Abstract
Sarcocystis is an obligatory intracellular protozoan parasite which can infect humans and animals. Sheep are intermediate hosts of four Sarcocystis species: Sarcocystis tenella, Sarcocystis gigantea, Sarcocystis arieticanis, and Sarcocystis medusiformis The purpose of this study was to perform a molecular identification of the macroscopic and microscopic cysts of Sarcocystis in sheep. In this investigation, the macroscopic and microscopic cysts of Sarcocystis were assessed in slaughtered sheep. The digestion method was used for bradyzoites observation in heart, liver, diaphragm and muscle samples. PCR analysis was conducted on macroscopic and microscopic cysts and also all other samples. Sequencing was performed for ten PCR products. Genotypes were identified by BLAST search and homology analysis. Macrocysts were seen in two muscle tissues. Digestion method and PCR analysis revealed positive results in all samples taken from heart, liver, diaphragm, and muscle. Genotyping of ten tissue samples proved that the genotype of macroscopic belonged to Sarcocystis gigantea and microscopic cysts to Sarcocystis tenella. Microscopic cysts are more prevalent than macroscopic cysts and they can cause enormous economic losses.
Key Words: Sarcocystis tenella, Sarcocystis gigantea, molecular analysis
Introduction
Sarcocystis species are intracellular protozoan parasites infecting a wide range of livestocks. Some of Sarcocystis genus are pathogenic for animals such as sheep and cattle which cause enormous economic losses (1).
Studies in different regions of the world indicate that the prevalence of Sarcocystis infection in slaughtered cattle and sheep are between 70% to 100% (2-6). Additionally, studies in Iran showed that the prevalence of this parasite in the animal was between 85% to 100% (7-13). For example, studies in Kerman and Ahwaz provinces indicated that 100% of animals were infected with Sarcocystis (12-13).
Different species of Sarcocystis have been isolated from animals worldwide. Sarcocystis tenella was isolated from sheep in Iran and Brazil (10, 14). In another study, Sarcocystis moulei was reported from reindeer (15). Also, Nourani et al. isolated Sarcocystis hominis from cattle (8) while Kalantari et al. separeted S. cruzi from cattle (16). Dalimi et al. determined S. gigantea and S. arieticanis in sheep (9).
Sheep are intermediate hosts of four Sarcocystis species: S. tenella, S.gigantea, S. arieticanis, and S. medusiformis (1). S. tenella and S. gigantea have worldwide distribution (1, 9-10, 14). S. gigantea and S. medusiformis which are non-pathogenic and transferred by cats, can create some macroscopic visible cysts (1). S. tenella and S. arieticanis are transmitted by dogs and can cause microscopic cysts (1). Pathogenic species in sheep may cause severe disease or abortion during the early phase of infection (1). Sheep will become infected with S. tenella by swallowing sporocysts in faces of dog. The first and second meront were created in the asexual stage of S. tenella (1). Cysts of S. tenella can be found in all sheep tissues. In addition, cysts of S. tenella may be formed in the cells of the central nervous system of sheep (1).
In official health control of slaughterhouses, the carcasses infected with macroscopic cysts are removed by veterinarians. But, the microscopic cysts remain because they are not visible. Since, some of microscopic cysts are pathogenic in sheep, thus, we decided to perform the molecular identification of microscopic and macroscopic cysts of this parasite in sheep.
Materials and Methods
Sampling method
This descriptive cross-sectional study was accomplished in eight months, from December 2012 to July 2013. 160 samples were collected from the heart (N= 40), liver (N= 40), diaphragm (N= 40) and muscle (N= 40) from 40 slaughtered sheep (20 males and 20 females) aged between 3 months to 3 years in the slaughterhouses of North Khorasan province.
Tissue digestion
Tissue digestion method was used for observing bradyzoite in tissue samples. Seventy grams of each tissue were ground and digested in 1.5% HCL and 0.5% Pepsin at 29°C overnight. The digested samples were filtered through mesh and centrifuged at 1500 rpm for 10 minutes. Then the supernatant was discarded and the pellet was stained by Giemsa and examined microscopically to detect bradyzoites of Sarcocystis. For observing bradyzoite in macroscopic cysts; macroscopic cysts were separated from intact tissues and then, both macroscopic cyst and intact tissues were digested, separately.
DNA extraction and PCR amplification
For DNA extraction, a small piece of each sample was selected, and DNAs were extracted using tissue DNA extraction kit (Cinnagen company). PCR analysis was performed on all tissue samples using Sar primers including Sar-F1 Forward 5'GCACTTGATGAATTCTGGCA3' and Sar-R1 Reverse 5'CACCACCCATAGAATCAAG 3' (9). PCR reaction was carried out in 30 ml of Ampliqone (Taq DNA polymerase master Mix RED, Denmark). Twenty-five microliters of Taq Master mix were used with 10 ng template DNA, 0.1 μM of each primer and distilled water. Cycles of PCR were set up as follows: Predenaturation step at 94 ° C for 5 min and 30 cycles of denaturation at 94 ° C for 45 s, annealing at 55 °C for 1 min and extension at 72 ° C for 1 min with an elongation step of 7 min at 72 °C at the last cycle. PCR product was electrophoresed on 2% agarose gel, stained with Ethidium Bromide (0.5 μg/ml) and visualized under the UV light.
Sequencing and genotyping of isolates
10 PCR products (2 hearts, 2 livers, 2 diaphragms and 4 muscles) were purified. using column-based purification kit and then sequenced through automatic sequencer of the Korean Macrogen Company.
The obtained sequences were edited using Bioedit software program. Genotype identification was performed by comparing with available Sarcocystis DNA sequences in the GenBank based on sequence analysis of 18s rRNA region.
Results
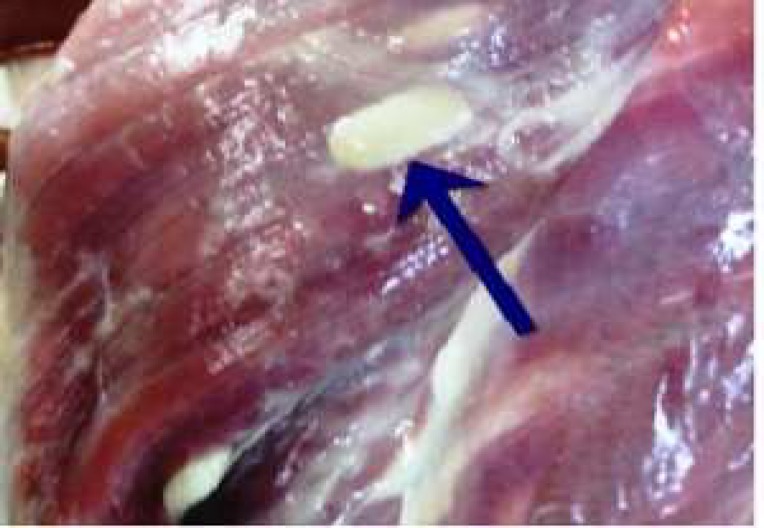
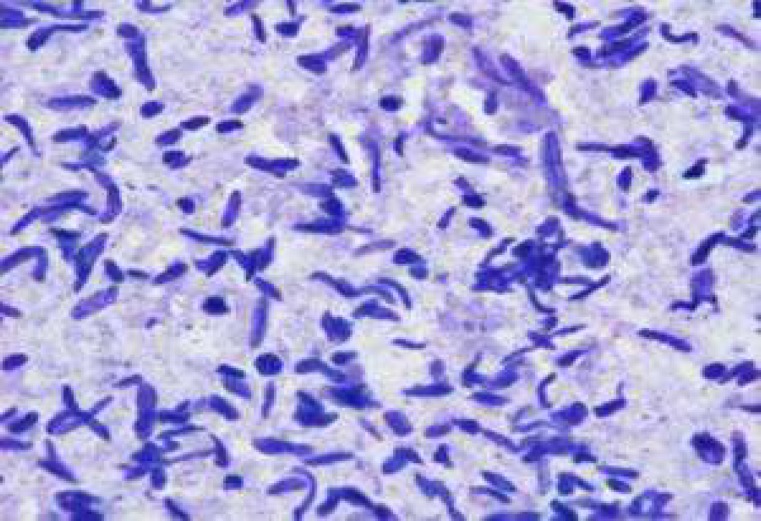
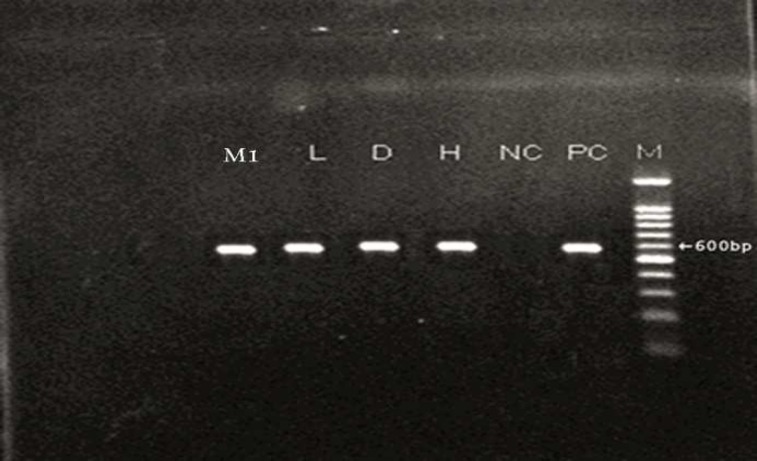
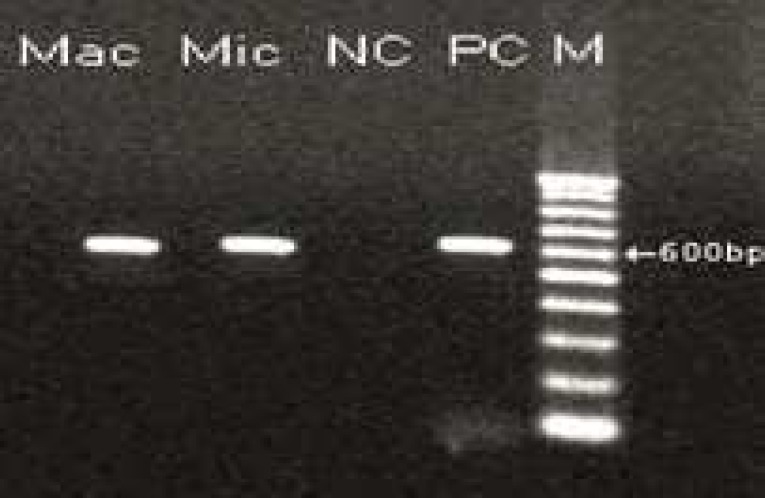
In this study, two samples of muscle had macroscopic cysts (Figure1 and table1). In addition, these muscles were found to be infected with microscopic cysts by digestion method. The results of digestion method showed that all samples of heart, diaphragm, muscle and liver were infected with bradyzoites of Sarcocystis (Figure 2). PCR analysis of macrocysts and microcysts as well as all samples showed a specific 600 bp band on the agarose gel (Figures 3 and 4). The results obtained from sequencing of ten samples (2 hearts, 2 diaphragms, 4 muscles and 2 livers) showed that the genotype of macroscopic and microscopic cysts correspond to Sarcocystis gigantea and Sarcocystis tenella, respectively.
Fig 1.
Macroscopic cyst in muscle of sheep
Table 1.
Number of macroscopic and microscopic cysts in sheep’s tissues and used methods
| Macroscopic cyst | Microscopic cyst | Digestion method | Molecular method | Negative | |
|---|---|---|---|---|---|
| Heart | 0 | 40 | 40 | 40 | 0 |
| Diaphragm | 0 | 40 | 40 | 40 | 0 |
| Muscle | 2 | 38 | 40 | 40 | 0 |
| Liver | 0 | 40 | 40 | 40 | 0 |
Fig 2.
Bradyzoites of Sarcocystis in sheep's muscles
Fig 3.
PCR Product from extracted DNA of Sarcocystis in different tissues.
M: Molecular weight marker (100bp), PC: Positive Control, NC: Negative Control, H: Heart, D: Diaphragm , L: Liver, M1: Muscle.
Fig 4.
PCR-product of microscopic and macroscopic cyst.
The genotypes and accession numbers are shown in table 2.
Table 2.
Genotypes and accession number of macroscopic and microscopic cyst
| ID | Macroscopic cyst | Macroscopic +Microscopic cyst | Accession number | Genotype | |
|---|---|---|---|---|---|
| H1 | - | - | KF489419 | S. tenella | |
| H2 | - | - | KF489427 | S. tenella | |
| D1 | - | - | KF489418 | S. tenella | |
| D2 | - | - | KF489422 | S. tenella | |
| M1 | + | + | KF489421 | Mac: S. gigantea | |
| KF489428 | Mic: S. tenella | ||||
| M2 | 2+ | 2+ | KF489425 | Mac: S. gigantea | |
| KF489429 | Mic: S. tenella | ||||
| L1 | - | - | KF489420 | S. tenella | |
| L2 | - | - | KF489424 | S. tenella | |
H: Heart, D: Diaphragm, M: Muscle, L: Liver, Mac: Macroscopic cyst, Mic: Microscopic cyst
Discussion
This investigation showed that two muscle tissues had macroscopic cysts. Also, all samples (heart, diaphragm, muscle and liver) were infected with Sarcocystisspp. The studies in Iran and other parts of the world indicated that livestocks are infected with Sarcocystisspp (2-11, 17-19). Other studies in different provinces of Iran showed that 85%-100% of cattle and sheep had Sarcocystis infection (7-11, 19).
In previous studies throughout the world, species of Sacocystis were isolated from different animals (8-10, 14-15). DaSilva and Langoni isolated S. tenella from the sheep in Brazil (14). Al-Hoost et al. reported S. moulei from the sheep in Saudi Arabia (20). Moreover, Gjerde isolated and characterized S. grueneri from reindeer based on molecular method (15). In other studies in Iran, Sarcocystis hominis and Sarcocystis cruzi were studied in cattle (8, 16). Dalimi et al. isolated S.gigantea and S. arieticanis from the sheep by PCR-RFLP method in Qazvin province, Iran (9). Furthermore, other researchers reported S. miescheriana from boar (21) and S. tenella from sheep in Iran (10). Additionally, Mahran in Egypt using morphometric method indicated that S. gigantea and S. tenella caused macroscopic and microscopic cysts (22). Using daub smear method, Bonyadian et al. showed that 91% of cows were infected with microscopic cyst and did not have any macroscopic cysts (23). Kargar Jahromi et al. using digestion method proved that goats had microscopic and macroscopic cysts (24). Molecular analysis was not performed in the above studies, but, the use of molecular methods in the present study showed that S. gigantea and S. tenella can cause macroscopic and microscopic cysts, respectively. The results of this investigation was in accordance with Anja and Astrid's study who reported that S. gigantea and S. tenella can cause macroscopic and microscopic cysts, respectively (1).
S. tenella is among the pathogenic species and can induce microscopic cysts (1). The severity of clinical symptoms caused by this species depends on the dose of ingested sporocysts and the immune system of the host (1, 25-26). S. tenella can lead to acute sarcocystosis in uninfected sheep (1). Non-specific infection symptoms include fever, anorexia, tachycardia and anemia could be observed following infection. In acute sarcocystosis, central nervous system will be involved, and it can cause encephalitis and encephalomyelitis and subsequ-ently death in sheep (27-29). In pregnant sheep, acute sarcocystosis can cause fetal death or premature birth of offspring. Chronic sarcocystosis can create economic problems due to reduced meat, milk and wool (1, 29-30). Also, Dubey reported that S. tenella caused symptoms such as inflammation, hepatitis and myocarditis in sheep inoculated with S. tenella sporocysts from canine feces (31).
In this study, S. tenella was isolated from the heart, diaphragm, muscle and liver of all tested samples. As noted above, S. tenella can cause severe clinical signs and dysfunction of organs in sheep, such as tachycardia and neurological disease (1). Furthermore, Sarcocystis tenella produces microscopic cyst which are neglected by veterinarians due to its invisible nature. Therefore, improvement of disease control and prevention strategies in sheep would be necessary.
S.tenella produces microscopic cyst and which are neglected by veterinarians due to its invisible nature. While S.gigantea which causes macroscopic cysts and is non-pathogenic are easily noted. For this reason, macroscopic cysts are removed, but, microscopic cysts do remain. Subsequently, the life cycle of Sarcocystis tenella is repeated and it causes enormous economical losses.
Acknowledgements
We are gratefully indebted to Mr. Koroosh Arzamani, the Vector-borne Diseases Research Center and the laboratory of North Khorasan veterinary head office.
Conflict of interest
The authors declared no Conflict of interests.
References
- 1.Heckeroth AR, Tenter AM. Comparison of immunological and molecular methods for the diagnosis of infections with pathogenic Sarcocystis species in sheep. Tokai J Exp Clin Med . 1998;23:293–302. [PubMed] [Google Scholar]
- 2.Pereira A, Bermejo M. Prevalence of Sarcocystis cysts in pigs and sheep in Spain. Vet Parasitol . 1988;27:353–5. doi: 10.1016/0304-4017(88)90049-0. [DOI] [PubMed] [Google Scholar]
- 3.Pena HF, Ogassawara S, Sinhorini IL. Occurrence of cattle Sarcocystis species in raw kibbe from Arabian food establishments in the city of Sao Paulo, Brazil, and experimental transmission to humans. J Parasitol . 2001;87:1459–65. doi: 10.1645/0022-3395(2001)087[1459:OOCSSI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 4.More G, Abrahamovich P, Jurado S, et al. Prevalence of Sarcocystis spp in Argentinean cattle. Vet Parasitol . 2011;177:162–5. doi: 10.1016/j.vetpar.2010.11.036. [DOI] [PubMed] [Google Scholar]
- 5.Britt DP, Baker JR. Causes of death and illness in the native sheep of North Ronaldsay, Orkney. I. Adult sheep. Br Vet J . 1990;146:129–42. doi: 10.1016/0007-1935(90)90005-N. [DOI] [PubMed] [Google Scholar]
- 6.Woldemeskel M, Gebreab F. Prevalence of sarcocysts in livestock of northwest Ethiopia. Zentralbl Veterinarmed B . 1996;43:55–8. doi: 10.1111/j.1439-0450.1996.tb00287.x. [DOI] [PubMed] [Google Scholar]
- 7.Oryan A, Ahmadi N, Mousavi SM. Prevalence, biology, and distribution pattern of Sarcocystis infection in water buffalo (Bubalus bubalis) in Iran. Trop Anim Health Prod. 2010;42:1513–8. doi: 10.1007/s11250-010-9601-7. [DOI] [PubMed] [Google Scholar]
- 8.Nourani H, Matin S, Nouri A, et al. Prevalence of thin-walled Sarcocystis cruzi and thick-walled Sarcocystis hirsuta or Sarcocystis hominis from cattle in Iran. Trop Anim Health Prod . 2010;42:1225–7. doi: 10.1007/s11250-010-9552-z. [DOI] [PubMed] [Google Scholar]
- 9.Dalimi Asl AH, Mutamedi G, Paykari H, et al. Detection of Sarcocystis spp. of slaughtered sheep in Gazvin Ziaran slaughter house by molecular assay. Journal of modarres Medical Science . 2008;11:65–72. [Google Scholar]
- 10.Shahbazi A, Falah S, Khanmohamadi M, et al. Identification of Sarcocystis tenella and Sarcocystis articanis from slaughtered sheep using PCR-RFLP in Tabriz province. Journal of pathobiology . 2013;10:959–64. [Google Scholar]
- 11.Valinezhad A, Oryan A, Ahmadi N. Sarcocystis and its complications in camels (Camelus dromedarius) of eastern provinces of Iran. Korean J Parasitol . 2008;46:229–34. doi: 10.3347/kjp.2008.46.4.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nourollahi Fard SR, Asghari M, Nouri F. Survey of Sarcocystis infection in slaughtered cattle in Kerman, Iran. Trop Anim Health Prod. 2009;41:1633–6. doi: 10.1007/s11250-009-9358-z. [DOI] [PubMed] [Google Scholar]
- 13.Hamidinejat H, Razi Jalali MH, Nabavi L. Survey on Sarcocystis Infection in Slaughtered Cattle in South-West of Iran, Emphasized on Evaluation of Muscle Squash in Comparison with Digestion Method. J Animal Vet Advances . 2010;9:1724–6. [Google Scholar]
- 14.da Silva RC, Su C, Langoni H. First identification of Sarcocystis tenella (Railliet, 1886) Moule, 1886 (Protozoa: Apicomplexa) by PCR in naturally infected sheep from Brazil. Vet Parasitol . 2009;165:332–6. doi: 10.1016/j.vetpar.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 15.Gjerde M. Ultrastructure of the cysts of Sarcocystis grueneri from cardiac muscle of reindeer (Rangifertarandustarandus) Z Parasitenkd . 1985;71:189–98. doi: 10.1007/BF00926269. [DOI] [PubMed] [Google Scholar]
- 16.Kalantari N, Bayani M, Ghaffari S. Sarcocystis cruzi: First molecular identification from cattle in Iran. Int J Mol Cell Med. 2013;2:125–30. [PMC free article] [PubMed] [Google Scholar]
- 17.Hamidinejat H, Hekmatimoghaddam SH, Jafari H, et al. Prevalence and distribution patterns of Sarcocystis in camels (Camelus dromedarius) in Yazd province, Iran. J parasit Dis . 2013;37:163–65. doi: 10.1007/s12639-012-0150-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Savini G, Dunsmore JD, Robertson ID, et al. The epidemiology of Sarcocystis spp. in cattle of Western Australia. Epidemiol Infect . 1992;108:107–13. doi: 10.1017/s0950268800049554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shekarforoush SS, Shakerian A, Hasanpoor MM. Prevalence of Sarcocystis in slaughtered one-humped camels (Camelus dromedarius) in Iran. Trop Anim Health Prod. 2006;38:301–3. doi: 10.1007/s11250-006-4362-z. [DOI] [PubMed] [Google Scholar]
- 20.Al-Hoot AS, Al-Qureishy SA, Al-Rashid K, et al. Microscopic study on Sarcocystis moulei from sheep and goats in Saudi Arabia. J Egypt Soc Parasitol . 2005;35:295–312. [PubMed] [Google Scholar]
- 21.Kia EB, Mirhendi H, Rezaeian M, et al. First molecular identification of Sarcocystis miescheriana (Protozoa, Apicomplexa) from wild boar (Sus scrofa) in Iran. Exp Parasitol . 2011;127:724–6. doi: 10.1016/j.exppara.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 22.Mahran OM. Sarcocystis infection in sheep and goats slaughtered in Shalatin Abattoir, Red Sea Governorate, Egypt. Assiut Veterinary Medical Journal . 2009;55:341–55. [Google Scholar]
- 23.Bonyadian M, Meshki B. Study on infection of cow cacasses to Sarcocyst Spp in slaughtered cows in Shahrei. Pajouhesh va sazandegi. 2006;19:14–8. [Google Scholar]
- 24.Kargar Jahromi Z, Solhjoo K, Zareian Jahromi M, et al. Investigation of Sarcocystis Infection in Slaughtered Goats in Jahrom Abattoir. JFUMS . 2012;2:163–7. [Google Scholar]
- 25.O'Donoghue P, Rommel M. Australian-German collaborative studies on the immunology of Sarcocystis infections. Angew Parasitol . 1992;33:102–19. [PubMed] [Google Scholar]
- 26.Uggla A, Buxton D. Immune responses against Toxoplasma and Sarcocystis infections in ruminants: diagnosis and prospects for vaccination. Rev Sci Tech . 1990;9:441–62. doi: 10.20506/rst.9.2.502. [DOI] [PubMed] [Google Scholar]
- 27.Jeffrey M. Sarcocystosis of sheep. Practice. 1993;15:2–8. [Google Scholar]
- 28.Leek RG, Fayer R, Johnson AJ. Sheep experimentally infected with sarcocystis from dogs. I. Disease in young lambs. J Parasitol . 1977;63:642–50. [PubMed] [Google Scholar]
- 29.Munday BL. The effect of Sarcocystis tenella on wool growth in sheep. Vet Parasitol . 1984;15:91–4. doi: 10.1016/0304-4017(84)90024-4. [DOI] [PubMed] [Google Scholar]
- 30.Collins GH, Charleston WA, Moriarty KM. Sarcocystis species in sheep. N Z Vet J. 1976;24:123–4. doi: 10.1080/00480169.1976.34299. [DOI] [PubMed] [Google Scholar]
- 31.Dubey JP. Lesions in sheep inoculated with Sarcocystis tenella sporocysts from canine feces. Vet Parasitol . 1988;26:237–52. doi: 10.1016/0304-4017(88)90092-1. [DOI] [PubMed] [Google Scholar]