Abstract
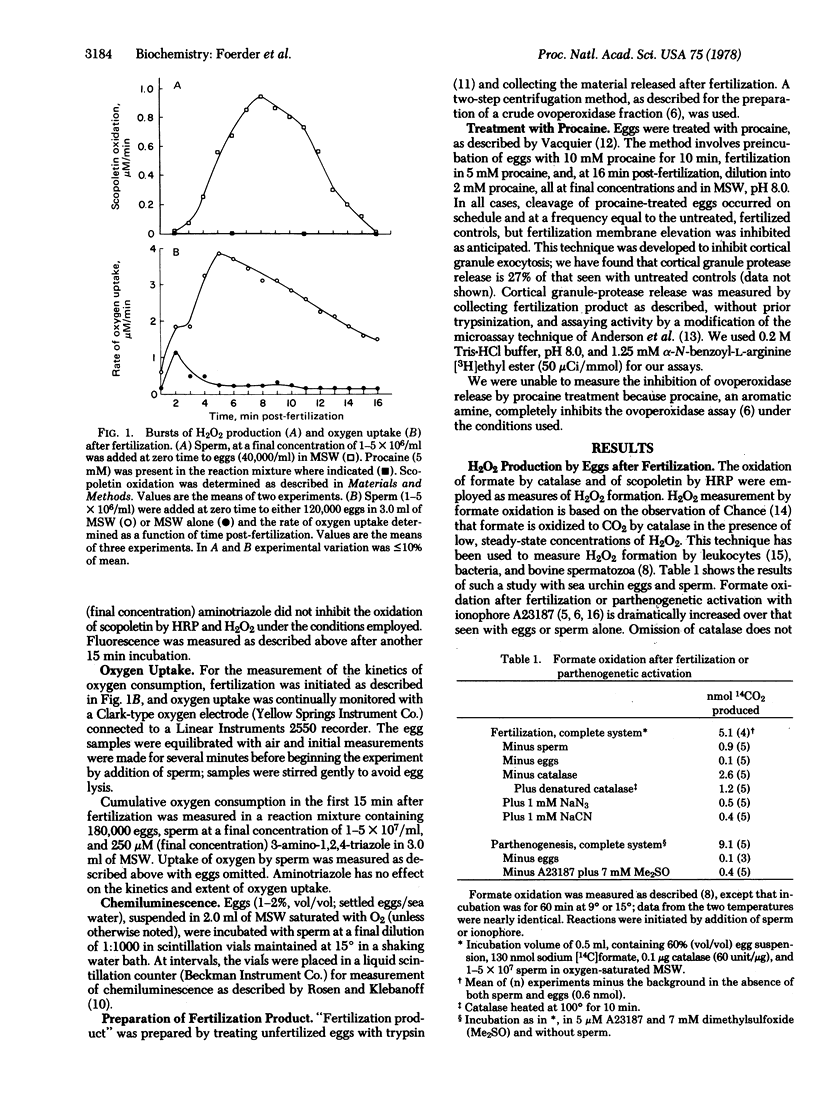
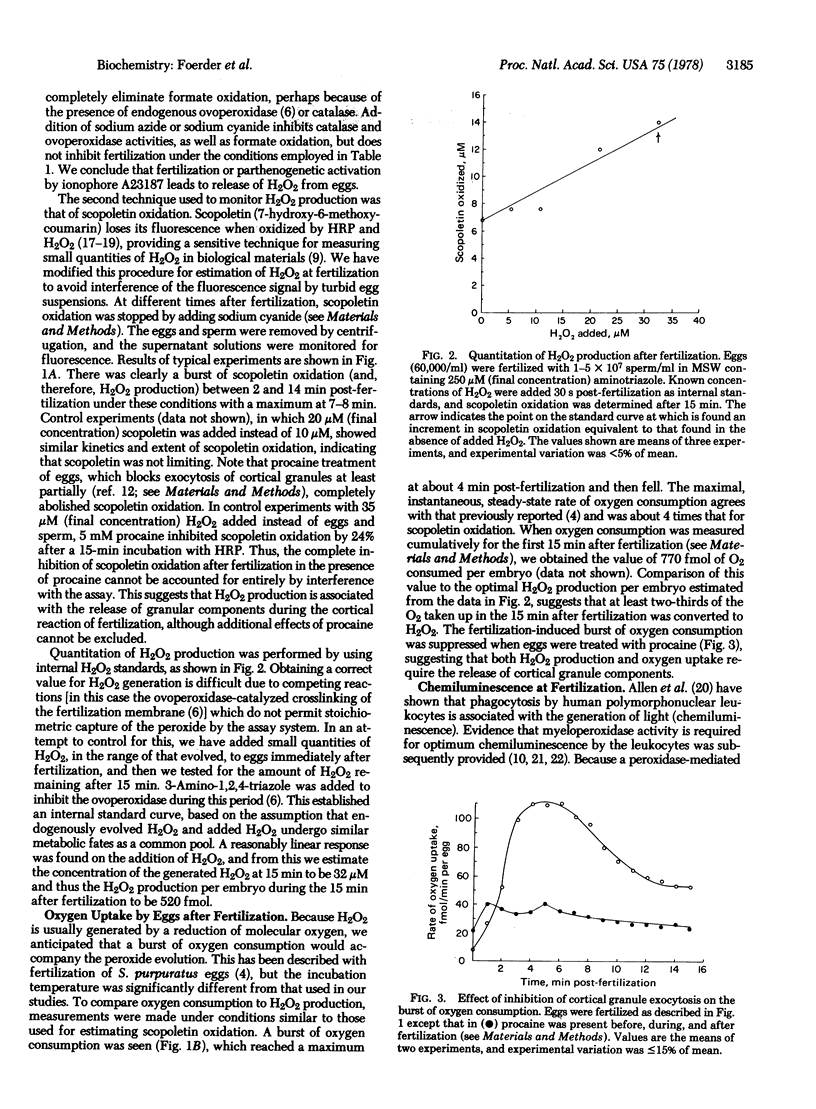
After fertilization of the sea urchin, Strongyl-ocentrotus purpuratus, a crosslinked fertilization membrane is formed; the crosslinks (dityrosine residues) are synthesized in a reaction catalyzed by an ovoperoxidase that is released from the cortical granules during fertilization. The substrate for ovoperoxidase activity, hydrogen peroxide, is generated by the egg coincident with the “respiratory burst” that follows parthenogenetic activation by the divalent ionophore A23187 or fertilization. This burst of oxygen consumption may be almost quantitatively accounted for by hydrogen peroxide evolution, as measured by the peroxidase-catalyzed quenching of scopoletin fluorescence. Neither the burst of oxygen consumption nor hydrogen peroxide production occurs when the inhibitor of cortical granule discharge, procaine, is present at fertilization.
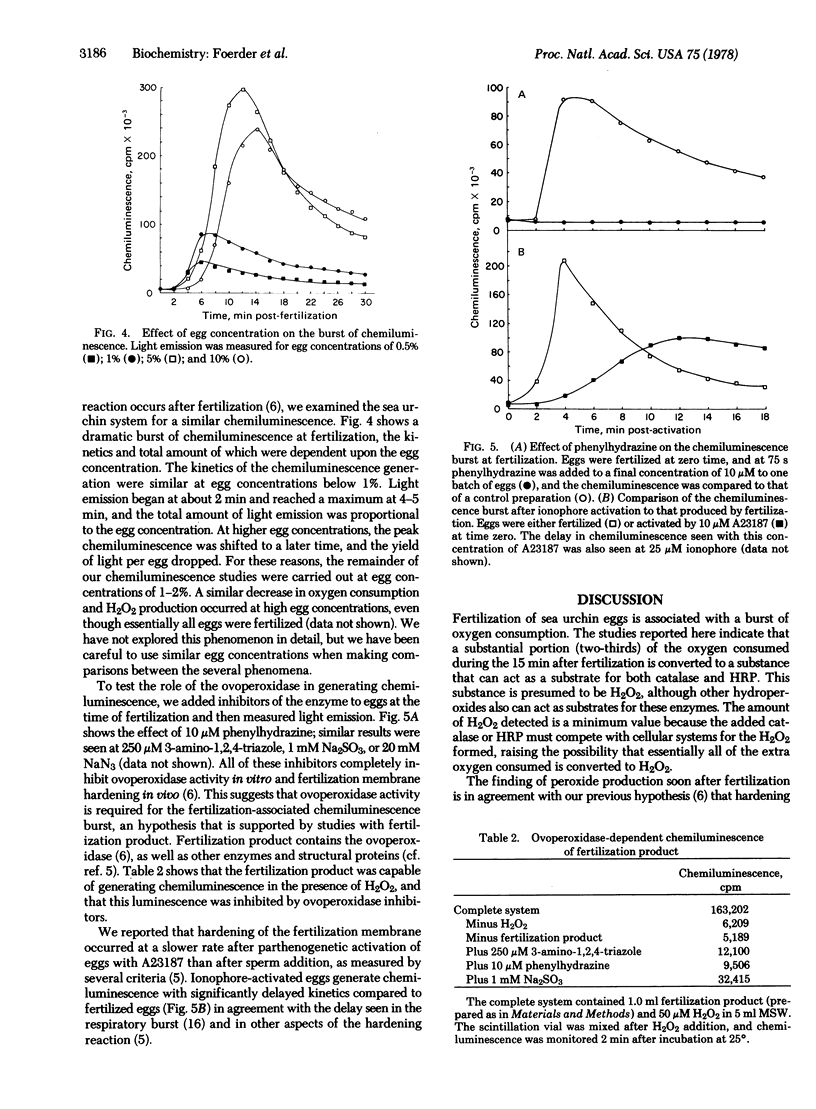
Fertilization or parthenogenetic activation with A23187 also is associated with a burst of light emission. This chemiluminescence is inhibited in vivo by inhibitors of the ovoperoxidase, such as 3-amino-1,2,4-triazole, phenylhydrazine, sulfite, or azide. A crude ovoperoxidase preparation catalyzes hydrogen peroxide-dependent chemiluminescence that is similarly inhibited. Thus, the bursts of oxygen uptake, peroxide production, and chemiluminescence appear to be several manifestations of the peroxidative system released at fertilization. This system may additionally be responsible for spermicidal activity and thus may act as a component of the block to polyspermy.
Keywords: peroxidase, oxygen, respiration, polyspermy
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDREAE W. A. A sensitive method for the estimation of hydrogen peroxide in biological materials. Nature. 1955 May 14;175(4463):859–860. doi: 10.1038/175859a0. [DOI] [PubMed] [Google Scholar]
- Allen R. C. Halide dependence of the myeloperoxidase-mediated antimicrobial system of the polymorphonuclear leukocyte in the phenomenon of electronic excitation. Biochem Biophys Res Commun. 1975 Apr 7;63(3):675–683. doi: 10.1016/s0006-291x(75)80437-2. [DOI] [PubMed] [Google Scholar]
- Allen R. C., Stjernholm R. L., Steele R. H. Evidence for the generation of an electronic excitation state(s) in human polymorphonuclear leukocytes and its participation in bactericidal activity. Biochem Biophys Res Commun. 1972 May 26;47(4):679–684. doi: 10.1016/0006-291x(72)90545-1. [DOI] [PubMed] [Google Scholar]
- Allen R. C. The role of PH in the chemiluminescent response of the myeloperoxidase-halide-HOOH antimicrobial system. Biochem Biophys Res Commun. 1975 Apr 7;63(3):684–691. doi: 10.1016/s0006-291x(75)80438-4. [DOI] [PubMed] [Google Scholar]
- Anderson L. E., Walsh K. A., Neurath H. Bovine enterokinase. Purification, specificity, and some molecular properties. Biochemistry. 1977 Jul 26;16(15):3354–3360. doi: 10.1021/bi00634a011. [DOI] [PubMed] [Google Scholar]
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boveris A., Martino E., Stoppani A. O. Evaluation of the horseradish peroxidase-scopoletin method for the measurement of hydrogen peroxide formation in biological systems. Anal Biochem. 1977 May 15;80(1):145–158. doi: 10.1016/0003-2697(77)90634-0. [DOI] [PubMed] [Google Scholar]
- Buege J. A., Aust S. D. Lactoperoxidase-catalyzed lipid peroxidation of microsomal and artificial membranes. Biochim Biophys Acta. 1976 Aug 24;444(1):192–201. doi: 10.1016/0304-4165(76)90236-1. [DOI] [PubMed] [Google Scholar]
- DURE L. S., CORMIER M. J. STUDIES ON THE BIOLUMINESCENCE OF BALANOGLOSSUS BIMINIENSIS EXTRACTS. 3. A KINETIC COMPARISON OF LUMINESCENT AND NONLUMINESCENT PEROXIDATION REACTIONS AND A PROPOSED MECHANISM FOR PEROXIDASE ACTION. J Biol Chem. 1964 Jul;239:2351–2359. [PubMed] [Google Scholar]
- Epel D. Methods for removal of the vitelline membrane of sea urchin eggs. II. Controlled exposure to trypsin to eliminate post-fertilization clumping of embryos. Exp Cell Res. 1970 Jul;61(1):69–70. doi: 10.1016/0014-4827(70)90258-2. [DOI] [PubMed] [Google Scholar]
- Foerder C. A., Shapiro B. M. Release of ovoperoxidase from sea urchin eggs hardens the fertilization membrane with tyrosine crosslinks. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4214–4218. doi: 10.1073/pnas.74.10.4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey E. N. THE MECHANISM OF LIGHT PRODUCTION IN ANIMALS. Science. 1916 Aug 11;44(1128):208–209. doi: 10.1126/science.44.1128.208. [DOI] [PubMed] [Google Scholar]
- Jones R., Mann T. Damage to ram spermatozoa by peroxidation of endogenous phospholipids. J Reprod Fertil. 1977 Jul;50(2):261–268. doi: 10.1530/jrf.0.0500261. [DOI] [PubMed] [Google Scholar]
- Jones R., Mann T. Lipid peroxidation in spermatozoa. Proc R Soc Lond B Biol Sci. 1973 Aug 31;184(1074):103–107. doi: 10.1098/rspb.1973.0035. [DOI] [PubMed] [Google Scholar]
- Jones R., Mann T. Toxicity of exogenous fatty acid peroxides towards spermatozoa. J Reprod Fertil. 1977 Jul;50(2):255–260. doi: 10.1530/jrf.0.0500255. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J., Smith D. C. The source of H2O2 for the uterine fluid-mediated sperm-inhibitory system. Biol Reprod. 1970 Oct;3(2):236–242. doi: 10.1093/biolreprod/3.2.236. [DOI] [PubMed] [Google Scholar]
- Krinsky N. I. Singlet excited oxygen as a mediator of the antibacterial action of leukocytes. Science. 1974 Oct 25;186(4161):363–365. doi: 10.1126/science.186.4161.363. [DOI] [PubMed] [Google Scholar]
- Kumar K. S., Walls R., Hochstein P. Lipid peroxidation and hemolysis induced by lactoperoxidase and thyroid hormones. Arch Biochem Biophys. 1977 Apr 30;180(2):514–521. doi: 10.1016/0003-9861(77)90067-4. [DOI] [PubMed] [Google Scholar]
- Root R. K., Metcalf J., Oshino N., Chance B. H2O2 release from human granulocytes during phagocytosis. I. Documentation, quantitation, and some regulating factors. J Clin Invest. 1975 May;55(5):945–955. doi: 10.1172/JCI108024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H., Klebanoff S. J. Chemiluminescence and superoxide production by myeloperoxidase-deficient leukocytes. J Clin Invest. 1976 Jul;58(1):50–60. doi: 10.1172/JCI108458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H., Klebanoff S. J. Formation of singlet oxygen by the myeloperoxidase-mediated antimicrobial system. J Biol Chem. 1977 Jul 25;252(14):4803–4810. [PubMed] [Google Scholar]
- SBARRA A. J., KARNOVSKY M. L. The biochemical basis of phagocytosis. I. Metabolic changes during the ingestion of particles by polymorphonuclear leukocytes. J Biol Chem. 1959 Jun;234(6):1355–1362. [PubMed] [Google Scholar]
- Smith D. C., Klebanoff S. J. A uterine fluid-mediated sperm-inhibitory system. Biol Reprod. 1970 Oct;3(2):229–235. doi: 10.1093/biolreprod/3.2.229. [DOI] [PubMed] [Google Scholar]
- Steinhardt R. A., Epel D. Activation of sea-urchin eggs by a calcium ionophore. Proc Natl Acad Sci U S A. 1974 May;71(5):1915–1919. doi: 10.1073/pnas.71.5.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacquier V. D. The isolation of intact cortical granules from sea urchin eggs: calcium lons trigger granule discharge. Dev Biol. 1975 Mar;43(1):62–74. doi: 10.1016/0012-1606(75)90131-1. [DOI] [PubMed] [Google Scholar]
- Veron M., Foerder C., Eddy E. M., Shapiro Sequential biochemical and morphological events during assembly of the fertilization membrane of the sea urchin. Cell. 1977 Feb;10(2):321–328. doi: 10.1016/0092-8674(77)90226-4. [DOI] [PubMed] [Google Scholar]
- WALES R. G., WHITE I. G., LAMOND D. R. The spermicidal activity of hydrogen peroxide in vitro and in vivo. J Endocrinol. 1959 May;18(3):236–244. doi: 10.1677/joe.0.0180236. [DOI] [PubMed] [Google Scholar]


