Abstract
Prolonged early life seizures are associated with disruptions of affective and cognitive function. Postictal disturbances, temporary functional deficits that persist for hours to days after seizures, have not yet been thoroughly characterized. Here, we used kainic acid (KA) to induce status epilepticus (SE) in immature rats at three developmental stages (postnatal day 15, 21, or 30) and subsequently assessed spatial learning and memory in a Barnes Maze, exploratory behavior in an open field and the spatiotemporal distribution of cell injury during the first 7-10 days of the postictal period. At 1 day post-SE, P15-SE rats showed no deficit on a Barnes mazes, but were hyperexploratory in an open field compared to their littermate controls. In contrast, P21- and P30-SE rats exhibited markedly impaired performance on a Barnes Maze and exhibited significantly reduced open field exploration suggestive of anxiety-like behavior. These behavioral changes were transient in P15 rats but more persistent in P21 and enduring in P30 rats after KA-SE. The time course of behavioral deficits in P21 and P30 rats was temporally correlated with the presence of neuronal injury in the lateral septal nuclei, amygdala, and ventral subiculum/CA1, regions involved in modulation of the hypothalamic-pituitary-adrenal stress response.
Keywords: Seizure, Epilepsy, Immature Brain, Anxiety, Cell death, Amygdala
1. INTRODUCTION
Seizures provide a profoundly aberrant input to the brain, perturbing normal patterns of neuronal activity and initiating structural and functional changes that persist well beyond the ictal event [1-6]. Not unexpectedly, recurrent seizures in childhood epilepsy are associated with deficits in cognition and attention, as well as of affect and mood [7-9]. Furthermore, it is increasingly recognized that such deficits may be evident on a much shorter timescale than previously appreciated. Individuals with epilepsy frequently report a “postictal state,” temporary affective and cognitive changes that last for hours to days after seizures, including deficits in attention, concentration and short term memory; anxiety, depression, lethargy, and confusion, and in rare cases, postictal psychosis [7, 8, 10].
Such short term postictal deficits have been deemed a “neglected entity” in epilepsy research [11], with scant published reports concerning human patients or animal models. Nearly all investigations of functional deficits in developing animals, for example, have postponed assessment until adulthood, typically testing at least 1-2 months after seizures [12-15]. Those that have investigated postictal symptoms have mainly focused on the period immediately after seizures [16, 17], failing to reflect that clinically, the emergence of neuropsychiatric symptoms may be delayed for several hours [10]. Therefore, little is known about the nature of behavioral deficits in the hours to days after seizures, termed the subacute postictal period, their modulation by age of seizure onset, or their etiopathogenesis. Similarly, regional quantification of KA-SE induced cell injury has been limited to acute time points (24-48 h) and has rarely included the subacute postictal period [18].
The postictal state and associated behavioral deficits resemble neuropsychiatric disorders that have been widely recognized to constitute the principle comorbidities associated with epilepsy. Replication of such deficits during the postictal state in chemoconvulsant animal models may suggest that recurrent or prolonged seizures contribute causally to the neuropsychiatric comorbidities of epilepsy [19, 20]. We used a systemic kainic acid (KA) model of status epilepticus (SE)/temporal lobe epilepsy to investigate cognitive and affective deficits in the subacute postictal period in developing rats. Because the developing brain propagates, responds to, and recovers from seizures uniquely at different developmental stages [21, 22], we induced seizures at three ages: postnatal day (P)15, 21, and 30. Subsequently, we assayed spatial learning/memory, exploratory behavior, and the spatiotemporal distribution of cell injury during the first 7-10 days of the postictal period. We demonstrate that the core postictal behavioral deficit after SE is anxiety-like behavior and that the persistence of SE-induced behavioral changes varies with developmental stage. Recovery from hyper-reactivity to stress after KA-SE is temporally correlated with age-dependent differences in cell injury in the limbic circuitry – most notably lateral septum, ventral hippocampus/subiculum and amygdala - brain regions implicated in cognitive and affective processing.
2. EXPERIMENTAL PROCEDURES
2.1. Animals
Male Long Evans rats (Charles River, Boston, MA) were used for all experiments. All rats arrived with their dams prior to weaning and were housed in our facility for 2-10 days prior to study commencement. Animals were housed multiply in plastic cages and exposed to 12 hour light-dark cycles with free access to food and water. All procedures were in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and approved by the International Care and Use Committee of Lurie Children's Hospital of Chicago Research Center.
2.2. Kainic Acid Seizure Induction
On P15, 21, or 30, pups from each litter were divided into control or experimental groups, and experimental rats were injected intraperitoneally with KA dissolved in phosphate buffered saline (PBS) (3 mg/kg, 10 mg/kg, 10 mg/kg, respectively). Littermate controls were injected with equivalent volumes of PBS and remained separated from their dams for the duration of the experiment. The doses of KA were chosen based on the age-dependent difference in threshold for KA-induced seizures. Each dose effectively induces seizures for 1-3 hours (h) while resulting in less than 20% lethality [23-25].
Behavioral limbic seizures were observed in animals of all age groups. Seizures began within 30 minutes of KA injection, and persisted for 2-3 h, manifesting as previously reported [21]. P15-SE animals exhibited nearly continuous forelimb and hindlimb clonus, tonic seizures and loss of balance, while P21-SE and P30-SE rats showed forceful clonic jerks, rearing and falling. Seizure severity and latency to the first sign of seizure were recorded. A seizure severity grade was assigned based on the maximal response achieved on a scale from 0 to V as follows: 0 — no response; I —behavioral arrest; II —staring, pawing, limb clonus, and head bobbing; III — clonic jerks, rearing and falling or tonic posturing and loss of balance; IV — continuous grade III seizures for longer than 30 min (status epilepticus); V — death. Only those animals experiencing grade IV seizures (>30 min convulsions) were included in the study.
2.3. Behavioral Assessments
Animals were returned to their cages (P15 animals were returned to their dams) and allowed to recover overnight. 1 day (d) after seizure induction, experimental and control animals from each age group were assessed for spatial learning and memory on a Barnes Maze or exploratory behavior in an open field. These tasks critically engage hippocampal and/or amygdalar circuitry and are accepted assessments of anxiety and memory, the principal postictal deficits reported by patients with temporal lobe epilepsy.
2.3.1. Spatial Learning and Memory
In order to investigate the effects of early life SE on hippocampal-dependent spatial learning and memory, a subset of animals of each age group (n=5-12 per group) was trained and assessed on Barnes Maze [26], an accepted hippocampal-dependent task [27, 28]. The maze consisted of a flat circular platform containing 20 equally spaced holes along its perimeter and elevated 105 cm above the ground (platform diameter=122 cm, hole diameter=10 cm). Visual cues were placed on the walls surrounding the maze. During each trial, the animal was placed in the center of the platform and allowed to search for the hole leading to a darkened escape box. A trial was completed when (1) the animal entered the escape box or (2) 4 minutes had elapsed, at which point the animal was led to and placed in the escape box. The animal then remained in the escape box for 2 minutes for habituation. Animals were trained for three trials per day for five consecutive days. Consecutive trials were separated by at least 20 minutes. Exploration latency (time taken to approach the first hole), escape latency (time taken to find and enter the escape box) and errors (total number of head deflections into an incorrect hole) were recorded for each trial, and search path [29] was noted each day. On day 10, 5 days after the last training trial, a retention trial was performed to assess spatial memory retention.
2.3.2. Exploratory Behavior
Because placement on an open, elevated platform is anxiogenic for rats, performance on the Barnes Maze may be influenced by affective factors [30]. In order to investigate the extent to which such factors might have contributed, a second subset of rats of each age group (n=6-12) was assessed for exploratory behavior in an open field. Exploration of an unfamiliar, open arena serves as an index of anxiety and emotional reactivity in the face of novelty [31]. Animals were placed individually into a 152.5 cm x 152.5 cm enclosed arena, marked off into 25, 30.5 cm x 30.5 cm squares. The path taken and number of line crosses occurring within a five minute period were recorded and quantified manually by two trained observers. Animals were tested daily for 3-5 consecutive days, or until exploratory behavior returned to the level of littermate controls. All animals were tested again on day 7.
2.3.3 Rotarod Testing
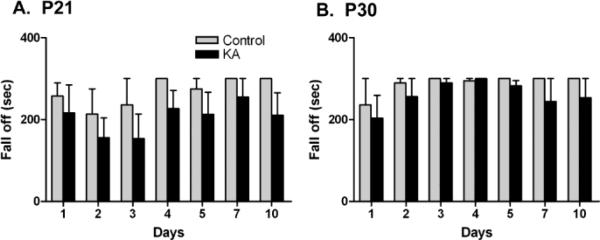
To rule out the possibility that performance on the Barnes Maze and open field were a result of motor dysfunction, a subgroup of P21 and P30 rats (n=4-5) was tested on a Rotarod apparatus (UgoBasile, VA, Italy). Rats were acclimated to the apparatus 1 day prior to induction of KA seizures. Rats were placed on the rotating cylinder (7 cm diameter, 15 rpm), and the number of seconds each rat remained on the cylinder before falling was recorded for up to 300 seconds. PBS and KA-SE rats were assessed on days 1-5, 7, and 10 after seizure induction.
2.4. Cell Injury
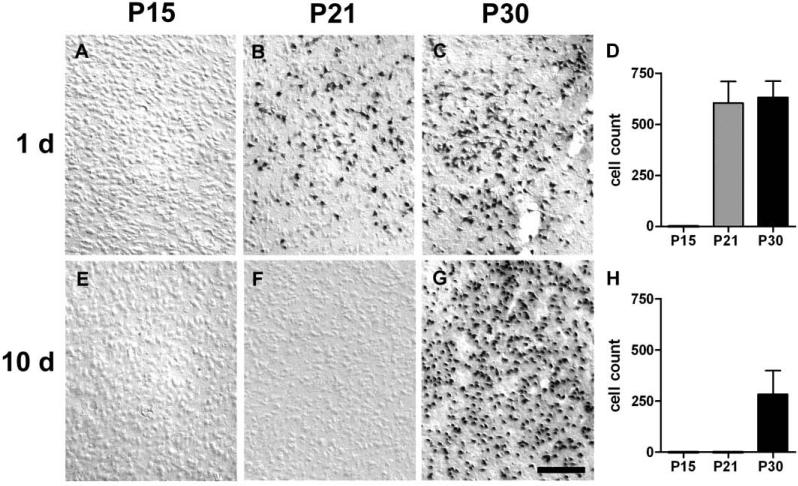
In order to assess the severity and duration of cell injury during the postictal period, the brains from a separate group of KA-treated and control animals from each age group (n=3-6 per condition/group, matched for seizure severity) were processed for in situ end labeling nick translation (ISEL), at 1 or 10 days after SE. Animals were deeply anesthetized with sodium pentobarbital (i.p.) and euthanized by transcardiac perfusion with 60 ml of cold PBS followed by 60 ml of cold 4% paraformaldahyde/0.1M sodium phosphate buffer. Brains were harvested, postfixed in the same fixative overnight, and cryoprotected in 30% sucrose for at least 24 hours. Serial 50-μm sections were cut coronally on a freezing microtome. Throughout the rostral-caudal extent of the brain including the septal area and dorsal and ventral hippocampus, at least six sections per animal were selected and processed for ISEL to detect DNA fragmentation, as previously described [25]. Location consistency was maintained by using the corpus callosum/lateral ventricles, dorsal hippocampus, and ventral hippocampus as anatomical landmarks to select sections located at the positions equivalent to approximately −0.3, −2.8, and −4.8 mm to Bregma in adult rats [32], respectively. For quantification of DNA fragmentation, representative images of regions of interest within the left and right lateral septum, basolateral amygdala (BLA, chosen as a representative nucleus), and ventral subiculum/ventral CA1 were captured digitally at 20x magnification. Positively-labeled cells within the 0.27 mm2 optical field were counted manually by an experimenter blind to group identity and averaged per animal. Mean and SD for each group (n=3-6) were calculated for comparison. Control P21 or P30 animals showed no DNA fragmentation. In total, 82 sections from 19 P21-SE and P30-SE animals were analyzed for quantification of DNA fragmentation. Images for Figures 4-6 were taken using differential interference contrast (DIC) imaging.
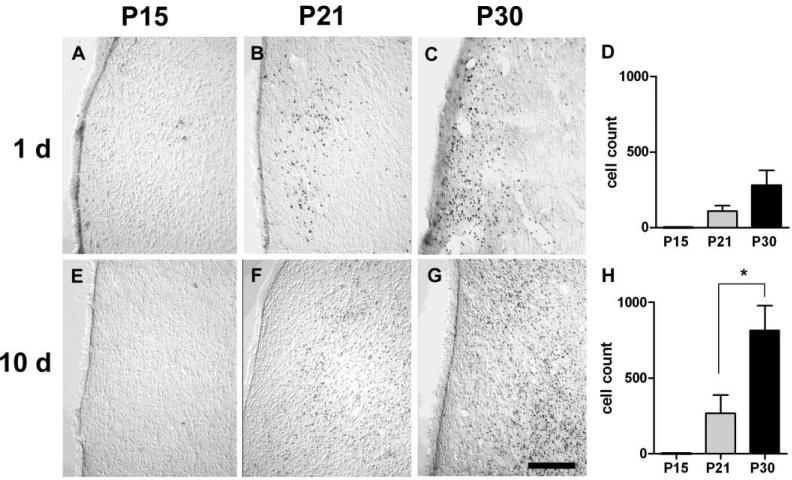
Figure 4.
Cell injury in the lateral septum at 1 and 10 days after SE. Differential interference contrast (DIC) view. A & E. No DNA fragmentation was present at 1 or 10 days in P15-SE animals. B & F. Scattered cells with fragmented DNA were present in P21-SE animals at both time points. C & G. DNA fragmentation was significantly more extensive in P30-SE compared to P21-SE animals by 10 days. Scale bar = 100μm. D & H. Quantification of DNA fragmentation. *p<0.05, Student's t-test.
Figure 6.
Cell injury in the ventral subiculum/CA1 region at 1 and 10 days after SE. A & E. No DNA fragmentation was present at 1 or 10 days in P15-SE animals. B & F. Scattered cells with fragmented DNA were present in P21-SE animals at both time points. C & G. DNA fragmentation was significantly more extensive in P30-SE compared to P21-SE animals by 10 days. Scale bar = 50μm. D & H. Quantification of DNA fragmentation. *p<0.01, Student's t-test.
2.5. Statistical Analyses
Statistical analyses were conducted using the GraphPad Prism 5.04 software package (GraphPad Software, San Diego). Group comparisons for the Barnes maze acquisition phase (days 1-5) were made using a two-way repeated measures ANOVA (RM-ANOVA) with Bonferroni post-tests. Data from open field and rota rod motor function experiments were analyzed using Student's t-tests for differences between SE and control animals at each time point. For analyses of ISEL data, Student's t-tests were conducted to compare the effect of age at SE (P21 vs. P30) on cell injury within each brain region at each time point.
3. RESULTS
3.1. Postictal Behavioral Deficits
3.1.1. Barnes Maze
3.1.1.1. Performance
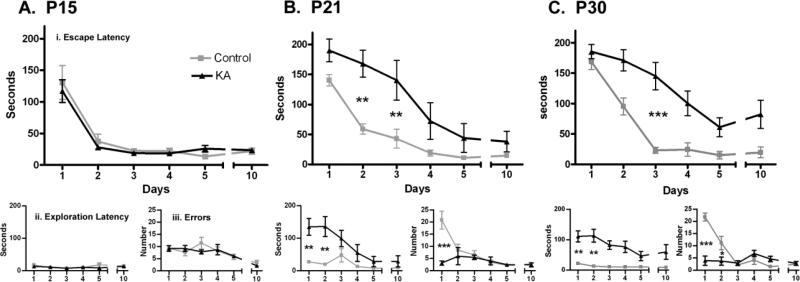
Status epilepticus at P15 had no effect on Barnes Maze performance. Animals experiencing SE on P15 demonstrated no differences compared to controls in escape latency, exploration latency, or errors on any day of training or testing on Barnes Maze (Figure 1A). In contrast, animals experiencing SE on P21 (Figure 1B.i) or P30 (Figure 1C.i) exhibited significant deficits in escape latency compared to respective controls (P21, p<0.02; P30, p<0.01, RM-ANOVA). SE animals remained frozen and immobile on the center of the maze for the duration of the early trials and engaged in significantly less searching than controls, as reflected in increased exploration latency (Figure 1B.ii & C.ii). Increased exploration latency also explains the decreased error rates for P21- and P30-SE rats compared to controls (Figure 1A.iii & C.iii), as fewer exploration attempts by SE rats resulted in fewer errors. During exploratory excursions, a fraction of the P21- (4/5), and P30-SE (2/12) animals attempted to jump off the platform. They were prevented from jumping and moved to the center of the platform. These behaviors contributed to increased escape latencies (Figure 1B.i & C.i). The escape latency of P21-SE animals began to improve by day 4. P30-SE animals had a more significant deficit on day 3 and trended towards worse performance compared to controls on Day 10 during the retention trial (p=0.09)
Figure 1.
Barnes Maze performance 1-10 days after SE. A. P15-SE animals (n=5) performed as well as controls (n=5). There was no difference in escape latency (i), exploration latency (ii), or errors (iii) on any day B. P21-SE animals demonstrated (i) significantly increased escape latencies compared to controls (p<0.02, RM-ANOVA; n=6, controls; n=6, SE) likely due to significant immobility and (ii) delayed initiation of exploration (p<0.01) which also led to (iii) significantly fewer errors (p<0.05). C. P30-SE animals also had (i) significantly longer escape latencies (p<0.01, RM-ANOVA; n=5, controls; n=12, SE) and (ii) exploration latencies (p<0.01). (**p<0.01, ***p<0.001, Bonferroni post-hoc test)
3.1.1.2 Escape Path
While P21 and P30 animals eventually progressed to a direct escape path, indicative of spatial cue utilization [33], P15 animals (both SE and control rats) failed to utilize such a path at any point during the trials. Rather than beginning increasingly closer to the escape box on successive trials, both P15-SE and P15-control animals initiated their search randomly, running serially over each hole until reaching the escape. This is reflected in the steady error rate of P15-SE and P15-control animals over time (Figure 1A.ii).
3.1.2. Exploratory Behavior
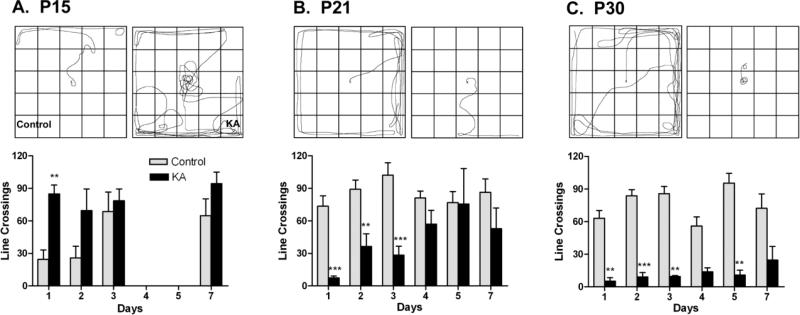
Status epilepticus caused acute alterations in exploratory behavior in all age groups. P15-SE animals were hyper-exploratory while P21- and P30-SE animals were nearly immobile. One day after SE, P15-SE rats engaged in frenzied exploration while P15-control rats exhibited low activity levels, as expected from pups soon after eye opening [34] (Figure 2A). While there is a trend toward increased activity even in day 2, the exploratory behavior of P15-SE animals (n=9) was not statistically different from P15-control animals (n=6) by day 2 and indistinguishable from P15-controls by day 3. In contrast to the postictal hyperexploration observed in P15-SE rats, P21-SE (Figure 2B) and P30-SE animals (Figure 2C) exhibited significantly reduced exploratory behavior compared to littermate controls beginning one day after SE, displaying behavioral inhibition characterized by freezing and cessation of activity. Deficits were transient in P21-SE animals, resolving by four days post-SE. In contrast, deficits persisted until day 7 in P30-SE animals.
Figure 2.
Open field exploration 1-7 days after SE. Rats who experienced SE at P15, P21, or P30 exhibited significant alterations in exploratory behavior compared to controls during the postictal period (p<0.05 in P15 and P21, p<0.001 in P30; paired t-tests). A. P15-SE rats were hyperexploratory relative to controls at 1 d after SE (*p<0.05, Student's t-test; n=6, controls; n= 9, SE), but recovered rapidly. B. P21-SE rats exhibited significantly decreased exploration compared to controls for 3 days post SE (**p<0.01, ***p<0.001, Student's t-test; n=12, controls; n=7, SE). C. P30-SE rats remained hypo-exploratory until 5 days post-SE (**p<0.01, ***p<0.001, Student's t-test, n=10, controls; n=6, SE).
3.1.3 Rotarod Testing
While the performance of P21 KA-SE rats was more variable than that of P30 KA-SE rats, neither KA-SE group showed significant deficits in motor function compared to controls on any day of testing.
3.2. Spontaneous Seizures during the Postictal Period
No spontaneous seizures were observed over a total of 15 hours of behavioral testing during the first seven days of the postictal period. This is consistent with previous studies that failed to detect any spontaneous seizures in young rats (under P20) over three months after KA [24], but detected a first spontaneous seizure at a mean of 11 days after KA in adult rats [35]. On the tenth day after SE, 2/12 P30-SE animals were noted to have brief clonic seizures immediately after being placed on the Barnes Maze during the retention trial. Because seizures occurred on the last day of Barnes Maze testing, data collected from these two animals were included in the analyses.
3.3. Seizure-induced Cell Injury
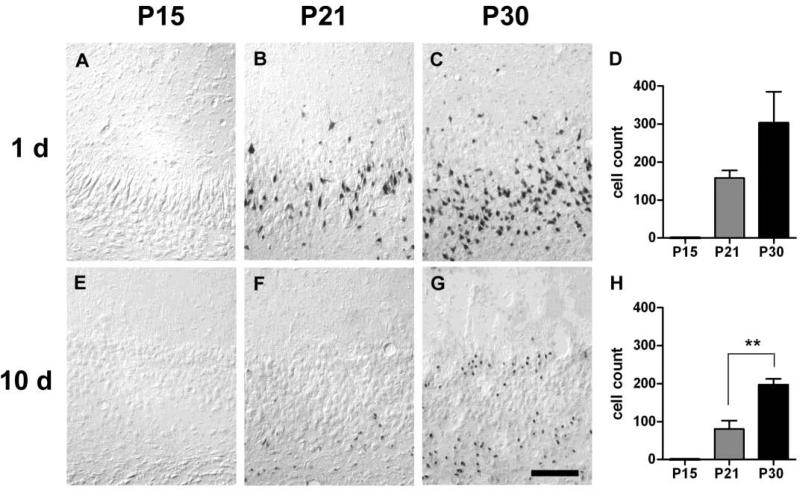
In situ end labeling (ISEL) was used to detect DNA fragmentation, indicative of cellular injury, following SE. In P15-SE animals, no discrete staining of cells beyond the background level seen in control animals was observed in any region at either 1 or 10 days after SE (Figures 4-6 A & E). At 1 d after SE in both P21- and P30-SE animals, extensive cell injury was observed bilaterally in the lateral septum (LS), amygdala, and ventral subiculum/ventral CA1 (vSub/CA1) (Figures 4-6 B & C). Cell injury appeared more extensive in P30-SE animals than in P21-SE animals in the LS and vSub/CA1. Quantification of positively-stained cells in the LS, basolateral amygdala (BLA; chosen as a representative nucleus), and vSub/CA1 showed these trends (p<0.09) (Figures 4-6 D). Regions affected to a lesser extent included the ventral dentate gyrus and CA3, entorhinal cortex, anterior thalamic nuclei, ventromedial hypothalamus, piriform cortex, and endopyriform nucleus (data not shown). At 10 d after SE, extensive cell injury continued to be present in all three limbic regions in P30 animals, and was significantly greater than in P21 animals. In P21-SE animals, cell injury was completely absent in the amygdala and decreased in the vSub/CA1.
4. DISCUSSION
The principal findings of the present study are: (1) Status epilepticus engenders behavioral deficits in the subacute postictal period (days following SE) in developing animals, the reversibility of which is faster at younger ages. (2) The postictal state is characterized predominantly by anxiety-like behavior. (3) Age specific postictal behavioral changes are correlated with the severity and persistence of cellular injury, specifically in the limbic system - most notably in the lateral septal nuclei, amygdala, and ventral hippocampal formation (vSub-CA1) - regions known to be involved in modulation of the stress response. We report here that a clinically-relevant “postictal state” exists following SE in developing animals and that heightened stress reactivity after status epilepticus is correlated with the age-dependent spatiotemporal extent of seizure-induced cell injury in the limbic area.
4.1. The reversibility of the postictal state depends on age of seizure induction
The immature brain is endowed with heightened plasticity, allowing nascent neuronal connections and resulting behavioral responses to be shaped by the particular patterns of activity experienced during early postnatal life [36-39]. Current theories suggest that such plasticity may confer either enhanced resilience from, or a heightened vulnerability to, adverse early life experiences, depending on the precise developmental stage at which an insult occurs [40-44]. Here, we demonstrated that beginning one day after SE, immature animals of all age groups exhibited behavioral alterations in a Barnes Maze and/or novel open field. Paralleling previous reports of age-dependent rates of recovery [45, 46], however, the reversibility of these deficits was strongly dependent on the developmental stage at which SE occurred. While behavioral alterations were highly transient in P15-SE rats and briefly persistent in P21-SE rats, they were more enduring in P30-SE rats. These new findings extend existing reports of long-term seizure-induced behavioral deficits [47-49] to the subacute postictal period, and demonstrate that developmental factors strongly modulate the duration of postictal symptoms.
4.2. The postictal state is characterized by age-dependent alterations in stress reactivity
Notably, there were striking differences between the youngest (P15) and older (P21 and P30) groups in the nature the behavioral changes observed. P15-SE animals displayed frenzied, hyperexploratory behavior in an open field, which may be interpreted as evidence of pure locomotor hyperactivity [50, 51], decreased [52], or increased emotional reactivity [53], or even accelerated maturation. Interestingly, associations between epilepsy and hyperactivity have been reported in both children and animals [54-57]. It is notable that P15-SE animals exhibited no deficits in the ability to search for and locate an escape on Barnes Maze. Successful task performance indicates that increased stress reactivity, if present, was not functionally relevant although the escape path of both P15-SE and P15-control animals appeared to suggest a non-spatial/hippocampal-dependent strategy, likely attributable to incomplete maturation of the hippocampus at this age [58-60],
In contrast to the P15 group, older (P21 and P30) animals demonstrated marked decreases in exploration following SE and exhibited freezing/behavioral arrest, signs of heightened stress reactivity or anxiety-like behavior [61, 62]. This parallels clinical reports of affective disturbances as the predominant postictal deficit in patients with epilepsy [10]. Increased emotional reactivity also resulted in markedly reduced attempts by P21- and P30-SE animals to search for the escape box on Barnes Maze, contributing to increased escape latencies during the first 3 days of the task. Interestingly, both groups’ improvement occurred only after anxiety-like behaviors reversed and exploration latency recovered, suggesting that learning occurred, but not evident in performance until hyper-reactive stress responses receded. While this precludes a conclusion regarding the existence of specific postictal deficits in hippocampal-dependent learning, it does contribute to a growing body of evidence documenting the detrimental effects of stress on performance of cognitive tasks [63-66], and raises the intriguing possibility that postictal cognitive difficulties in humans may also be exacerbated by co-occurring affective symptoms such as fear and anxiety [10]. Also of note is that the behavior of P30-SE animals recovered more quickly on Barnes Maze than in the open field. This may be attributable to the differences in the nature of the defensive response when the potential for escape does (Barnes Maze) and does not (open field) exist [67].
4.3. Postictal neuropathology is evident in regions involved in stress response
KA-induced seizures are known to recruit a network of limbic regions [68, 69] and to result in a variety of structural and functional neuronal sequelae [2, 70-72]. Given that stress hyper-reactivity was the most prominent deficit observed in P21- and P30-SE animals, and that the limbic network is known to modulate emotional behavior [73, 74], we hypothesized that the postictal phenotype might bear a direct relationship to seizure-induced dysfunction in limbic structures. Employing neuronal cell injury as an indicator of the spatiotemporal extent of postictal neuropathology, we observed that the most extensive and consistent cell injury occurred in the lateral septum, amygdala, and ventral subiculum/CA1. Appropriately, these structures are major components of a CNS network involved in modulation of the hypothalamic-pituitary-adrenal (HPA) axis [75-78], the primary controller of neuroendocrine and autonomic stress responses.
Specifically, the amygdala is known to be intimately involved in the control of anxiety, threat-induced behavioral arousal, and emotion [79, 80], to be recruited in response to psychological stressors such as spatial novelty [81], and to be implicated in neuropsychiatric disorders of mood and anxiety [82-84]. Additionally, the basolateral amygdala (BLA), the nucleus in which damage was most prominently and consistently observed, has been implicated in the expression of freezing behavior [85], an accepted manifestation of fear in rats. Similarly, although much attention has been given to alterations in the hippocampus proper following limbic seizures, damage to the vSub-CA1 is well-poised to play a role in postictal stress responsiveness. While the dorsal hippocampus is likely involved in spatial learning and modulation of basal and circadian HPA activity, but not acute stress reactivity [86, 87], damage to the vSub-CA1 has been found to result in increased HPA activation, specifically in response to innately-programmed stressors such as spatial novelty [88]. Finally, abnormalities in the lateral septum may also contribute to a variety of motivational and affective disorders, including fear and anxiety, as well as drug addition, schizophrenia, and depression [89]. Taken together, these results are suggestive of a potential functional relationship between the observed postictal deficits and SE-induced neuropathology. A direct causal link between cell degeneration and observed enhanced stress reactivity, however, cannot be made based solely on spatio-temporal correlation. Further investigation will be required to establish a causal link. It remains to be determined whether there exists a certain threshold up to which neuronal loss can be compensated or affected glial cells are subsequently replaced. In the present study, animals undergoing behavioral tests were not processed for quantification of neuronal injury. To determine whether a direct correlation exists between the amount of cell injury and behavioral deficit, assessment of both of anatomical and behavioral changes would need to be done on the same animals. Future studies may also include exploration of the sequence of events that occur over time after status epilepticus such as glial activation, oxidative stress, upregulation of transcriptional factors and inflammatory genes, cytokine release, and comprised integrity of the blood-brain barrier.
4.4. The reversibility of postictal deficits coincides with the persistence of limbic cell injury
The temporal correspondence between altered postictal stress reactivity and seizure-induced cell injury further strengthens this relationship. In agreement with previous studies [90, 91], SE did not result in substantial neuronal injury in the P15-SE group at either 1 d or 10 d after seizures, but did produce marked limbic cell injury in P21-SE and P30-SE groups by 1 d. Paralleling behavioral deficits, cell injury persisted to a greater extent in P30 than in P21-SE animals. Overall, the strong spatiotemporal relationship between postictal behavior and neuropathology suggests that seizure-induced alterations in neuronal populations specifically involved in regulation of the HPA axis may play a role in the development of heightened stress reactivity during the postictal period. Active and ongoing cell injury as indicated by fragmented DNA 10 days after SE in the P30 rats may have been, at least in part, due to the occurrence of recurrent spontaneous seizures that may further trigger cell degeneration. Alternatively, clinical or subclinical seizure activity that has been missed during behavioral observations could be responsible for both the ongoing cell injury and the heightened stress reactivity in the P30 rats. While others have established the paucity of spontaneous seizures before 10 days after status epilepticus in developing rats [24, 35], the possibility of subclinical or brief convulsions needs to be considered and cannot be ruled out in the absence of continuous EEG monitoring.
4.5. Conclusions
Our results establish the existence of postictal affective disturbances, long recognized clinically [92, 93], in developing animals in the subacute period after SE. Our findings suggest that young age is a protective factor in resilience from both seizure-induced neuropathology and postictal neuropsychiatric symptoms and that dysregulation of the stress response as a result of limbic network disruption may in part underlie affective disturbances. As cell death is only one of many neuronal changes to occur following SE in developing animals [94], the precise mechanism by which seizures induce hyper-reactive postictal stress responses remains to be characterized. Establishment of the present animal model will enable further investigation of the postictal state and its etiogenesis, potentially facilitating treatment of its underlying substrates in advance of their progression into the chronic neuropsychiatric disorders associated with repeated seizures over time.
HIGHLIGHTS.
Status epilepticus causes acute anxiety-like behavior in developing rats.
Seizure-induced behavioral deficits are age-dependent and can last for days.
Status epilepticus causes age-dependent cell injury in the limbic system.
Persistence of behavioral deficits is temporally correlated with limbic cell injury.
Figure 3.
Rotarod testing 1-10 days after SE. A. P21-SE animals and B. P30-SE animals performed as well as controls (p>0.05, Student's t-test; n=4, controls; n= 5 SE).
Figure 5.
Cell injury in the basolateral amygdala (BLA) at 1 and 10 days after SE. A & E. No DNA fragmentation was present at 1 or 10 days in P15-SE animals. B & F. Extensive DNA fragmentation present in P21-SE animals one day after SE was no longer observed by day 10. C & G. BLA of P30-SE animals were densely packed with cells containing fragmented DNA at both time points. Scale bar = 50μm. D & H. Quantification of DNA fragmentation.
ACKNOWLEDGMENTS
The authors wish to thank Sarada Alla, Hyokwon Chung, Patrick Fox Cassandra Kazl, Min Jung Kim, and Fatima Zaheer for their assistance in this research. This work is supported by NIH/NINDS R01NS073768.
ABBREVIATIONS
- BLA
basolateral amygdala
- HPA
hypothalamic-pituitary-adrenal
- ISEL
in situ end labeling
- KA
kainic acid
- LS
lateral septum
- P
postnatal
- PBS
phosphate buffered saline
- SE
status epilepticus
- vSub/CA1
ventral subiculum/cornu ammonis 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Coulter DA. Chronic epileptogenic cellular alterations in the limbic system after status epilepticus. Epilepsia. l1999;40(Suppl 1):S23–33. doi: 10.1111/j.1528-1157.1999.tb00875.x. discussion S40-1. [DOI] [PubMed] [Google Scholar]
- 2.Wilson DN, Chung H, Elliott RC, Bremer E, George D, Koh S. Microarray analysis of postictal transcriptional regulation of neuropeptides. J Mol Neurosci. l2005;25:285–98. doi: 10.1385/JMN:25:3:285. [DOI] [PubMed] [Google Scholar]
- 3.Chen JW, Naylor DE, Wasterlain CG. Advances in the pathophysiology of status epilepticus. Acta Neurol Scand Suppl. l2007;186:7–15. [PubMed] [Google Scholar]
- 4.Majores M, Schoch S, Lie A, Becker AJ. Molecular neuropathology of temporal lobe epilepsy: complementary approaches in animal models and human disease tissue. Epilepsia. l2007;48(Suppl 2):4–12. doi: 10.1111/j.1528-1167.2007.01062.x. [DOI] [PubMed] [Google Scholar]
- 5.Scantlebury MH, Heida JG, Hasson HJ, Veliskova J, Velisek L, Galanopoulou AS, Moshe SL. Age-dependent consequences of status epilepticus: animal models. Epilepsia. l2007;48(Suppl 2):75–82. doi: 10.1111/j.1528-1167.2007.01069.x. [DOI] [PubMed] [Google Scholar]
- 6.Avignone E, Ulmann L, Levavasseur F, Rassendren F, Audinat E. Status epilepticus induces a particular microglial activation state characterized by enhanced purinergic signaling. J Neurosci. l2008;28:9133–44. doi: 10.1523/JNEUROSCI.1820-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Devinsky O. Effects of Seizures on Autonomic and Cardiovascular Function. Epilepsy Curr. l2004;4:43–46. doi: 10.1111/j.1535-7597.2004.42001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pellock JM. Understanding co-morbidities affecting children with epilepsy. Neurology. l2004;62:S17–23. doi: 10.1212/wnl.62.5_suppl_2.s17. [DOI] [PubMed] [Google Scholar]
- 9.Jones JE, Austin JK, Caplan R, Dunn D, Plioplys S, Salpekar JA. Psychiatric disorders in children and adolescents who have epilepsy. Pediatr Rev. l2008;29:e9–14. doi: 10.1542/pir.29-2-e9. [DOI] [PubMed] [Google Scholar]
- 10.Kanner AM, Soto A, Gross-Kanner H. Prevalence and clinical characteristics of postictal psychiatric symptoms in partial epilepsy. Neurology. l2004;62:708–13. doi: 10.1212/01.wnl.0000113763.11862.26. [DOI] [PubMed] [Google Scholar]
- 11.Fisher RS, Schachter SC. The Postictal State: A Neglected Entity in the Management of Epilepsy. Epilepsy Behav. l2000;1:52–59. doi: 10.1006/ebeh.2000.0023. [DOI] [PubMed] [Google Scholar]
- 12.Stafstrom CE, Chronopoulos A, Thurber S, Thompson JL, Holmes GL. Age-dependent cognitive and behavioral deficits after kainic acid seizures. Epilepsia. l1993;34:420–32. doi: 10.1111/j.1528-1157.1993.tb02582.x. [DOI] [PubMed] [Google Scholar]
- 13.Holmes GL. Effects of early seizures on later behavior and epileptogenicity. Ment Retard Dev Disabil Res Rev. l2004;10:101–5. doi: 10.1002/mrdd.20019. [DOI] [PubMed] [Google Scholar]
- 14.Kubova H, Mares P, Suchomelova L, Brozek G, Druga R, Pitkanen A. Status epilepticus in immature rats leads to behavioural and cognitive impairment and epileptogenesis. Eur J Neurosci. l2004;19:3255–65. doi: 10.1111/j.0953-816X.2004.03410.x. [DOI] [PubMed] [Google Scholar]
- 15.Kleen JK, Wu EX, Holmes GL, Scott RC, Lenck-Santini PP. Enhanced oscillatory activity in the hippocampal-prefrontal network is related to short-term memory function after early-life seizures. J Neurosci. l2011;31:15397–406. doi: 10.1523/JNEUROSCI.2196-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ehlers CL, Koob GF, Bloom FE. Post-ictal locomotor activity in three different rat models of epilepsy. Brain Res. l1982;250:178–82. doi: 10.1016/0006-8993(82)90966-0. [DOI] [PubMed] [Google Scholar]
- 17.Mikulecka A, Mares P. Postictal behavior after two types of cortical epileptic afterdischarges in rats. Epilepsy Behav. l2007;10:213–8. doi: 10.1016/j.yebeh.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 18.Weiss S, Cataltepe O, Cole AJ. Anatomical studies of DNA fragmentation in rat brain after systemic kainate administration. Neuroscience. l1996;74:541–51. doi: 10.1016/0306-4522(96)00148-0. [DOI] [PubMed] [Google Scholar]
- 19.Sayin U, Sutula TP, Stafstrom CE. Seizures in the developing brain cause adverse long-term effects on spatial learning and anxiety. Epilepsia. l2004;45:1539–48. doi: 10.1111/j.0013-9580.2004.54903.x. [DOI] [PubMed] [Google Scholar]
- 20.Cardoso A, Carvalho LS, Lukoyanova EA, Lukoyanov NV. Effects of repeated electroconvulsive shock seizures and pilocarpine-induced status epilepticus on emotional behavior in the rat. Epilepsy Behav. l2009;14:293–9. doi: 10.1016/j.yebeh.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 21.Tremblay E, Nitecka L, Berger ML, Ben-Ari Y. Maturation of kainic acid seizure-brain damage syndrome in the rat. I. Clinical, electrographic and metabolic observations. Neuroscience. l1984;13:1051–72. doi: 10.1016/0306-4522(84)90288-4. [DOI] [PubMed] [Google Scholar]
- 22.Lado FA, Sankar R, Lowenstein D, Moshe SL. Age-dependent consequences of seizures: relationship to seizure frequency, brain damage, and circuitry reorganization. Ment Retard Dev Disabil Res Rev. l2000;6:242–52. doi: 10.1002/1098-2779(2000)6:4<242::AID-MRDD3>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 23.Albala BJ, Moshe SL, Okada R. Kainic-acid-induced seizures: a developmental study. Brain Res. l1984;315:139–48. doi: 10.1016/0165-3806(84)90085-3. [DOI] [PubMed] [Google Scholar]
- 24.Stafstrom CE, Thompson JL, Holmes GL. Kainic acid seizures in the developing brain: status epilepticus and spontaneous recurrent seizures. Brain Res Dev Brain Res. l1992;65:227–36. doi: 10.1016/0165-3806(92)90184-x. [DOI] [PubMed] [Google Scholar]
- 25.Koh S, Storey TW, Santos TC, Mian AY, Cole AJ. Early-life seizures in rats increase susceptibility to seizure-induced brain injury in adulthood. Neurology. l1999;53:915–21. doi: 10.1212/wnl.53.5.915. [DOI] [PubMed] [Google Scholar]
- 26.Barnes CA. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J Comp Physiol Psychol. l1979;93:74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- 27.Paylor R, Zhao Y, Libbey M, Westphal H, Crawley JN. Learning impairments and motor dysfunctions in adult Lhx5-deficient mice displaying hippocampal disorganization. Physiol Behav. l2001;73:781–92. doi: 10.1016/s0031-9384(01)00515-7. [DOI] [PubMed] [Google Scholar]
- 28.Deacon RM, Rawlins JN. Learning impairments of hippocampal-lesioned mice in a paddling pool. Behav Neurosci. l2002;116:472–8. doi: 10.1037//0735-7044.116.3.472. [DOI] [PubMed] [Google Scholar]
- 29.Bach ME, Hawkins RD, Osman M, Kandel ER, Mayford M. Impairment of spatial but not contextual memory in CaMKII mutant mice with a selective loss of hippocampal LTP in the range of the theta frequency. Cell. l1995;81:905–15. doi: 10.1016/0092-8674(95)90010-1. [DOI] [PubMed] [Google Scholar]
- 30.Holscher C. Stress impairs performance in spatial water maze learning tasks. Behav Brain Res. l1999;100:225–35. doi: 10.1016/s0166-4328(98)00134-x. [DOI] [PubMed] [Google Scholar]
- 31.Walsh RN, Cummins RA. The Open-Field Test: a critical review. Psychol Bull. l1976;83:482–504. [PubMed] [Google Scholar]
- 32.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 2nd ed. Academic Press; Sydney: 1986. [Google Scholar]
- 33.Harrison FE, Reiserer RS, Tomarken AJ, McDonald MP. Spatial and nonspatial escape strategies in the Barnes maze. Learn Mem. l2006;13:809–19. doi: 10.1101/lm.334306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laviola G, Renna G, Bignami G, Cuomo V. Ontogenetic and pharmacological dissociation of various components of locomotor activity and habituation in the rat. Int J Dev Neurosci. l1988;6:431–8. doi: 10.1016/0736-5748(88)90049-4. [DOI] [PubMed] [Google Scholar]
- 35.Williams PA, White AM, Clark S, Ferraro DJ, Swiercz W, Staley KJ, Dudek FE. Development of spontaneous recurrent seizures after kainate-induced status epilepticus. J Neurosci. l2009;29:2103–12. doi: 10.1523/JNEUROSCI.0980-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shatz CJ. Impulse activity and the patterning of connections during CNS development. Neuron. l1990;5:745–56. doi: 10.1016/0896-6273(90)90333-b. [DOI] [PubMed] [Google Scholar]
- 37.Joseph R. Environmental influences on neural plasticity, the limbic system, emotional development and attachment: a review. Child Psychiatry Hum Dev. l1999;29:189–208. doi: 10.1023/a:1022660923605. [DOI] [PubMed] [Google Scholar]
- 38.Stiles J. Neural plasticity and cognitive development. Dev Neuropsychol. l2000;18:237–72. doi: 10.1207/S15326942DN1802_5. [DOI] [PubMed] [Google Scholar]
- 39.Khazipov R, Luhmann HJ. Early patterns of electrical activity in the developing cerebral cortex of humans and rodents. Trends Neurosci. l2006;29:414–8. doi: 10.1016/j.tins.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 40.Anand KJ, Scalzo FM. Can adverse neonatal experiences alter brain development and subsequent behavior? Biol Neonate. l2000;77:69–82. doi: 10.1159/000014197. [DOI] [PubMed] [Google Scholar]
- 41.Sanchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: evidence from rodent and primate models. Dev Psychopathol. l2001;13:419–49. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- 42.McEwen BS. Early life influences on life-long patterns of behavior and health. Ment Retard Dev Disabil Res Rev. l2003;9:149–54. doi: 10.1002/mrdd.10074. [DOI] [PubMed] [Google Scholar]
- 43.Gleissner U, Sassen R, Schramm J, Elger CE, Helmstaedter C. Greater functional recovery after temporal lobe epilepsy surgery in children. Brain. l2005;128:2822–9. doi: 10.1093/brain/awh597. [DOI] [PubMed] [Google Scholar]
- 44.Jacobs R, Harvey AS, Anderson V. Executive function following focal frontal lobe lesions: impact of timing of lesion on outcome. Cortex. l2007;43:792–805. doi: 10.1016/s0010-9452(08)70507-0. [DOI] [PubMed] [Google Scholar]
- 45.Duhaime AC, Hunter JV, Grate LL, Kim A, Golden J, Demidenko E, Harris C. Magnetic resonance imaging studies of age-dependent responses to scaled focal brain injury in the piglet. J Neurosurg. l2003;99:542–8. doi: 10.3171/jns.2003.99.3.0542. [DOI] [PubMed] [Google Scholar]
- 46.Sandstrom MI, Nelson CL, Bruno JP. Neurochemical correlates of sparing from motor deficits in rats depleted of striatal dopamine as weanlings. Dev Psychobiol. l2003;43:373–83. doi: 10.1002/dev.10148. [DOI] [PubMed] [Google Scholar]
- 47.Liu Z, Gatt A, Werner SJ, Mikati MA, Holmes GL. Long-term behavioral deficits following pilocarpine seizures in immature rats. Epilepsy Res. l1994;19:191–204. doi: 10.1016/0920-1211(94)90062-0. [DOI] [PubMed] [Google Scholar]
- 48.Holmes GL. Influence of brain development on status epilepticus. Epilepsia. l2007;48(Suppl 8):19–20. doi: 10.1111/j.1528-1167.2007.01339.x. [DOI] [PubMed] [Google Scholar]
- 49.Sankar R, Rho JM. Do seizures affect the developing brain? Lessons from the laboratory. J Child Neurol. l2007;22:21S–9S. doi: 10.1177/0883073807303072. [DOI] [PubMed] [Google Scholar]
- 50.Accili D, Fishburn CS, Drago J, Steiner H, Lachowicz JE, Park BH, Gauda EB, Lee EJ, Cool MH, Sibley DR, Gerfen CR, Westphal H, Fuchs S. A targeted mutation of the D3 dopamine receptor gene is associated with hyperactivity in mice. Proc Natl Acad Sci U S A. l1996;93:1945–9. doi: 10.1073/pnas.93.5.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Murphy CA, DiCamillo AM, Haun F, Murray M. Lesion of the habenular efferent pathway produces anxiety and locomotor hyperactivity in rats: a comparison of the effects of neonatal and adult lesions. Behav Brain Res. l1996;81:43–52. doi: 10.1016/s0166-4328(96)00041-1. [DOI] [PubMed] [Google Scholar]
- 52.Holmes A. Targeted gene mutation approaches to the study of anxiety-like behavior in mice. Neurosci Biobehav Rev. l2001;25:261–73. doi: 10.1016/s0149-7634(01)00012-4. [DOI] [PubMed] [Google Scholar]
- 53.Alaverdashvili M, Kubova H, Mares P. Motor performance and behavior of immature rats are not compromised by a high dose of topiramate. Epilepsy Behav. l2005;7:222–30. doi: 10.1016/j.yebeh.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 54.Richer LP, Shevell MI, Rosenblatt BR. Epileptiform abnormalities in children with attention-deficit-hyperactivity disorder. Pediatr Neurol. l2002;26:125–9. doi: 10.1016/s0887-8994(01)00370-8. [DOI] [PubMed] [Google Scholar]
- 55.Dunn DW, Kronenberger WG. Childhood epilepsy, attention problems, and ADHD: review and practical considerations. Semin Pediatr Neurol. l2005;12:222–8. doi: 10.1016/j.spen.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 56.Schubert R. Attention deficit disorder and epilepsy. Pediatr Neurol. l2005;32:1–10. doi: 10.1016/j.pediatrneurol.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 57.McIntyre DC, Gilby KL. Genetically seizure-prone or seizure-resistant phenotypes and their associated behavioral comorbidities. Epilepsia. l2007;48(Suppl 9):30–2. doi: 10.1111/j.1528-1167.2007.01398.x. [DOI] [PubMed] [Google Scholar]
- 58.Michelson HB, Lothman EW. An in vivo electrophysiological study of the ontogeny of excitatory and inhibitory processes in the rat hippocampus. Brain Res Dev Brain Res. l1989;47:113–22. doi: 10.1016/0165-3806(89)90113-2. [DOI] [PubMed] [Google Scholar]
- 59.Dumas TC. Late postnatal maturation of excitatory synaptic transmission permits adult-like expression of hippocampal-dependent behaviors. Hippocampus. l2005;15:562–78. doi: 10.1002/hipo.20077. [DOI] [PubMed] [Google Scholar]
- 60.Danglot L, Triller A, Marty S. The development of hippocampal interneurons in rodents. Hippocampus. l2006;16:1032–60. doi: 10.1002/hipo.20225. [DOI] [PubMed] [Google Scholar]
- 61.Blanchard RJ, Blanchard DC. Crouching as an index of fear. J Comp Physiol Psychol. l1969;67:370–5. doi: 10.1037/h0026779. [DOI] [PubMed] [Google Scholar]
- 62.Henry JP. Biological basis of the stress response. Integr Physiol Behav Sci. l1992;27:66–83. doi: 10.1007/BF02691093. [DOI] [PubMed] [Google Scholar]
- 63.McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. l1999;22:105–22. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- 64.Garcia O, Massieu L. Strategies for neuroprotection against L-trans-2,4-pyrrolidine dicarboxylate-induced neuronal damage during energy impairment in vitro. Journal of Neuroscience Research. l2001;64:418–28. doi: 10.1002/jnr.1093. [DOI] [PubMed] [Google Scholar]
- 65.Sandi C, Pinelo-Nava MT. Stress and memory: behavioral effects and neurobiological mechanisms. Neural Plast. l20072007:78970. doi: 10.1155/2007/78970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bisaz R, Conboy L, Sandi C. Learning under stress: a role for the neural cell adhesion molecule NCAM. Neurobiol Learn Mem. l2009;91:333–42. doi: 10.1016/j.nlm.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 67.van Dijken HH, Mos J, van der Heyden JA, Tilders FJ. Characterization of stress-induced long-term behavioural changes in rats: evidence in favor of anxiety. Physiol Behav. l1992;52:945–51. doi: 10.1016/0031-9384(92)90375-c. [DOI] [PubMed] [Google Scholar]
- 68.Schwob JE, Fuller T, Price JL, Olney JW. Widespread patterns of neuronal damage following systemic or intracerebral injections of kainic acid: a histological study. Neuroscience. l1980;5:991–1014. doi: 10.1016/0306-4522(80)90181-5. [DOI] [PubMed] [Google Scholar]
- 69.Lothman EW, Collins RC. Kainic acid induced limbic seizures: metabolic, behavioral, electroencephalographic and neuropathological correlates. Brain Res. l1981;218:299–318. doi: 10.1016/0006-8993(81)91308-1. [DOI] [PubMed] [Google Scholar]
- 70.Chang D, Baram TZ. Status epilepticus results in reversible neuronal injury in infant rat hippocampus: novel use of a marker. Brain Res Dev Brain Res. l1994;77:133–6. doi: 10.1016/0165-3806(94)90220-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ravizza T, Rizzi M, Perego C, Richichi C, Veliskova J, Moshe SL, De Simoni MG, Vezzani A. Inflammatory response and glia activation in developing rat hippocampus after status epilepticus. Epilepsia. l2005;46(Suppl 5):113–7. doi: 10.1111/j.1528-1167.2005.01006.x. [DOI] [PubMed] [Google Scholar]
- 72.Friedman LK, Saghyan A, Peinado A, Keesey R. Age- and region-dependent patterns of Ca2+ accumulations following status epilepticus. Int J Dev Neurosci. l2008;26:779–90. doi: 10.1016/j.ijdevneu.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 73.Dalgleish T. The emotional brain. Nat Rev Neurosci. l2004;5:583–9. doi: 10.1038/nrn1432. [DOI] [PubMed] [Google Scholar]
- 74.de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. l2005;6:463–75. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- 75.Uhlir I, Seggie J, Brown GM. The effect of septal lesions on the threshold of adrenal stress response. Neuroendocrinology. l1974;14:351–5. doi: 10.1159/000122279. [DOI] [PubMed] [Google Scholar]
- 76.Dobrakovova M, Kvetnansky R, Torda T, Murgas K. Changes of plasma and adrenal catecholamines and corticosterone in stressed rats with septal lesions. Physiol Behav. l1982;29:41–5. doi: 10.1016/0031-9384(82)90363-8. [DOI] [PubMed] [Google Scholar]
- 77.Feldman S, Conforti N, Weidenfeld J. Limbic pathways and hypothalamic neurotransmitters mediating adrenocortical responses to neural stimuli. Neurosci Biobehav Rev. l1995;19:235–40. doi: 10.1016/0149-7634(94)00062-6. [DOI] [PubMed] [Google Scholar]
- 78.Herman JPMN, Figueiredo H, Cullinan WE. Neurocircuit regulation of the hypothalamo-pituitary-adrenocortical stress response - an overview. In: Steckler TKN, Reul JMHM, editors. Handbook of Stress and the Brain: Part 1. Elsevier B.V.; 2005. pp. 405–418. [Google Scholar]
- 79.Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. l2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- 80.LeDoux J. The emotional brain, fear, and the amygdala. Cell Mol Neurobiol. l2003;23:727–38. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dayas CV, Buller KM, Crane JW, Xu Y, Day TA. Stressor categorization: acute physical and psychological stressors elicit distinctive recruitment patterns in the amygdala and in medullary noradrenergic cell groups. Eur J Neurosci. l2001;14:1143–52. doi: 10.1046/j.0953-816x.2001.01733.x. [DOI] [PubMed] [Google Scholar]
- 82.Drevets WC. Neuroimaging abnormalities in the amygdala in mood disorders. Ann N Y Acad Sci. l2003;985:420–44. doi: 10.1111/j.1749-6632.2003.tb07098.x. [DOI] [PubMed] [Google Scholar]
- 83.Rauch SL, Shin LM, Wright CI. Neuroimaging studies of amygdala function in anxiety disorders. Ann N Y Acad Sci. l2003;985:389–410. doi: 10.1111/j.1749-6632.2003.tb07096.x. [DOI] [PubMed] [Google Scholar]
- 84.Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. l2005;48:175–87. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 85.Potegal M. Aggressive arousal: The amygdala connection. In: Potegal M, Knutson JF, editors. The Dynamics of Aggression: Biological and Social Processes in Dyads and Groups. Lawrence Erlbaum Associates; Hillsdale, NJ: 1994. pp. 73–108. [Google Scholar]
- 86.Herman JP, Schafer MK, Young EA, Thompson R, Douglass J, Akil H, Watson SJ. Evidence for hippocampal regulation of neuroendocrine neurons of the hypothalamo-pituitary-adrenocortical axis. J Neurosci. l1989;9:3072–82. doi: 10.1523/JNEUROSCI.09-09-03072.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Moser MB, Moser EI. Functional differentiation in the hippocampus. Hippocampus. l1998;8:608–19. doi: 10.1002/(SICI)1098-1063(1998)8:6<608::AID-HIPO3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 88.Herman JP, Dolgas CM, Carlson SL. Ventral subiculum regulates hypothalamo-pituitary-adrenocortical and behavioural responses to cognitive stressors. Neuroscience. l1998;86:449–59. doi: 10.1016/s0306-4522(98)00055-4. [DOI] [PubMed] [Google Scholar]
- 89.Sheehan TP, Chambers RA, Russell DS. Regulation of affect by the lateral septum: implications for neuropsychiatry. Brain Res Brain Res Rev. l2004;46:71–117. doi: 10.1016/j.brainresrev.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 90.Nitecka L, Tremblay E, Charton G, Bouillot JP, Berger ML, Ben-Ari Y. Maturation of kainic acid seizure-brain damage syndrome in the rat. II. Histopathological sequelae. Neuroscience. l1984;13:1073–94. doi: 10.1016/0306-4522(84)90289-6. [DOI] [PubMed] [Google Scholar]
- 91.Jarvela JT, Lopez-Picon FR, Holopainen IE. Age-dependent cyclooxygenase-2 induction and neuronal damage after status epilepticus in the postnatal rat hippocampus. Epilepsia. l2008;49:832–41. doi: 10.1111/j.1528-1167.2007.01454.x. [DOI] [PubMed] [Google Scholar]
- 92.Torta R, Keller R. Behavioral, psychotic, and anxiety disorders in epilepsy: etiology, clinical features, and therapeutic implications. Epilepsia. l1999;40(Suppl 10):S2–20. doi: 10.1111/j.1528-1157.1999.tb00883.x. [DOI] [PubMed] [Google Scholar]
- 93.Beyenburg S, Mitchell AJ, Schmidt D, Elger CE, Reuber M. Anxiety in patients with epilepsy: systematic review and suggestions for clinical management. Epilepsy Behav. l2005;7:161–71. doi: 10.1016/j.yebeh.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 94.Holopainen IE. Seizures in the developing brain: cellular and molecular mechanisms of neuronal damage, neurogenesis and cellular reorganization. Neurochem Int. l2008;52:935–47. doi: 10.1016/j.neuint.2007.10.021. [DOI] [PubMed] [Google Scholar]