Abstract
Objectives
We sought to evaluate the incidence, correlates, and clinical implications of periprocedural myocardial injury (PMI) during percutaneous coronary intervention (PCI) of chronic total occlusions (CTOs).
Background
The risk of PMI during CTO PCI may be underestimated because systematic cardiac biomarker measurement was not performed in published studies.
Methods
We retrospectively examined PMI among 325 consecutive CTO PCIs performed at our institution between 2005 and 2012. Creatine kinase MB fraction (CK-MB) and troponin were measured before PCI and 8–12 and 18–24 hours after PCI in all patients. PMI was defined as CK-MB increase ≥3x upper limit of normal. Major adverse cardiac events (MACE) during mid-term follow-up was evaluated.
Results
Mean age was 64±8 years. The retrograde approach was used in 26.8% of all procedures. The technical and procedural success was 77.8% and 76.6%, respectively. PMI occurred in 28 patients (8.6%, 95% confidence intervals 5.8%, 12.2%), with symptomatic ischemia in 7 of those patients. The incidence of PMI was higher in patients treated with the retrograde compared to the antegrade approach (13.8% vs. 6.7%, p=0.04). During a median follow-up of 2.3 years, compared to patients without PMI those with PMI had higher incidence of MACE (hazard ratio=2.25, p=0.006). Patients with only asymptomatic PMI also had higher incidence of MACE on follow-up (HR=2.26, p=0.013).
Conclusions
Systematic measurement of cardiac biomarkers post CTO PCI demonstrates that PMI occurs in 8.6% of patients, is more common with the retrograde approach, and is associated with worse subsequent clinical outcomes during mid-term follow-up.
Keywords: chronic total occlusion, percutaneous coronary intervention, complications, acute myocardial infarction, periprocedural myocardial injury
Periprocedural myocardial injury (PMI) is a known complication of percutaneous coronary intervention (PCI) and has been associated with higher mortality, even when patients do not develop symptoms or electrocardiographic changes (1,2). PCI of chronic total occlusions (CTOs) can be technically challenging and may require use of advanced crossing techniques that could result in high rates of PMI (3). In a weighted meta-analysis of 18,061 patients from 65 studies reporting complications after CTO PCI the incidence of PMI was 2.5% (95% CI: 1.9%–3.0%) and the incidence of Q-wave myocardial infarction (MI) was 0.2% (95% CI: 0.1%–0.3%) (4). However, systematic cardiac biomarker measurements were not performed in these studies, hence the true incidence of PMI may be underestimated. Furthermore, while in non-CTO PCI PMI is associated with higher immediate and long-term morbidity, the prognostic implications of PMI in CTO PCI remain unclear (4). To overcome these limitations we performed the present study aiming to (a) evaluate the incidence of both symptomatic and asymptomatic PMI in CTO PCI using serial biomarker measurements; (b) assess the association of PMI with various CTO PCI techniques; and (c) determine the impact of PMI on subsequent clinical outcomes.
Methods
Patients
Between 2005 and 2012, 325 consecutive patients who underwent CTO PCI at our institution had serial post-PCI cardiac biomarker measurements (which is standard policy at our institution for all PCIs) and were included in the present study. Their medical records, electrocardiograms, and coronary angiograms were reviewed. The study was approved by the institutional review board of our institution.
Definitions
Coronary CTOs were defined as coronary lesions with thrombolysis in myocardial infarction (TIMI) grade 0 flow for a duration of at least 3 months. Estimation of the occlusion duration was based on first onset of anginal symptoms, prior history of MI in the target vessel territory, or comparison with a prior angiogram.
Technical success of CTO PCI was defined as successful CTO revascularization with achievement of <30% residual diameter stenosis within the treated segment and restoration of TIMI grade 3 antegrade flow. Procedural success was defined as achievement of technical success with no in-hospital major adverse cardiac events (MACE).
For in-hospital events, MACE was defined as the composite of death and clinical MI (symptoms or signs suggestive of ischemia in addition to increase and fall of cardiac biomarker levels). For events during follow-up, MACE was defined as the composite of all-cause death, MI (defined according to the Universal Definition of MI 2012 version), or any coronary revascularization (5). All patients underwent CK-MB and troponin measurement before PCI and at 8–12 and 18–24 hours after PCI. PMI was defined as CK-MB increase ≥3x upper limit of normal (ULN), if the baseline CK-MB levels were below ULN. If the baseline CK-MB levels were higher than the ULN, PMI was defined as a CK-MB increase ≥3x ULN if the relative increase of the highest post-PCI CK-MB was >20% above the baseline level. Periprocedural MI was defined as the subset of PMI patients who had evidence of prolonged ischemia as demonstrated by persistent chest pain (>20 minutes), or new pathological Q waves seen on the electrocardiogram (5). Cardiac troponin level elevation was reported using various cutoffs (≥3x, ≥10x, and ≥20x ULN). The ULN for CK-MB and troponin at our institution was 6.3 ng/ml and 0.03 ng/ml, respectively.
Statistical analysis
Continuous variables were presented as mean ± standard deviation or median with interquartile range and compared using the t-test or Wilcoxon rank-sum test, as appropriate. Categorical variables were reported as percentages and compared using the chi-square or Fisher’s exact test, as appropriate. A two sided p-value of <0.05 was considered statistically significant. Logistic regression analysis was performed to identify predictors of PMI in CTO PCI using the SAS macro written by Bursac et al (6). Any variable having a significant univariate association with PMI (p-value 0.25 or less) was selected as a candidate for the multivariable analysis. Age, gender, diabetes, hypertension, prior coronary artery bypass graft (CABG) surgery, use of the retrograde approach, and procedure time were candidates for the multivariable model. In the iterative process of variable selection, covariates were removed from the model if they were non-significant and not a confounder. Significance was evaluated at the 0.1 alpha level and confounding as a change in any parameter estimate greater than 15%. At the end of this iterative process, the model contained significant covariates and confounders. At this point any variable not selected for the original multivariable model was added back one at a time, with significant covariates and confounders retained earlier. Any that were significant at the 0.1 level were put in the model, and the model was iteratively reduced as before, but only for the variables that were additionally added.
The incidence of MACE was calculated using the Kaplan-Meier method and compared using the log-rank test, with hazard ratio calculated using Cox proportional hazard. Sensitivity and specificity of post-PCI troponin values for the prediction of MACE were evaluated with receiver operating characteristic (ROC) curves, and the Youden index was calculated for each troponin cutoff value. Statistical analyses were performed using JMP version 9.0 (SAS Institute, Cary, North Carolina), SAS version 9.2 for Linux (SAS Institute, Cary, North Carolina), and Stata release 11 (StataCorp, College Station, TX).
Results
Patient characteristics
The clinical and procedural characteristics of the study patients are presented in Table 1. As is common in CTO PCI series, most patients were men with high prevalence of atherosclerosis risk factors. Approximately 1 in 4 patients had prior CABG and 1 in 3 patients had prior PCI. The most common CTO target vessel was the right coronary artery. The retrograde approach was used in 26.8% of all procedures and the technical and procedural success was 77.8% and 76.6%, respectively. Procedure time, fluoroscopy time, air kerma radiation exposure, and total contrast utilization were higher in patients treated with the retrograde approach (p=0.001).
Table 1.
Clinical and angiographic characteristics and outcomes of the study patients, classified according to whether they underwent chronic total occlusion percutaneous coronary intervention using the antegrade or the retrograde approach
| All patients (n=325) |
Antegrade (n=238) |
Retrograde (n=87) |
p | |
|---|---|---|---|---|
| Age (years)* | 64 ± 8.4 | 64 ± 8.8 | 64 ± 7.4 | 0.704 |
| Men (%) | 98.7 | 98.7 | 98.8 | 0.935 |
| Hypertension (%) | 90.0 | 89.5 | 91.9 | 0.501 |
| Hyperlipidemia (%) | 89.0 | 87.8 | 91.9 | 0.278 |
| Diabetes (%) | 47.0 | 48.2 | 43.7 | 0.447 |
| Heart failure (%) | 38.4 | 39.5 | 35.6 | 0.527 |
| History of MI (%) | 47.3 | 45.4 | 53.9 | 0.231 |
| History of CABG (%) | 26.0 | 20.7 | 40.2 | 0.001 |
| History of stroke (%) | 4.3 | 3.8 | 5.7 | 0.452 |
| Prior PCI (%) | 36.4 | 40.5 | 25.3 | 0.011 |
| Initial presentation with | 20.9 | 23.5 | 13.8 | 0.048 |
| ACS (%) | ||||
| CTO target vessel | 0.001 | |||
| RCA (%) | 66 | 46.8 | 78.2 | |
| LCx (%) | 18 | 25.5 | 9.2 | |
| LAD (%) | 15.5 | 25.9 | 10.3 | |
| LM/graft (%) | 0.5 | 1.4 | 1.3 | |
| Number of stents implanted† |
2 (0–3) | 2 (1–3) | 3 (0–4) | 0.387 |
| Procedure time (min)† | 124 (88–177) | 107.5 (84.3–141.7) | 192 (151–238) | 0.001 |
| Fluoroscopy time (min)† | 34.7 (21.6–52.7) | 28.6 (18.5–40.3) | 55.2 (44.6–71.7) | 0.001 |
| Air kerma radiation exposure (Gray)† |
4.4 (3.0–5.9) | 3.4 (2.4–5.0) | 5.7 (4.5–7.3) | 0.001 |
| Contrast volume (ml)† | 338 (250–430) | 310 (230–400) | 400 (300–500) | 0.001 |
| Post-PCI CK-MB increase ≥3xULN (%) |
8.6 | 6.7 | 13.8 | 0.044 |
| Post-PCI Troponin ≥ 3xULN (%) |
60.6 | 51.7 | 85.1 | <0.0001 |
| Post-PCI Troponin ≥ 10xULN (%) |
43.1 | 33.2 | 70.1 | <0.0001 |
| Post-PCI Troponin ≥ 20xULN (%) |
31.4 | 24.8 | 49.4 | <0.0001 |
| Technical success (%) | 77.8 | 80.7 | 70.1 | 0.047 |
| Procedural success (%) | 76.6 | 80.3 | 66.7 | 0.014 |
Mean ± standard deviation.
Median and interquartile range.
MI, myocardial infarction; CABG, coronary artery bypass graft; PCI, percutaneous coronary intervention; ACS, acute coronary syndrome; CTO, chronic total occlusion; RCA, right coronary artery; Lcx, left circumflex; LAD, left anterior descending; LM, left main artery; ULN, upper limit of normal; min, minute; ml, milliliter
The clinical presentation of patients who underwent CTO PCI is shown in Table 2. Approximately 80% of patients presented with stable angina and 20% with recent acute coronary syndrome (ACS), mainly non-ST-segment elevation acute myocardial infarction (NSTEMI) or unstable angina. More patients presenting with ACS were treated using the antegrade than the retrograde approach (23.5% vs. 13.5%, p=0.048, Table 1).
Table 2.
Clinical presentation of patients who underwent chronic total occlusion percutaneous coronary intervention during the study period
| Clinical Presentation | Number (n=325) |
Percent of total (%) |
|---|---|---|
| Stable Angina | 257 | 79.1 |
| Acute Coronary Syndrome | 68 | 20.9 |
| Unstable Angina | 31 | 9.5 |
| NSTEMI | 36 | 11.1 |
| STEMI | 1 | 0.31 |
NSTEMI, non-ST-segment elevation acute myocardial infarction; STEMI, ST-segment elevation acute myocardial infarction
Frequency of periprocedural myocardial injury
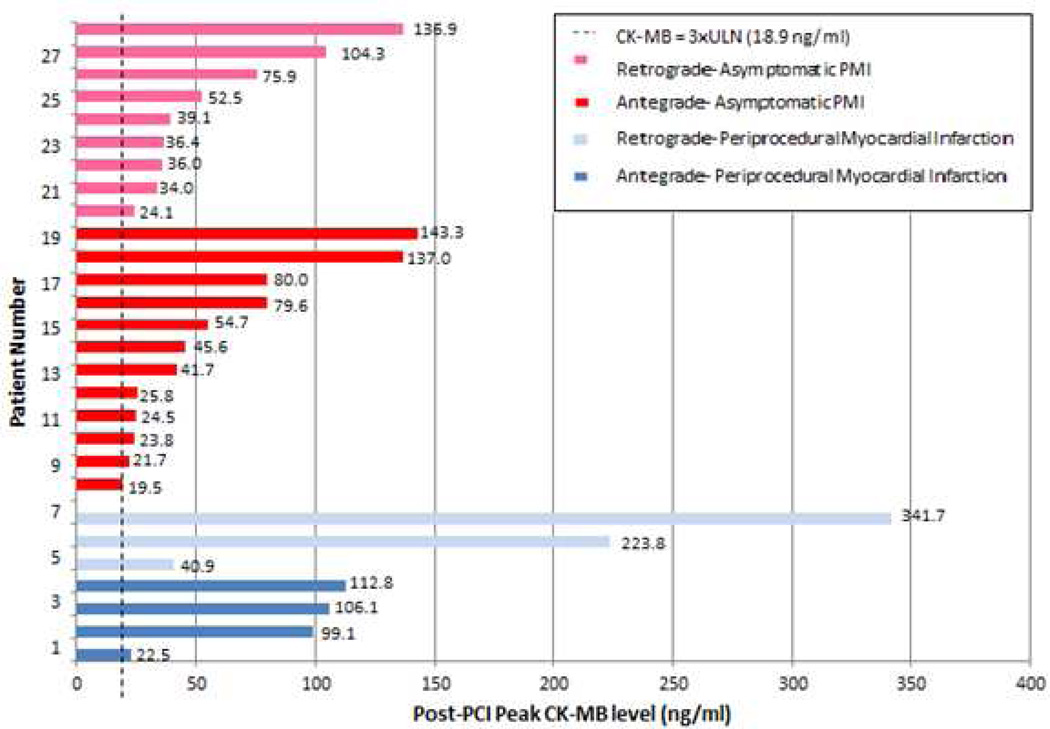
PMI occurred in 28 patients (8.6%, 95% CI: 5.8%–12.2%). The mean and median CK-MB level was 69 ± 73 and 39 (IQR: 22–103) ng/ml, respectively (Figure 1). The baseline and maximum CK-MB for these 28 patients are presented in Table 3. Of the 325 CTO PCI patients studied, 30 had elevated baseline CK-MB ≥6.3 ng/ml (ULN): 9 of these 30 had peak post-PCI CK-MB ≥3xULN, but 3 patients had peak post-PCI CK-MB that were less than baseline CK-MB and thus were not categorized as PMI. Therefore, 6 patients, or 21.4% of patients with PMI, had baseline CK-MB ≥ULN and still met the criteria for PMI (Table 3).
Figure 1. Post-PCI peak CK-MB levels of the 28 patients who developed PMI during CTO PCI.
Periprocedural MI occurred in 7 of the 28 patients with PMI: 6 patients had prolonged ischemic symptoms >20 minutes, and 1 patient (patient 7) had both prolonged chest pain and new Q-wave MI.
PCI, percutaneous coronary intervention; CK-MB, creatine kinase myocardial band fraction; PMI, periprocedural myocardial injury; CTO, chronic total occlusion; ULN, upper limit of normal; MI, myocardial infarction
Table 3.
Baseline and peak post percutaneous coronary intervention CK-MB levels for all patients with periprocedural myocardial injury
| Patient Number |
Baseline CK-MB (ng/ml) |
Peak post-PCI CK-MB (ng/ml) |
|---|---|---|
| 1 | 6.9 | 22.5 |
| 2 | 1.9 | 99.1 |
| 3 | 1.1 | 106.1 |
| 4 | 1.9 | 112.8 |
| 5 | 6.6 | 40.9 |
| 6 | 2.4 | 223.8 |
| 7 | 12.3 | 341.7 |
| 8 | 4.1 | 19.5 |
| 9 | 1.7 | 21.7 |
| 10 | 5.0 | 23.8 |
| 11 | 3.0 | 24.5 |
| 12 | 3.3 | 25.8 |
| 13 | 2.6 | 41.7 |
| 14 | 6.2 | 45.6 |
| 15 | 7.2 | 54.7 |
| 16 | 2.1 | 79.6 |
| 17 | 1.2 | 80.0 |
| 18 | 2.5 | 137.0 |
| 19 | 24.9 | 143.3 |
| 20 | 1.1 | 24.1 |
| 21 | 6.8 | 34.0 |
| 22 | 4.3 | 36.0 |
| 23 | 2.9 | 36.4 |
| 24 | 1.3 | 39.1 |
| 25 | 1.8 | 52.5 |
| 26 | 3.0 | 75.9 |
| 27 | 5.6 | 104.3 |
| 28 | 1.0 | 136.9 |
Periprocedural MI occurred in 7 of the 28 patients with PMI (25% of PMI patients or 2.1% of CTO PCI patients) as 6 patients had prolonged ischemic symptoms > 20 minutes, and 1 patient (patient number 7) had both prolonged chest pain and new Q-wave MI (Q-wave MI developed in 3.6% of PMI patients or 0.3% of total CTO PCI patients) (Figure 1). The frequency of periprocedural cardiac troponin elevation ≥3x, ≥10x, and ≥20x ULN was 61%, 43% and 31%, respectively. Irrespective of the troponin cut-off used to define PMI, the frequency of periprocedural troponin elevation was greater when the retrograde approach was used (p<0.0001, Table 1).
Correlates of periprocedural myocardial injury
The frequency of PMI among 21 patients who had a procedural complication (emergency CABG, tamponade etc) was 19% vs. 8% among patients who did not have a complication (p=0.12). PMI was numerically higher among patients in whom CTO PCI failed compared to those in whom CTO PCI was successful (11.8% vs. 7.6%, p=0.268).
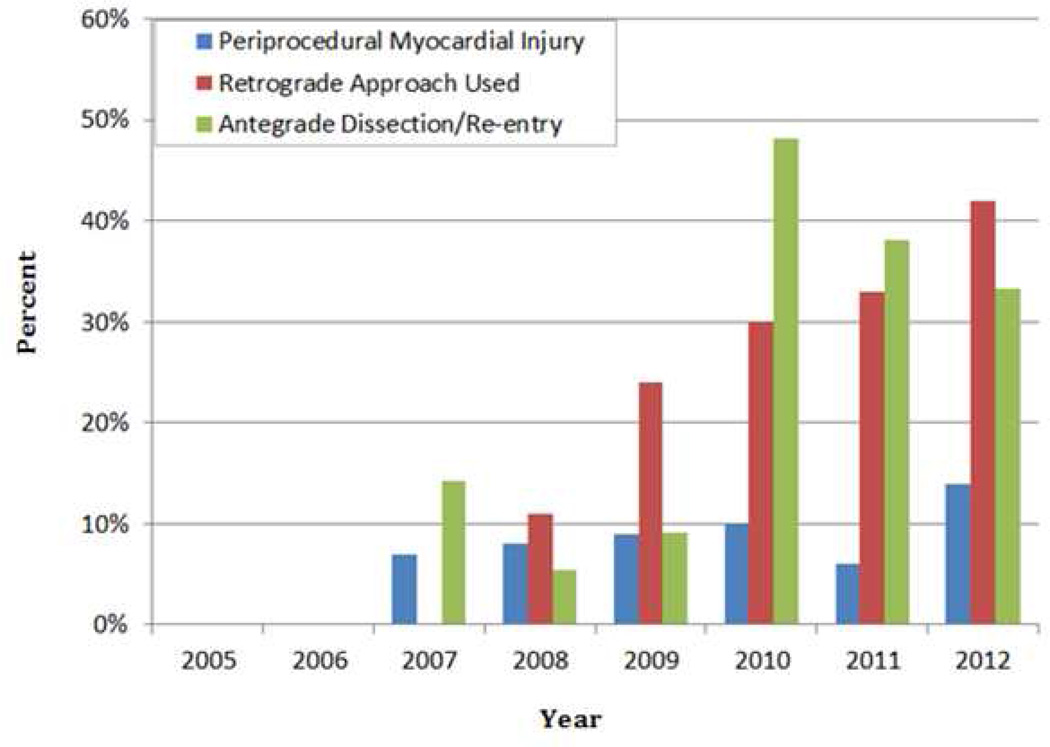
The clinical characteristics and outcomes of patients with PMI vs. those without PMI are shown in Table 4. Patients with PMI were more likely to have hypertension and prior CABG (p=0.014). They were also more likely to have had CTO PCI using the retrograde approach: PMI occurred in 13.8% of patients in whom the retrograde approach was used compared to 6.7% of patients in whom only antegrade crossing was performed (p=0.044). Antegrade dissection/reentry was used in 29% of cases and antegrade wire escalation was used in 90.3% of cases. The incidence of PMI when antegrade dissection/re-entry crossing strategies were used was 11.2% vs. 7.7% when such strategies were not used (antegrade wire escalation and/or retrograde approach, p=0.311). If retrograde CTO PCI cases were excluded from the analysis, the PMI rate with antegrade dissection/re-entry was similar to that of antegrade wire escalation (8.0% of 50 cases vs. 7.0% of 213 cases, p=0.821). On multivariable analysis, results of the purposeful selection model yielded two variables associated with PMI: diabetes (adjusted OR=0.45, 95% CI: 0.19–1.04, p=0.061) and prior CABG (adjusted OR=3.03, 95% CI: 1.36–6.75, p=0.007). The incidence of PMI tended to increase over time in conjunction with an increase in the use of retrograde crossing strategies and was unrelated to the utilization of antegrade dissection/re-entry strategies (Figure 2).
Table 4.
Clinical and angiographic characteristics and outcomes of the study patients, classified according to whether they had periprocedural myocardial injury
| All patients (n=325) |
PMI (n=28) | No PMI (n=297) | p | |
|---|---|---|---|---|
| Age (years)* | 64 ± 8.4 | 63 ± 5.6 | 64 ± 8.7 | 0.281 |
| Men (%) | 98.7 | 100.0 | 98.6 | 0.394 |
| Hypertension (%) | 90.0 | 100.0 | 89.2 | 0.014 |
| Hyperlipidemia (%) | 89.0 | 92.9 | 88.6 | 0.464 |
| Diabetes (%) | 47.0 | 32.1 | 48.5 | 0.094 |
| Heart failure (%) | 38.4 | 35.7 | 38.7 | 0.754 |
| History of MI (%) | 47.3 | 50.0 | 47.1 | 0.772 |
| History of CABG (%) | 26.0 | 46.4 | 24.0 | 0.014 |
| History of stroke (%) | 4.3 | 3.6 | 4.4 | 0.837 |
| Prior PCI (%) | 36.4 | 46.4 | 35.5 | 0.256 |
| Retrograde approach (%) | 26.8 | 43.9 | 25.3 | 0.044 |
| CTO target vessel | 0.271 | |||
| RCA (%) | 66 | |||
| LCx (%) | 18 | |||
| LAD (%) | 15.5 | |||
| LM/graft (%) | 0.5 | |||
| Technical success (%) | 77.8 | 67.9 | 78.8 | 0.201 |
| Procedural success (%) | 76.6 | 67.9 | 77.4 | 0.268 |
| Number of stents implanted† |
2 (0–3) | 3 (0–4) | 2 (0–3) | 0.778 |
| Procedure time (min)† | 124 (88–177) | 175 (147–241) | 120 (88–174) | <0.0001 |
| Fluoroscopy time (min)† | 34.7 (21.6–52.7) | 64.5 (43.5–72.7) | 31.9 (21.1–50.0) | <0.0001 |
| Air kerma radiation exposure (Gray)† |
4.4 (3.0–5.9) | 5.9 (5.3–7.4) | 4.2 (2.8–5.6) | 0.012 |
| Contrast volume (ml)† | 338 (250–430) | 450 (375–545) | 325 (245–415) | <0.0001 |
Mean ± standard deviation.
Median and interquartile range.
PMI, periprocedural myocardial injury; MI, myocardial infarction; CABG, coronary artery bypass graft; PCI, percutaneous coronary intervention; CTO, chronic total occlusion; RCA, right coronary artery; Lcx, left circumflex; LAD, left anterior descending; LM, left main artery; ULN, upper limit of normal; min, minute; ml, milliliter
Figure 2. Temporal trends.
Temporal trends in the incidence of periprocedural myocardial injury, use of the retrograde approach, and use of the antegrade dissection/re-entry in CTO PCIs.
CTO, chronic total occlusion; PCI, percutaneous coronary intervention
PMI and clinical outcomes after CTO PCI
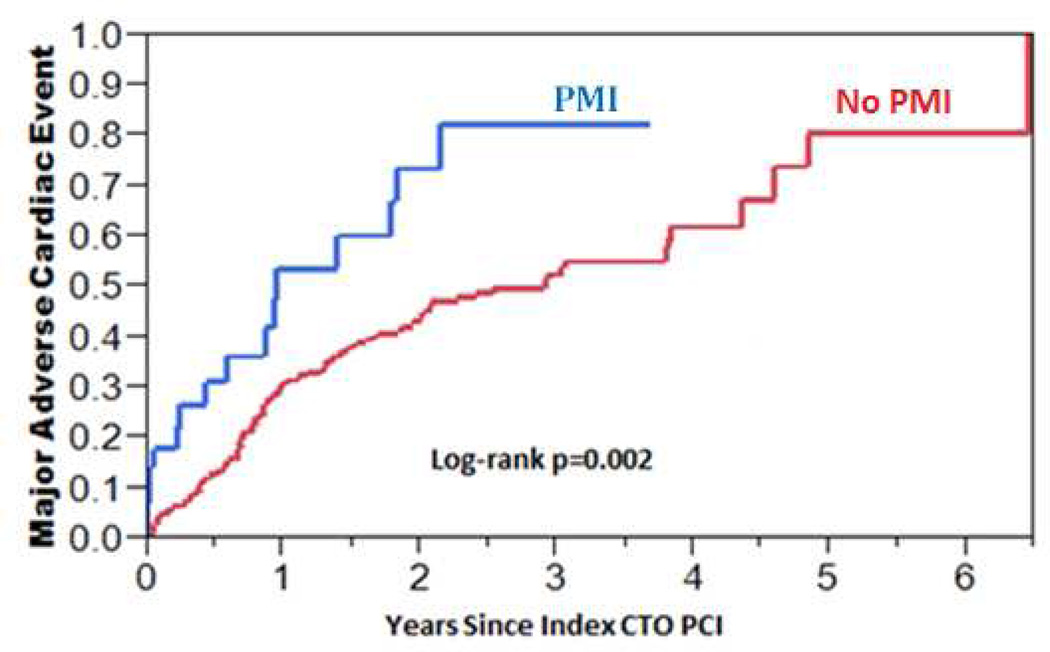
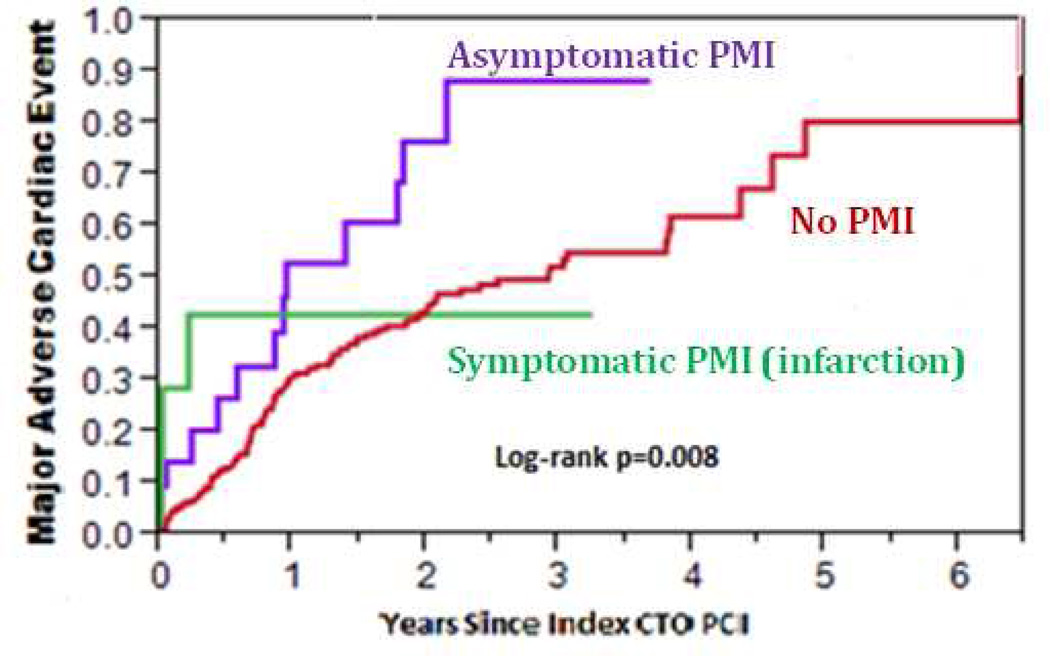
During mid-term follow-up (median 2.3 years, IQR: 0.8–4.6 years), 40% of patients experienced MACE: 11% died, 9% had an ACS, 28.5% required PCI, and 3.4% underwent CABG. The incidence of MACE during mid-term follow-up was higher among patients who had PMI during the index PCI compared to those who did not (HR = 2.25, 95% CI: 1.28–3.70, p=0.006, Figure 3). When patients with asymptomatic PMI were separately analyzed from those with periprocedural MI, the incidence of MACE was still higher among patients who had asymptomatic PMI compared to those who had no PMI (HR = 2.26, 95% CI: 1.21–3.88, p=0.013, Figure 4). Patients with periprocedural MI had a trend toward higher incidence of MACE compared to patients without PMI (HR = 2.23, 95% CI: 0.55–5.92, p=0.226, Figure 4). As shown in Table 5, patients with asymptomatic PMI, periprocedural MI, and no PMI were discharged on similar medication regimens.
Figure 3. Kaplan-Meier curves of the incidence of major adverse cardiac events (MACE) in patients with and without periprocedural myocardial injury (PMI) after chronic total occlusion percutaneous coronary intervention.
The incidence of MACE during a median of 2.3 years (IQR: 0.8–4.6 years) was higher among patients who had PMI during CTO PCI compared to those who did not have PMI (HR = 2.25, 95% CI: 1.28–3.70, p = 0.006).
MACE, major adverse cardiac events; PMI, periprocedural myocardial injury; IQR, interquartile range; CTO, chronic total occlusion; PCI, percutaneous coronary intervention; HR, hazard ratio; CI, confidence interval
Figure 4. Incidence of major adverse cardiac events (MACE) among study patients, classified according to the occurrence of periprocedural myocardial injury (PMI).
Kaplan-Meier curves describing the incidence of MACE among patients with asymptomatic PMI, symptomatic PMI, and no PMI
MACE, major adverse cardiac events; PMI, periprocedural myocardial injury
Table 5.
Discharge medications of patients undergoing CTO PCI during the study period.
| All Patients (n=325) |
Asymptomatic PMI (n=21) |
Periprocedural MI (n=7) |
No PMI or periproceduralM I (n=297) |
p | |
|---|---|---|---|---|---|
| Beta- Blocker (%) |
93.5 | 95.2 | 100 | 93.2 | 0.579 |
| ACEI or ARB (%) |
79.3 | 66.7 | 71.4 | 80.3 | 0.325 |
| Statin (%) | 96.9 | 100 | 100 | 88.2 | 0.398 |
| Aspirin (%) | 98.8 | 100 | 100 | 98.6 | 0.694 |
| Thieno- pyridine (%) |
90.1* | 81.0 | 85.7 | 90.9 | 0.383 |
a thienopyridine was administered in 98.8% of successful CTO PCI procedures vs. 61.3% of failed CTO PCI procedures.
PMI, periprocedural myocardial injury; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker
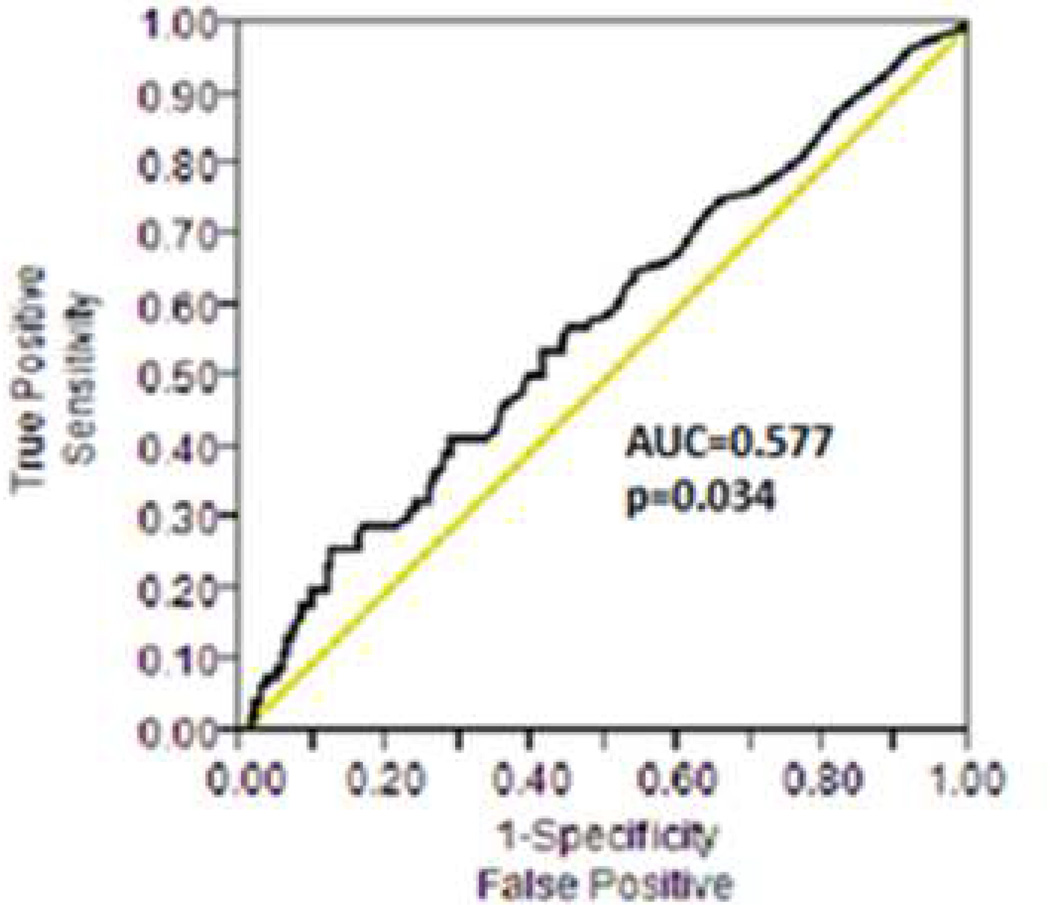
Receiver operating characteristic (ROC) curves were used to determine a threshold for post-PCI peak troponin association with MACE (Figure 5). The area under the curve (AUC) for the 1-year incidence of MACE was 0.577 (95% CI: 0.506–0.647, p=0.034). The discriminatory capacity of troponin for the 1-year incidence of MACE was similarly low among patients undergoing antegrade (AUC 0.579, 95% CI: 0.497–0.660, p=0.0649) or retrograde (AUC 0.588, 95% CI: 0.447–0.729, p=0.200) CTO PCI. The optimal troponin value for 1-year incidence of MACE as defined using the maximum Youden index was 1.64 ng/ml (approximately 50xULN). This cutoff value provided 26% sensitivity and 88% specificity for the prediction of MACE and a positive likelihood ratio (LR) of 2.12 and negative LR of 0.84, suggesting poor discriminatory capacity.
Figure 5. Peak post-PCI troponin levels receiver operating characteristic (ROC) curve.
ROC curve of peak post-PCI troponin levels for determining the one-year incidence of major adverse cardiac events
PCI, percutaneous coronary intervention; ROC, receiver operating characteristic; AUC, area under the curve
Discussion
The main findings of our study that used systematic evaluation of cardiac biomarkers after PCI are that PMI (1) occurs more commonly than previously reported after CTO PCI, (2) is more frequent when the retrograde approach is used, and (3) is associated with worse subsequent clinical outcomes.
Incidence and presentation of PMI after CTO PCI
A systematic review and meta-analysis of 65 studies that reported complications after CTO PCI found wide variability in the incidence of post PCI MI (from 0 to 19.4%), however the pooled estimate was low (2.8%, 95% CI: 1.5%–4.1%). The incidence of Q-wave MI was very low (0.2%, 95% CI: 0%–0.9%) (4). However, a major limitation of prior studies is that they did not perform systematic cardiac biomarker measurements, as is true for most contemporary PCI procedures in the US (only 7% of patients had cardiac biomarker measurement post-PCI in the National Cardiovascular Data Registry) (7).
This limitation was addressed by our study, in which cardiac biomarkers were measured twice during the first 24 hours post PCI and revealed that the actual incidence of PMI was approximately three-fold higher (8.6%, 95% CI: 5.8%–12.2%) than suggested by previous reports. However, the incidence of Q-wave MI was 0.2%, suggesting that most PMIs occurring during CTO PCI may affect limited areas of myocardium. Accordingly, 75% of all patients with PMI in our series did not have any ischemic symptoms.
Correlates of PMI after CTO PCI
The retrograde approach has revolutionized CTO PCI by enabling high success rates even in patients with very complex coronary anatomy (8, 9). The retrograde approach entails insertion of a coronary guidewire via a collateral vessel in the coronary vessel distal to the distal cap followed either by retrograde true to true lumen crossing or by using a dissection/re-entry strategy, such as the controlled antegrade and retrograde tracking and dissection (CART) or reverse CART technique (9). Previous studies had shown a trend for higher PMI with the retrograde approach: in an analysis of 1983 lesions in 1914 patients from the European registry of CTOs, the retrograde approach was used in 11.8% of cases and a trend for higher frequency of post-PCI myocardial infarction was observed in patients undergoing retrograde CTO PCI (2.1% vs. 1.0%, p=0.08) (10). In the meta-analysis of 65 studies reporting on CTO PCI complications among 884 patients undergoing CTO PCI of 886 lesions using a retrograde approach, PMI occurred in 1.8% (95% CI: 1.5%–4.1%) (4). Major complications such as death, emergent CABG, and stroke were rare, each occurring in 0.1% of patients.
In our study, the incidence of PMI was approximately two-fold higher among patients treated using a retrograde vs. an antegrade approach (13.8% vs. 6.7%, p=0.044). The higher incidence of PMI with the retrograde approach may be due to injury of the myocardium along the collateral vessel used for retrograde crossing or could be related to the use of dissection strategies that could disrupt small coronary artery side branches. In a recent report, magnetic resonance imaging post retrograde CTO PCI via a septal collateral demonstrated a new area of delayed hyperenhancement in the septum, suggesting injury from guidewire or other equipment passage (11). The higher incidence of PMI with the retrograde approach may also be related to higher lesion complexity, as in most cases the retrograde approach was used after antegrade crossing failure. Accordingly, the prevalence of prior CABG was twice as high among patients in whom the retrograde approach was used (Table 1), and prior CABG is known to be associated with higher rates of procedural failure (12). Similarly, right coronary artery CTOs, which are more challenging to cross, were more likely to be approached using the retrograde approach (Table 1).
Implications of periprocedural myocardial injury and infarction after CTO PCI
While cardiac biomarker elevation after routine PCI has been associated with higher immediate and long-term mortality, the clinical and therapeutic implications of CTO PCI PMI remain controversial (5). Our study demonstrates higher incidence of MACE during a median follow-up of 2.3 years among CTO PCI patients who developed PMI. Possible explanations include compromise of distal collateral vessels from microembolization, heightened inflammatory state post-PCI PMI, predisposition to arrhythmias after infarction and higher rates of index CTO PCI failure (Table 4) (11, 13–14). Moreover, PMI patients may be at higher risk for subsequent adverse events because of comorbidities, or higher coronary lesion complexity.
Troponin release in CTO PCI
Our study shows that troponin release is common after both antegrade and retrograde CTO PCI. The threshold value of troponin above the ULN whereby an adverse prognosis is evident is not well defined (5,15). Currently, guidelines suggest that troponin elevation >5xULN should be considered evidence of cardiac injury or infarction, but this threshold was arbitrarily chosen (5). In our population post CTO PCI troponin levels had limited association with MACE. A high troponin threshold (50x ULN) provided the best association with 1-year MACE, suggesting that further studies are needed for determining a clinically useful troponin threshold.
Study limitations
Our study is limited by the retrospective single-center design and relatively small number of patients, however it is the first study of its kind to be reported. Our sample size may not have accommodated a larger set of predictors, thereby potentially limiting replication. However, our final model revealed only two significant predictors and therefore had adequate statistical power. As is typical in veteran populations, most of the included patients were men, although men constitute the majority of patients in most CTO PCI series. Moreover, the study patients often had prior CABG, prior MI, and prior PCI, suggesting that this may be a higher risk group compared to the general population. In the United States the use of CK-MB is currently declining and hence CK-MB may not be available for clinical use at all institutions.
Conclusions
In summary, we found that PMI occurs in 8.6% of patients undergoing CTO PCI, is more common with the retrograde approach, and is associated with worse clinical outcomes during mid-term follow-up. Studies to better assess the pathophysiology of PMI in the CTO PCI setting, its implications, and preventive and treatment measures are needed.
Acknowledgments
Dr. Michael: Cardiovascular Training Grant from the National Institutes of Health Award Number T32HL007360.
Dr. Banerjee: Speakers’ Bureau for St. Jude Medical Center, Medtronic Corp., and Johnson & Johnson; research grant from Boston Scientific.
Dr. Brilakis: Speaker honoraria from St Jude Medical, Terumo, and Bridgepoint Medical; research support from Guerbet; spouse is an employee of Medtronic.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest:
Remaining Authors: none
References
- 1.Tardiff BE, Califf RM, Tcheng JE, et al. Clinical outcomes after detection of elevated cardiac enzymes in patients undergoing percutaneous intervention: IMPACT-II Investigators Integrilin (eptifibatide) to Minimize Platelet Aggregation and Coronary Thrombosis-II. J Am Coll Cardiol. 1999;33:88–96. doi: 10.1016/s0735-1097(98)00551-8. [DOI] [PubMed] [Google Scholar]
- 2.Kong TQ, Davidson CJ, Meyers SN, Tauke JT, Parker MA, Bonow RO. Prognostic implication of creatine kinase elevation following elective coronary artery interventions. JAMA. 1997;277:461–466. [PubMed] [Google Scholar]
- 3.Brilakis ES, Grantham JA, Rinfret S, et al. A percutaneous treatment algorithm for crossing coronary chronic total occlusions. J Am Coll Cardiol Intv. 2012;5:367–379. doi: 10.1016/j.jcin.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 4.Patel VG, Brayton K, Tamayo A, et al. Incidence of angiographic success and procedural complications in patients undergoing percutaneous coronary chronic total occlusion interventions: a weighted meta-analysis of 18,061 patients from 65 studies. J Am Coll Cardiol Intv. 2013;6:128–136. doi: 10.1016/j.jcin.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 5.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD. Third universal definition of myocardial infarction. Circulation. 2012;126:2020–2035. doi: 10.1016/j.gheart.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Bursac Z, Gauss CH, Williams DK, Hosmer DW. Purposeful selection of variables in logistic regression. Source Code in Biology and Medicine. 2008;3:1–8. doi: 10.1186/1751-0473-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang TY, Peterson ED, Dai D, et al. Patterns of cardiac marker surveillance after elective percutaneous coronary intervention and implications for the use of periprocedural myocardial infarction as a quality metric: a report from the National Cardiovascular Data Registry (NCDR) J Am Coll Cardiol. 2008;51:2068–2074. doi: 10.1016/j.jacc.2008.01.054. [DOI] [PubMed] [Google Scholar]
- 8.Karmpaliotis D, Michael TT, Brilakis ES, et al. Retrograde coronary chronic total occlusion revascularization: procedural and in-hospital procedural outcomes from a multicenter registry in the United States. J Am Coll Cardiol Intv. 2012;5(12):1273–1279. doi: 10.1016/j.jcin.2012.06.025. [DOI] [PubMed] [Google Scholar]
- 9.Brilakis ES, Grantham JA, Thompson CA, et al. The retrograde approach to coronary artery chronic total occlusions: A Practical Approach. Catheter Cardiovasc Interv. 2012;79:3–19. doi: 10.1002/ccd.23004. [DOI] [PubMed] [Google Scholar]
- 10.Galassi AR, Tomasello SD, Reifart N, et al. In-hospital outcomes of percutaneous coronary intervention in patients with chronic total occlusion: insights from the ERCTO (European Registry of Chronic Total Occlusion) registry. EuroIntervention. 2011;7:472–479. doi: 10.4244/EIJV7I4A77. [DOI] [PubMed] [Google Scholar]
- 11.Kim SM, Gwon HC, Lee HJ, et al. Periprocedural myocardial infarction after retrograde approach for chronic total occlusion of coronary artery: demonstrated by cardiac magnetic resonance imaging. Korean Circ J. 2011;41:747–749. doi: 10.4070/kcj.2011.41.12.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Michael TT, Karmpaliotis D, Brilakis ES, et al. Impact of prior coronary artery bypass graft surgery on chronic total occlusion revascularization: insights from a multicenter US registry. Heart. 2013 doi: 10.1136/heartjnl-2013-303763. [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13.Califf RM, Abdelmequid AE, Kuntz RE, et al. Myonecrosis after revascularization procedures. J Am Coll Cardiol. 1998;31:241–251. doi: 10.1016/s0735-1097(97)00506-8. [DOI] [PubMed] [Google Scholar]
- 14.Paizis I, Manginas A, Voudris V, et al. Percutaneous coronary intervention for chronic total occlusions: the role of side-branch occlusions. EuroIntervention. 2009;4:600–606. doi: 10.4244/eijv4i5a101. [DOI] [PubMed] [Google Scholar]
- 15.Damman P, Wallentin L, Fox K, et al. Long-term cardiovascular mortality after procedure-related or spontaneous myocardial infarction in patients with non-ST-segment elevation acute coronary syndrome: a collaborative analysis of individual patient data from the FRISC II, ICTUS, and RITA-3 trials (FIR) Circulation. 2012;125:568–576. doi: 10.1161/CIRCULATIONAHA.111.061663. [DOI] [PubMed] [Google Scholar]