar]
- 3.De Magistris MT, Alexander J, Coggeshall M, Altman A, Gaeta FC, Grey HM, Sette A. Antigen analog-major histocompatibility complexes act as antagonists of the T cell receptor. Cell. 1992;68(4):625–634. doi: 10.1016/0092-8674(92)90139-4. [DOI] [PubMed] [Google Scholar]
- 4.Bachmann MF, McKall-Faienza K, Schmits R, Bouchard D, Beach J, Speiser DE, Mak TW, Ohashi PS. Distinct roles for LFA-1 and CD28 during activation of naive T cells: adhesion versus costimulation. Immunity. 1997;7(4):549–557. doi: 10.1016/s1074-7613(00)80376-3. [DOI] [PubMed] [Google Scholar]
- 5.Van Seventer GA, Shimizu Y, Horgan KJ, Shaw S. The LFA-1 ligand ICAM-1 provides an important costimulatory signal for T cell receptor-mediated activation of resting T cells. J Immunol. 1990;144(12):4579–4586. [PubMed] [Google Scholar]
- 6.Springer TA, Dustin ML, Kishimoto TK, Marlin SD. The lymphocyte function-associated LFA-1, CD2, and LFA-3 molecules: cell adhesion receptors of the immune system. Annu Rev Immunol. 1987;5:223–252. doi: 10.1146/annurev.iy.05.040187.001255. [DOI] [PubMed] [Google Scholar]
- 7.Kobayashi N, Kobayashi H, Gu L, Malefyt T, Siahaan TJ. Antigen-specific suppression of experimental autoimmune encephalomyelitis by a novel bifunctional peptide inhibitor. J Pharmacol Exp Ther. 2007;322(2):879–886. doi: 10.1124/jpet.107.123257. [DOI] [PubMed] [Google Scholar]
- 8.Ridwan R, Kiptoo P, Kobayashi N, Weir S, Hughes M, Williams T, Soegianto R, Siahaan TJ. Antigen-specific suppression of experimental autoimmune encephalomyelitis by a novel bifunctional peptide inhibitor: structure optimization and pharmacokinetics. J Pharmacol Exp Ther. 2010;332(3):1136–1145. doi: 10.1124/jpet.109.161109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carrasco MR, Alvarado CI, Dashner ST, Wong AJ, Wong MA. Synthesis of aminooxy and N-alkylaminooxy amines for use in bioconjugation. J Org Chem. 2010;75(16):5757–5759. doi: 10.1021/jo101066c. [DOI] [PubMed] [Google Scholar]
- 10.Mezo G, Szabo I, Kertesz I, Hegedus R, Orban E, Leurs U, Bosze S, Halmos G, Manea M. Efficient synthesis of an (aminooxy) acetylated-somatostatin derivative using (aminooxy)acetic acid as a 'carbonyl capture' reagent. J Pept Sci. 2011;17(1):39–46. doi: 10.1002/psc.1294. [DOI] [PubMed] [Google Scholar]
- 11.Sestak J, Mullins M, Northrup L, Thati S, Siahaan T, Berkland C. Single-Step Grafting of Aminooxy-Peptides to Hyaluronan: A Simple Approach to Multifunctional Therapeutics for Experimental Autoimmune Encephalomyelitis. Journal of Controlled Release. 2013;168(3):334–340. doi: 10.1016/j.jconrel.2013.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mempel TR, Henrickson SE, von Andrian UH. T-cell priming by dendriticcells in lymph nodes occurs in three distinct phases. Nature. 2004;427(6970):154–159. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- 13.Sherman L, Sleeman J, Herrlich P, Ponta H. Hyaluronate receptors: key players in growth, differentiation, migration and tumor progression. Curr Opin Cell Biol. 1994;6(5):726–733. doi: 10.1016/0955-0674(94)90100-7. [DOI] [PubMed] [Google Scholar]
- 14.Haegel-Kronenberger H, de la Salle H, Bohbot A, Oberling F, Cazenave JP, Hanau D. Adhesive and/or signaling functions of CD44 isoforms in human dendritic cells. J Immunol. 1998;161(8):3902–3911. [PubMed] [Google Scholar]
- 15.Dadaglio G, Nelson CA, Deck MB, Petzold SJ, Unanue ER. Characterization and Quantitation of Peptide–MHC Complexes Produced from Hen Egg Lysozyme Using a Monoclonal Antibody. Immunity. 1997;6(6):727–738. doi: 10.1016/s1074-7613(00)80448-3. [DOI] [PubMed] [Google Scholar]
- 16.Viner NJ, Nelson CA, Deck B, Unanue ER. Complexes generated by the binding of free peptides to class II MHC molecules are antigenically diverse compared with those generated by intracellular processing. J Immunol. 1996;156(7):2365–2368. [PubMed] [Google Scholar]
- 17.Babbitt BP, Allen PM, Matsueda G, Haber E, Unanue ER. Binding of immunogenic peptides to Ia histocompatibility molecules. 1985. J Immunol. 2005;175(7):4163–4165. [PubMed] [Google Scholar]
- 18.Babbitt BP, Matsueda G, Haber E, Unanue ER, Allen PM. Antigenic competition at the level of peptide-Ia binding. Proc Natl Acad Sci U S A. 1986;83(12):4509–4513. doi: 10.1073/pnas.83.12.4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Commins SP, Borish L, Steinke JW. Immunologic messenger molecules: cytokines, interferons, and chemokines. J Allergy Clin Immunol. 2010;125(2 Suppl 2):S53–S72. doi: 10.1016/j.jaci.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 20.Nagar M, Jacob-Hirsch J, Vernitsky H, Berkun Y, Ben-Horin S, Amariglio N, Bank I, Kloog Y, Rechavi G, Goldstein I. TNF activates a NF-kappaB-regulated cellular program in human CD45RA-regulatory T cells that modulates their suppressive function. J Immunol. 2010;184(7):3570–3581. doi: 10.4049/jimmunol.0902070. [DOI] [PubMed] [Google Scholar]
- 21.Blanco P, Palucka AK, Pascual V, Banchereau J. Dendritic cells and cytokines in human inflammatory and autoimmune diseases. Cytokine Growth Factor Rev. 2008;19(1):41–52. doi: 10.1016/j.cytogfr.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mills KH. Induction, function and regulation of IL-17-producing T cells. Eur J Immunol. 2008;38(10):2636–2649. doi: 10.1002/eji.200838535. [DOI] [PubMed] [Google Scholar]
- 23.Song C, Luo L, Lei Z, Li B, Liang Z, Liu G, Li D, Zhang G, Huang B, Feng ZH. IL-17-producing alveolar macrophages mediate allergic lung inflammation related to asthma. J Immunol. 2008;181(9):6117–6124. doi: 10.4049/jimmunol.181.9.6117. [DOI] [PubMed] [Google Scholar]
- 24.Lutz MB, Kukutsch N, Ogilvie ALJ, Rosner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. Journal of Immunological Methods. 1999;223(1):77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.