Abstract
Subgroups of patients with breast cancer may be at greater risk for cytokine-induced changes in cognitive function after diagnosis and during treatment. The purposes of this study were to identify subgroups of patients with distinct trajectories of attentional function and evaluate for phenotypic and genotypic (i.e., cytokine gene polymorphisms) predictors of subgroup membership. Self-reported attentional function was evaluated in 397 patients with breast cancer using the Attentional Function Index before surgery and for six months after surgery (i.e., seven time points). Using growth mixture modeling, three attentional function latent classes were identified: High (41.6%), Moderate (25.4%), and Low-moderate (33.0%). Patients in the Low-moderate class were significantly younger than those in the High class, with more comorbidities and lower functional status than the other two classes. No differences were found among the classes in years of education, race/ethnicity, or other clinical characteristics. DNA was recovered from 302 patients’ samples. Eighty-two single nucleotide polymorphisms among 15 candidate genes were included in the genetic association analyses. After controlling for age, comorbidities, functional status, and population stratification due to race/ethnicity, IL1R1 rs949963 remained a significant genotypic predictor of class membership in the multivariable model. Carrying the rare “A” allele (i.e., GA+AA) was associated with a two-fold increase in the odds of belonging to a lower attentional function class (OR: 1.98; 95% CI: 1.18, 3.30; p=.009). Findings provide evidence of subgroups of women with breast cancer who report distinct trajectories of attentional function and of a genetic association between subgroup membership and an IL1R1 promoter polymorphism.
Keywords: attention, breast cancer, inflammation, cytokine genes, interleukin 1 receptor, type I, growth mixture modeling
1. Introduction1
Self-reported attentional function is an important aspect of quality of life for patients with breast cancer [1–3]. Perceived changes in attentional function after diagnosis and during treatment negatively impact women’s ability to maintain meaningful activities that require the direction of attention for sustained periods of time [1]. Attentional function is closely tied to working memory [4] and is a component of executive function [5]. Therefore, changes in attentional function interfere with planning and goal-directed activities [1]. Patients may report these changes because of increased mental effort exerted to compensate for cancer- and cancer-treatment-related cognitive changes [6]. Functional magnetic resonance imaging studies support the hypothesis that subjective reports of cognitive changes are associated with increased mental effort [6, 7].
A recent report of the International Cognition and Cancer Task Force highlighted a consistent finding in the literature that subgroups of patients are more vulnerable to cognitive changes [8]. Characterization of vulnerable subgroups would allow clinicians to target education and interventions to patients most likely to benefit. Phenotypic characteristics (e.g., age, functional status) and clinical characteristics (e.g., disease stage, treatment), as well as differences in peripheral inflammatory processes [9], may be associated with vulnerability to changes in attentional function in these women.
Cytokines and their receptors regulate inflammatory processes [9]. Peripheral inflammation, due to cancer and its treatment, could induce inflammation in the central nervous system (CNS) through activation of afferent nerves such as the vagus nerve [10, 11], peripheral cytokine interactions with circumventricular organs [12], active transport of cytokines across the blood-brain barrier [13], activation of second messengers [14], and/or direct entry of peripherally activated monocytes into the CNS [10, 15]. Microglial cells within the CNS respond by producing central pro-inflammatory cytokines that contribute to oxidative stress [16], dysregulation of hypothalamic-pituitary-adrenal axis function [17], and diminished growth factor signaling [18, 19]. Therefore, the effects of peripheral cytokines could have a negative impact on cognitive function [20]. Given this hypothesized relationship, variations in genes that encode for inflammatory cytokines and their receptors may explain some of the variability in attentional function reported by women with breast cancer.
In a previous study using growth mixture modeling (GMM) [21], we identified three subgroups of participants with clinically meaningful differences in trajectories of attentional function during and after radiation therapy. In these patients with breast, prostate, brain, or lung cancer and their family caregivers, a single nucleotide polymorphism (SNP) in IL6 (rs1800795) predicted subgroup membership. In the current study, we evaluate the same SNP and attempt to identify novel associations in a larger, more homogenous sample. Therefore, the purposes of this study, in a sample of women with breast cancer, were to identify latent classes (i.e., subgroups of patients) with distinct trajectories of attentional function and to evaluate for phenotypic and genotypic characteristics associated with latent class membership.
2. Material and Methods
This analysis is part of a larger study that evaluated for multiple symptoms in patients who underwent surgery for breast cancer [22]. Patients were recruited from breast care centers located in a Comprehensive Cancer Center, two public hospitals, and four community practices. Patients were eligible to participate if they were ≥18 years of age; were scheduled to undergo surgery on one breast; were able to read, write, and understand English; and gave written informed consent. Patients with distant metastases at the time of diagnosis were excluded. Of the 516 patients who were approached, 410 enrolled in the study (79.5% response rate) and 397 completed baseline assessments. The most common reasons for refusal were being too busy or feeling overwhelmed.
2.1. Study procedures
The study was approved by the Committee on Human Research at the University of California, San Francisco and by the institutional review boards at each of the other study sites. During preoperative visits, a clinical staff member explained the study and invited patients to participate. Those women who were willing to participate were introduced to a research nurse, who determined eligibility. After providing written informed consent, patients completed baseline questionnaires a mean of four days prior to surgery. Follow-up questionnaires were completed each month for six months after surgery (i.e., seven assessments over six months). Medical records were reviewed for disease and treatment information.
Patients completed a demographic questionnaire, the Karnofsky Performance Status (KPS) scale [23], the Self-administered Comorbidity Questionnaire (SCQ) [24], and the Attentional Function Index (AFI). The AFI consists of 13 items designed to measure self-reported attentional function (i.e., ability to voluntarily direct and sustain attention) [1]. Higher mean scores on a 0 to 10 numeric rating scale indicate greater capacity to direct attention. Scores are grouped into categories of attentional function (i.e., <5.0 low function, 5.0 to 7.5 moderate function, >7.5 high function) [25]. Multiple studies have used the AFI in patients with breast cancer before [25–27] and after [28] surgery and chemotherapy [29]. Additional studies have used the measure across multiple treatment modalities [30] and in long-term survivors [31]. The AFI has established reliability, as well as construct and convergent validity [1]. In this study, Cronbach’s alpha was .93.
2.2. Phenotypic analyses
Data were analyzed using SPSS 19 (IBM, Armonk, New York) and Mplus 6.11 (Muthén & Muthén, Los Angeles). Descriptive statistics and frequency distributions were generated for sample characteristics and AFI scores. GMM with robust maximum likelihood estimation identified latent classes of patients with distinct trajectories of attentional function. The GMM methods are described in detail elsewhere [32].
Analyses of variance and Chi-square analyses were used to evaluate for differences in patient characteristics among classes. The cohort of patients for each analysis was dependent on the largest set of available data across classes. Differences were considered statistically significant at p<.05. Post hoc contrasts used the Bonferroni correction to control the overall family alpha. For any one of three possible pairwise contrasts, p<.017 was considered statistically significant. Effect sizes were determined using Cohen’s d [33].
2.3. Genotypic analyses
Genomic DNA was extracted from archived buffy coats using the Puregene DNA Isolation System (Invitrogen, Carlsbad, CA). Of 397 patients who completed the baseline assessment, DNA was recovered for 302.
DNA was quantitated using spectrophotometry and normalized to a concentration of 50 ng/μL (diluted in 10 mM Tris/1 mM EDTA). Genotyping was performed blinded to clinical status. Samples were genotyped using the GoldenGate genotyping platform and processed using GenomeStudio (Illumina, San Diego). Genotype calls for each SNP were visually inspected by two blinded reviewers. Disagreements were adjudicated by a third reviewer.
2.3.1. Gene and SNP selection
Genes that encode for pro-inflammatory cytokines and their receptors include interleukin 2 (IL2), IL8, IL17A, and tumor necrosis factor alpha (TNFA, also referred to in the literature as TNF), as well as interferon gamma receptor 1 (IFNGR1) and IL1 receptor, type 1 (IL1R1). Genes that encode for anti-inflammatory cytokines and their receptors include IL4, IL10, and IL13, as well as IL1R2. Genes that encode for cytokines with both pro- and anti-inflammatory functions include IFNG, IL1 beta (IL1B), and IL6. Genes that encode for transcription factors, which moderate the levels of cytokine production, include nuclear factor kappa B 1 (NFKB1) and NFKB2.[9]
A combination of tagging SNPs and literature-driven SNPs (i.e., associated with altered function, symptoms) for these genes were selected for analysis. Tagging SNPs were required to have minor allele frequencies (MAFs) ≥5% in public databases. SNPs with call rates <95% or Hardy-Weinberg expectation p<.001 were excluded. Rare alleles are defined as having allele frequencies of less than 50% in the sample.
2.3.2. Statistical analyses
Allele and genotype frequencies were determined by gene counting. Hardy-Weinberg expectation was assessed by the Chi-square or Fisher Exact test. Measures of linkage disequilibrium (LD) (i.e., D′ and r2) were computed from patients’ genotypes with Haploview 4.2 (Broad Institute, Cambridge, Massachusetts). LD-based haplotype block definition was based on the D′ confidence interval (CI) method [34]. Haplotypes were constructed using PHASE 2.1 [35], as described previously [36]. One hundred six ancestry informative markers (AIMs) were included in the analyses, as described previously [36]. A backwards stepwise approach was used to create the most parsimonious phenotypic regression model. Except for self-reported race/ethnicity and AIMs, which were included to minimize confounding due to population stratification [37–39], only predictors with a p-value of <.05 were retained in the final model.
Additive, dominant, and recessive genetic models were assessed in association tests for each SNP. Barring trivial improvements (i.e., delta <10%) from the additive model, the model that best fit the data, by maximizing the significance of the p-value, was selected for inclusion in the multivariable analyses. To estimate the magnitude (i.e., odds ratio, OR) and precision (i.e., 95% CI) of the association of genotype with class membership, logistic regression models were fit that treated class as a discrete categorical variable. Model fit and both unadjusted and covariate-adjusted ORs were estimated using Stata 9 (StataCorp, College Station, Texas). If the overall model included a statistically significant genotype term, pairwise post hoc models (e.g., High versus Moderate attentional function) were fit. Only post hoc models with Bonferroni-corrected statistically significant genotype terms were retained.
As was done in our previous studies [21, 36, 40] and based on recommendations in the literature [41, 42], the implementation of rigorous quality controls for genomic data, the non-independence of SNPs/haplotypes in LD, and the exploratory nature of the analyses, adjustments were not made for multiple testing. Significant SNPs identified in the bivariate analyses were evaluated further using regression analyses that controlled for differences in phenotypic characteristics, potential confounding due to population stratification, and variation in other SNPs/haplotypes within the same gene. Only SNPs that remained significant were included in the final results. Therefore, the significant independent genetic association reported is unlikely to be due solely to chance. In addition, unadjusted (i.e., bivariate) associations are reported for all SNPs passing quality control criteria to allow for subsequent comparisons and meta-analyses.
3. Results
3.1. GMM classes
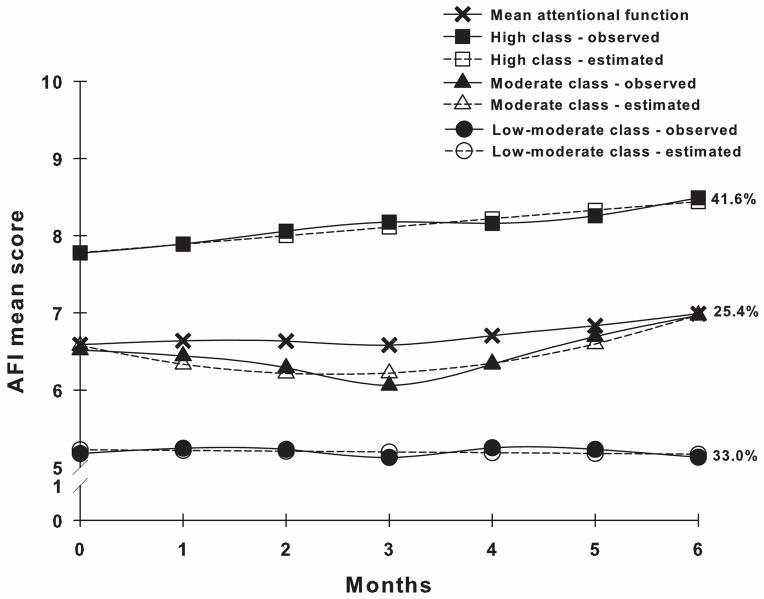
Three distinct classes of attentional function trajectories were identified (Figure 1). A three-class solution provided the best model fit because it had the smallest Bayesian information criterion (BIC) and a significant bootstrapped likelihood ratio test (BLRT), as well as greater entropy and more differentiating growth trajectories than the two-class solution, with each class maintaining reasonable size and interpretability (Table 1). Further, the Vuong-Lo-Mendell-Rubin likelihood ratio test (VLMR) was not significant for the four-class solution [43].
Figure 1.
Observed and estimated attentional function trajectories for patients in each latent class (High class, n=165; Moderate class, n=101; Low-moderate class, n=131), as well as mean Attentional Function Index (AFI) scores for the total sample.
Table 1.
Fit indices for attentional function growth mixture model solutions over seven assessments.
| Model | LL | AIC | BIC | Entropy | VLMRc | BLRTd |
|---|---|---|---|---|---|---|
| 1-classa | −4286.758 | 8597.516 | 8645.323 | n/a | n/a | n/a |
| 2-class | −4239.001 | 8518.003 | 8597.682 | 0.461 | 135.636** | 135.636** |
| 3-classb | −4218.660 | 8485.321 | 8580.935 | 0.554 | 40.682* | 40.682** |
| 4-class | −4220.918 | 8497.836 | 8609.386 | 0.642 | 23.514ns | 23.514**e |
LL = log likelihood, AIC = Akaike information criterion, BIC = Bayesian information criterion, VLMR = Vuong-Lo-Mendell-Rubin likelihood ratio test, BLRT = bootstrapped likelihood ratio test, n/a = not applicable, ns = not significant, CFI = comparative fit index, RMSEA = root mean square error of approximation.
p<.05,
p<.001.
Random intercepts latent growth curve model with linear components; χ2=49.470, 23 df, p<.01, CFI=.975, RMSEA=.054.
A three-class solution provided the best model fit because it had the smallest BIC and a significant BLRT, as well as greater entropy and more differentiating growth trajectories than the two-class solution, with each class maintaining reasonable size and interpretability. Further, VLMR was not significant for the four-class solution.
This statistic is the Chi-square statistic for VLMR. When significant, this test provides evidence that the K-class solution fits the data better than the K-1-class solution.
BLRT p-values are approximated based on varying numbers of bootstrap draws.
Although BLRT was significant, one of the classes comprised <5% of the sample (n=18), indicating an unreliable model.
Patients in the High Attentional Function (“High”) class (41.6%) had estimated AFI scores of 7.78 at enrollment that increased significantly and remained high throughout the study (Table 2). Patients in the Moderate Attentional Function (“Moderate”) class (25.4%) had estimated AFI scores of 6.58 at enrollment that decreased and then increased significantly but remained moderate throughout the study. Patients in the Low-moderate Attentional Function (“Low-moderate”) class (33.0%) had estimated AFI scores of 5.23 at enrollment that did not change significantly during the study.
Table 2.
Growth mixture model parameter estimates.
| Parameter estimatesa | High attentional function n=165b (41.6%) |
Moderate attentional function n=101 (25.4%) |
Low-moderate attentional function n=131 (33.0%) |
|---|---|---|---|
|
| |||
| Mean (SE) | Mean (SE) | Mean (SE) | |
| Intercept | 7.779*** (0.222) | 6.576*** (0.196) | 5.227*** (0.231) |
| Linear slope | 0.110*** (0.017) | −0.304* (0.129) | −0.010 (0.027) |
| Quadratic slope | 0c | 0.062** (0.021) | 0c |
| Intercept variance | 0.678*** (0.174) | 2.329*** (0.486) | 1.020** (0.354) |
| Linear slope variance | 0c | 0.761*** (0.191) | 0c |
| Quadratic slope variance | 0c | 0.019*** (0.005) | 0c |
SE = standard error.
p<.05,
p<.01,
p<.001.
Parameter estimates were obtained with robust maximum likelihood.
Trajectory class sizes are for classification of individuals based on most likely latent class membership.
Fixed at zero to improve estimation.
3.2. Phenotypic differences among classes
Patients in the Low-moderate class were significantly younger than those in the High class (Table 3). They had significantly more comorbidities and lower functional status than the other two classes. While a significant difference in body mass index (BMI) was found among the classes, post hoc contrasts were not significant. Although no differences were found among the classes in the proportion of patients who worked for pay, a greater proportion of patients making <$30,000/year than ≥$30,000/year were in the Low-moderate class compared to the High class. Likewise, a greater proportion of patients making <$30,000/year than ≥$100,000/year were in the Low-moderate class compared to the Moderate class. No differences were found among the classes in years of education, race/ethnicity, or other clinical characteristics.
Table 3.
Differences in demographic and clinical characteristics among the three latent classes for attentional function.
| Characteristic | High attentional function (0) | Moderate attentional function (1) | Low-moderate attentional function (2) | Statistics and post hoc comparisons |
|---|---|---|---|---|
|
| ||||
| Mean (SD) | Mean (SD) | Mean (SD) | ||
| Age (years) | 56.7 (11.2) | 55.2 (10.3) | 52.6 (12.6) | F(2,394)=4.9, p=.008; 2<0 |
| Education (years) | 15.8 (2.7) | 15.8 (2.9) | 15.6 (2.4) | ns |
| SCQ score | 3.8 (2.5) | 4.0 (2.6) | 5.1 (3.2) | F(2,393)=8.8, p<.001; 2>0,1 |
| BMI (kg/m2) | 25.9 (5.7) | 27.6 (6.6) | 27.4 (6.3) | F(2,388)=3.4, p=.033; no significant post hoc contrasts |
| KPS score | 95.7 (8.6) | 94.9 (6.9) | 88.8 (12.8) | F(2,387)=19.6, p<.001; 2<0,1 |
| n (%) | n (%) | n (%) | ||
| Race/ethnicity | ns | |||
| White | 113 (69.3) | 70 (69.3) | 72 (55.0) | |
| Black | 15 (9.2) | 7 (6.9) | 18 (13.7) | |
| Asian/Pacific Islander | 17 (10.4) | 14 (13.9) | 19 (14.5) | |
| Hispanic/mixed/other | 18 (11.0) | 10 (9.9) | 22 (16.8) | |
| Live alone (% yes) | 39 (23.9) | 19 (19.2) | 36 (27.7) | ns |
| Married or partnered (% yes) | 69 (42.1) | 36 (36.0) | 60 (46.2) | ns |
| Work for pay (% yes) | 84 (51.5) | 52 (51.5) | 53 (40.8) | ns |
| Household income level | KW=13.2, p=.001a | |||
| <$30,000 | 19 (14.3) | 15 (16.7) | 36 (34.0) | |
| $30,000–$99,999 | 61 (45.9) | 33 (36.7) | 40 (37.7) | |
| ≥$100,000 | 53 (39.8) | 42 (46.7) | 30 (28.3) | |
| Regular exercise (% yes) | 118 (71.5) | 74 (74.0) | 82 (63.6) | ns |
| Stage of disease | ns | |||
| Stage 0 | 30 (19.1) | 18 (18.8) | 16 (12.8) | |
| Stage I | 65 (41.4) | 38 (39.6) | 40 (32.0) | |
| Stage II | 50 (31.8) | 35 (36.5) | 53 (42.4) | |
| Stages III and IV | 12 (7.6) | 5 (5.2) | 16 (12.8) | |
| Postmenopausal (% yes) | 106 (65.4) | 64 (66.7) | 78 (60.9) | ns |
| Neoadjuvant therapy (% yes) | 27 (16.4) | 17 (17.0) | 35 (26.7) | ns |
| Type of surgery (% mastectomy) | 30 (18.2) | 24 (23.8) | 25 (19.1) | ns |
| Postoperative complications (% yes) | 32 (19.4) | 19 (19.0) | 32 (24.8) | ns |
| Adjuvant chemotherapy (% yes) | 48 (29.1) | 40 (39.6) | 45 (34.4) | ns |
| Radiation therapy (% yes) | 95 (57.6) | 52 (51.5) | 77 (58.8) | ns |
| Estrogen receptor (% positive) | 137 (83.5) | 74 (73.3) | 96 (73.3) | ns |
| Progesterone receptor (% positive) | 124 (75.6) | 70 (69.3) | 85 (64.9) | ns |
| HER2/neu (% positive) | 21 (14.3) | 15 (17.0) | 23 (18.7) | ns |
| HRT before diagnosis (% yes) | 22 (13.3) | 24 (24.0) | 21 (16.2) | ns |
SD = standard deviation, ns = not significant, SCQ = Self-administered Comorbidity Questionnaire, BMI = body mass index, KPS = Karnofsky Performance Status, KW = Kruskal-Wallis test, HER2/neu = human epidermal growth factor receptor 2, HRT = hormone replacement therapy.
Post hoc contrasts revealed that a greater proportion of patients making <$30,000/year than ≥$30,000/year were in the Low-moderate class compared to the High class. Likewise, a greater proportion of patients making <$30,000/year than ≥$100,000/year were in the Low-moderate class compared to the Moderate class.
Using a backwards stepwise approach, only age, comorbidities (i.e., SCQ score), and functional status (i.e., KPS score) significantly predicted class membership in multivariable models unadjusted for genotype. For each five-year increase in age, patients had a 12% decrease in the odds of belonging to a lower attentional function class (OR: 0.88; 95% CI: 0.80, 0.97; p=.012). For every one point increase in SCQ score (i.e., indicating a greater number, severity, and/or functional impact of comorbidities), patients had a 14% increase in the odds of belonging to a lower attentional function class (OR: 1.14; 95% CI: 1.04, 1.24; p=.004). For every ten-point increase in KPS score (i.e., indicating a clinically meaningful increase in functional status), patients had a 30% decrease in the odds of belonging to a lower attentional function class (OR: 0.70; 95% CI: 0.55, 0.90; p=.006).
3.3. Genotypic differences among classes
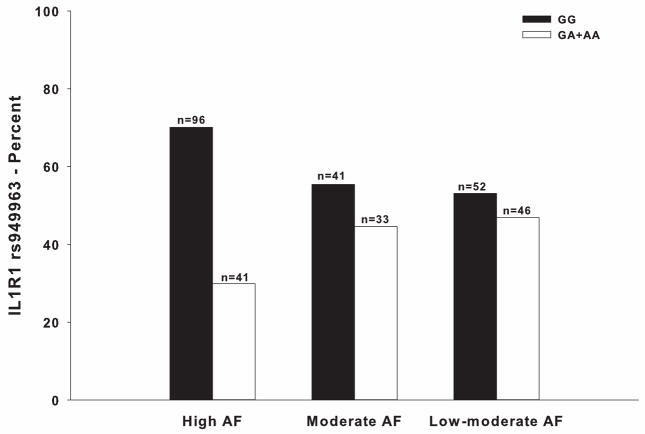
Eighty-two SNPs among 15 candidate genes passed all of the quality control filters. Genotype distributions differed significantly among classes for four SNPs and one haplotype (Table 4). Controlling for age, comorbidities, functional status, and population stratification due to race/ethnicity, the model fit for IL1R1 rs949963 remained significant (p<.001) (Table 5). Pairwise post hoc contrasts did not meet Bonferroni-corrected thresholds for significant between-class differences by genotype (OR: 2.10; 95% CI: 1.12, 3.94; p=.021 for High versus Moderate classes. OR: 2.01; 95% CI: 1.10, 3.68; p=.023 for High versus Low-moderate classes. OR: .90; 95% CI: .46, 1.75; p=.750 for Moderate versus Low-moderate classes). See Figure 2 for allelic distributions.
Table 4.
Differences in cytokine gene single nucleotide polymorphism (SNP) allele and haplotype frequencies among the latent classes of attentional function.
| Gene | SNP | Position | Chr | MAF | Alleles | Chi-square | p-value | Model |
|---|---|---|---|---|---|---|---|---|
| IFNG | rs2069728 | 66834051 | 12 | .110 | G>A | 1.27 | .867 | A |
| IFNG | rs2069727 | 66834490 | 12 | .384 | A>G | 2.65 | .617 | A |
| IFNG | rs2069718 | 66836429 | 12 | .494 | C>T | 3.17 | .530 | A |
| IFNG | rs1861493 | 66837463 | 12 | .266 | A>G | 0.92 | .921 | A |
| IFNG | rs1861494 | 66837676 | 12 | .273 | T>C | 0.67 | .955 | A |
| IFNG | rs2069709 | 66839970 | 12 | .003 | G>T | n/a | n/a | n/a |
| IFNG | HapA3 | 0.80 | .939 | - | ||||
| IFNG | HapA5 | 2.79 | .593 | - | ||||
| IFNGR1 | rs9376268 | 137574444 | 6 | .254 | G>A | 3.75 | .441 | A |
| IL1B | rs1071676 | 106042060 | 2 | .189 | G>C | 4.87 | .301 | A |
| IL1B | rs1143643 | 106042929 | 2 | .383 | G>A | 1.64 | .802 | A |
| IL1B | rs1143642 | 106043180 | 2 | .082 | C>T | 1.14 | .887 | A |
| IL1B | rs1143634 | 106045017 | 2 | .187 | C>T | 4.66 | .324 | A |
| IL1B | rs1143633 | 106045094 | 2 | .392 | G>A | 2.03 | .731 | A |
| IL1B | rs1143630 | 106046282 | 2 | .115 | C>A | 6.82 | .146 | A |
| IL1B | rs3917356 | 106046990 | 2 | .450 | G>A | 4.48 | .345 | A |
| IL1B | rs1143629 | 106048145 | 2 | .389 | T>C | 6.64 | .156 | A |
| IL1B | rs1143627 | 106049014 | 2 | .397 | T>C | 5.09 | .278 | A |
| IL1B | rs16944 | 106049494 | 2 | .386 | G>A | 5.63 | .229 | A |
| IL1B | rs1143623 | 106050452 | 2 | .277 | G>C | 6.61 | .158 | A |
| IL1B | rs13032029 | 106055022 | 2 | .448 | C>T | 3.02 | .554 | A |
| IL1B | HapA1 | 6.20 | .185 | - | ||||
| IL1B | HapA4 | 1.55 | .818 | - | ||||
| IL1B | HapA6 | 4.84 | .304 | - | ||||
| IL1B | HapB1 | 2.72 | .605 | - | ||||
| IL1B | HapB6 | 5.93 | .205 | - | ||||
| IL1B | HapB8 | 3.37 | .498 | - | ||||
| IL1R1 | rs949963a | 96533648 | 2 | .223 | G>A | 8.32 | .016 | D |
| IL1R1 | rs2228139 | 96545511 | 2 | .053 | C>G | 2.09 | .720 | A |
| IL1R1 | rs3917320 | 96556738 | 2 | .047 | A>C | n/a | n/a | n/a |
| IL1R1 | rs2110726 | 96558145 | 2 | .317 | C>T | 1.11 | .893 | A |
| IL1R1 | rs3917332 | 96560387 | 2 | .187 | A>T | 3.75 | .441 | A |
| IL1R1 | HapA1 | 1.67 | .797 | - | ||||
| IL1R1 | HapA2 | 2.26 | .689 | - | ||||
| IL1R1 | HapA3 | 3.81 | .432 | - | ||||
| IL1R2 | rs4141134 | 96370336 | 2 | .362 | T>C | 2.62 | .623 | A |
| IL1R2 | rs11674595 | 96374804 | 2 | .258 | T>C | 4.50 | .343 | A |
| IL1R2 | rs7570441 | 96380807 | 2 | .408 | G>A | 5.21 | .266 | A |
| IL1R2 | HapA1 | 6.36 | .174 | - | ||||
| IL1R2 | HapA2 | 3.84 | .147 | - | ||||
| IL1R2 | HapA4 | 1.50 | .827 | - | ||||
| IL2 | rs1479923 | 119096993 | 4 | .308 | C>T | 1.30 | .862 | A |
| IL2 | rs2069776 | 119098582 | 4 | .184 | T>C | n/a | n/a | n/a |
| IL2 | rs2069772 | 119099739 | 4 | .241 | A>G | 5.08 | .279 | A |
| IL2 | rs2069777 | 119103043 | 4 | .047 | C>T | n/a | n/a | n/a |
| IL2 | rs2069763 | 119104088 | 4 | .277 | T>G | 5.17 | .270 | A |
| IL2 | HapA1 | 2.46 | .653 | - | ||||
| IL2 | HapA2 | 4.99 | .289 | - | ||||
| IL2 | HapA3 | 5.08 | .279 | - | ||||
| IL4 | rs2243248 | 127200946 | 5 | .086 | T>G | 2.93 | .570 | A |
| IL4 | rs2243250 | 127201455 | 5 | .269 | C>T | n/a | n/a | n/a |
| IL4 | rs2070874 | 127202011 | 5 | .245 | C>T | n/a | n/a | n/a |
| IL4 | rs2227284 | 127205027 | 5 | .387 | C>A | n/a | n/a | n/a |
| IL4 | rs2227282 | 127205481 | 5 | .390 | C>G | n/a | n/a | n/a |
| IL4 | rs2243263 | 127205601 | 5 | .124 | C>G | 4.28 | .369 | A |
| IL4 | rs2243266 | 127206091 | 5 | .237 | G>A | n/a | n/a | n/a |
| IL4 | rs2243267 | 127206188 | 5 | .237 | G>C | n/a | n/a | n/a |
| IL4 | rs2243274 | 127207134 | 5 | .261 | G>A | n/a | n/a | n/a |
| IL4 | HapA1 | 0.39 | .983 | - | ||||
| IL4 | HapA3 | 2.18 | .704 | - | ||||
| IL4 | HapX1 | 3.64 | .457 | - | ||||
| IL6 | rs4719714 | 22643793 | 7 | .255 | A>T | 0.88 | .927 | A |
| IL6 | rs2069827 | 22648536 | 7 | .069 | G>T | 1.11 | .892 | A |
| IL6 | rs1800796 | 22649326 | 7 | .134 | C>G | n/a | n/a | n/a |
| IL6 | rs1800795 | 22649725 | 7 | .285 | C>G | 3.10 | .542 | A |
| IL6 | rs2069835 | 22650951 | 7 | .061 | T>C | n/a | n/a | n/a |
| IL6 | rs2066992 | 22651329 | 7 | .049 | G>T | 4.11 | .391 | A |
| IL6 | rs2069840 | 22651652 | 7 | .333 | C>G | 4.51 | .341 | A |
| IL6 | rs1554606 | 22651787 | 7 | .319 | G>T | 2.21 | .697 | A |
| IL6 | rs2069845 | 22653229 | 7 | .319 | A>G | 2.44 | .655 | A |
| IL6 | rs2069849 | 22654236 | 7 | .024 | C>T | n/a | n/a | n/a |
| IL6 | rs2069861 | 22654734 | 7 | .056 | C>T | 7.92 | .094 | A |
| IL6 | rs35610689 | 22656903 | 7 | .259 | A>G | 2.49 | .646 | A |
| IL6 | HapA1 | 4.39 | .356 | - | ||||
| IL6 | HapA5 | 4.85 | .303 | - | ||||
| IL6 | HapA8 | 2.70 | .610 | - | ||||
| IL8 | rs4073 | 70417508 | 4 | .455 | T>A | 2.39 | .665 | A |
| IL8 | rs2227306 | 70418539 | 4 | .366 | C>T | 3.76 | .440 | A |
| IL8 | rs2227543 | 70419394 | 4 | .368 | C>T | 3.36 | .500 | A |
| IL8 | HapA1 | 2.39 | .665 | - | ||||
| IL8 | HapA4 | 3.91 | .419 | - | ||||
| IL10 | rs3024505 | 177638230 | 1 | .129 | C>T | 4.13 | .389 | A |
| IL10 | rs3024498 | 177639855 | 1 | .204 | A>G | 2.66 | .617 | A |
| IL10 | rs3024496 | 177640190 | 1 | .421 | T>C | 4.87 | .301 | A |
| IL10 | rs1878672 | 177642039 | 1 | .416 | G>C | 4.71 | .319 | A |
| IL10 | rs3024492 | 177642438 | 1 | .190 | T>A | n/a | n/a | n/a |
| IL10 | rs1518111 | 177642971 | 1 | .303 | G>A | 4.36 | .359 | A |
| IL10 | rs1518110 | 177643187 | 1 | .301 | G>T | 4.69 | .321 | A |
| IL10 | rs3024491 | 177643372 | 1 | .408 | G>T | 4.39 | .356 | A |
| IL10 | HapA1 | 4.25 | .373 | - | ||||
| IL10 | HapA2 | 2.94 | .567 | - | ||||
| IL10 | HapA8 | 1.99 | .738 | - | ||||
| IL13 | rs1881457 | 127184713 | 5 | .210 | A>C | 6.44 | .169 | A |
| IL13 | rs1800925 | 127185113 | 5 | .233 | C>T | 1.56 | .816 | A |
| IL13 | rs2069743 | 127185579 | 5 | .019 | A>G | n/a | n/a | n/a |
| IL13 | rs1295686 | 127188147 | 5 | .265 | G>A | 5.70 | .223 | A |
| IL13 | rs20541 | 127188268 | 5 | .212 | C>T | 4.18 | .383 | A |
| IL13 | HapA1 | 5.27 | .261 | - | ||||
| IL13 | HapA4 | 4.20 | .379 | - | ||||
| IL17A | rs4711998 | 51881422 | 6 | .346 | G>A | 2.96 | .565 | A |
| IL17A | rs8193036 | 51881562 | 6 | .327 | T>C | 6.08 | .193 | A |
| IL17A | rs3819024 | 51881855 | 6 | .372 | A>G | 4.97 | .291 | A |
| IL17A | rs2275913 | 51882102 | 6 | .361 | G>A | 4.06 | .398 | A |
| IL17A | rs3804513 | 51884266 | 6 | .023 | A>T | n/a | n/a | n/a |
| IL17A | rs7747909 | 51885318 | 6 | .217 | G>A | 4.06 | .398 | A |
| NFKB1 | rs3774933 | 103645369 | 4 | .409 | T>C | 6.29 | .043 | R |
| NFKB1 | rs170731 | 103667933 | 4 | .358 | A>T | 0.93 | .920 | A |
| NFKB1 | rs17032779 | 103685279 | 4 | .011 | T>C | n/a | n/a | n/a |
| NFKB1 | rs230510 | 103695201 | 4 | .410 | T>A | 7.14 | .028 | D |
| NFKB1 | rs230494 | 103706005 | 4 | .434 | A>G | 4.37 | .358 | A |
| NFKB1 | rs4648016 | 103708706 | 4 | .010 | C>T | n/a | n/a | n/a |
| NFKB1 | rs4648018 | 103709236 | 4 | .018 | G>C | n/a | n/a | n/a |
| NFKB1 | rs3774956 | 103727564 | 4 | .435 | C>T | 3.84 | .429 | A |
| NFKB1 | rs10489114 | 103730426 | 4 | .018 | A>G | n/a | n/a | n/a |
| NFKB1 | rs4648068 | 103737343 | 4 | .363 | A>G | 1.55 | .818 | A |
| NFKB1 | rs4648095 | 103746914 | 4 | .052 | T>C | 5.92 | .052 | A |
| NFKB1 | rs4648110 | 103752867 | 4 | .170 | T>A | 1.23 | .873 | A |
| NFKB1 | rs4648135 | 103755716 | 4 | .061 | A>G | 6.05 | .049 | A |
| NFKB1 | rs4648141 | 103755947 | 4 | .180 | G>A | 2.61 | .625 | A |
| NFKB1 | rs1609798 | 103756488 | 4 | .337 | C>T | 2.83 | .587 | A |
| NFKB1 | HapA1 | 10.11 | .039 | - | ||||
| NFKB1 | HapA9 | 1.09 | .895 | - | ||||
| NFKB2 | rs12772374 | 104146901 | 10 | .168 | A>G | 7.51 | .112 | A |
| NFKB2 | rs7897947 | 104147701 | 10 | .221 | T>G | 5.70 | .223 | A |
| NFKB2 | rs11574849 | 104149686 | 10 | .070 | G>A | 3.73 | .444 | A |
| NFKB2 | rs1056890 | 104152760 | 10 | .305 | C>T | 1.24 | .872 | A |
| TNFA | rs2857602 | 31533378 | 6 | .341 | T>C | 3.41 | .492 | A |
| TNFA | rs1800683 | 31540071 | 6 | .390 | G>A | 1.25 | .871 | A |
| TNFA | rs2239704 | 31540141 | 6 | .335 | G>T | 3.37 | .497 | A |
| TNFA | rs2229094 | 31540556 | 6 | .278 | T>C | 2.81 | .591 | A |
| TNFA | rs1041981 | 31540784 | 6 | .386 | C>A | 1.07 | .900 | A |
| TNFA | rs1799964 | 31542308 | 6 | .224 | T>C | 2.66 | .616 | A |
| TNFA | rs1800750 | 31542963 | 6 | .016 | G>A | n/a | n/a | n/a |
| TNFA | rs1800629 | 31543031 | 6 | .149 | G>A | 1.30 | .861 | A |
| TNFA | rs1800610 | 31543827 | 6 | .100 | C>T | 8.12 | .087 | A |
| TNFA | rs3093662 | 31544189 | 6 | .074 | A>G | 7.38 | .117 | A |
| TNFA | HapA1 | 1.22 | .874 | - | ||||
| TNFA | HapA5 | 3.18 | .528 | - | ||||
| TNFA | HapA6 | 2.15 | .708 | - |
Chr = chromosome, MAF = minor allele frequency, A = additive model, D = dominant model, R = recessive model, n/a = not assayed because SNP violated Hardy-Weinberg expectation (p<.001) or because MAF<.05, Hap = haplotype.
Only IL1R1 rs949963 was retained in multivariable analyses.
Table 5.
Multiple logistic regression for IL1R1 rs949963.
| GMM class comparison | Predictora | Odds ratio | Standard error | 95% CI | z | p-value |
|---|---|---|---|---|---|---|
| High versus Moderate and Low-moderate attentional function classes (n=300) | Genotype | 1.98 | 0.52 | 1.18, 3.30 | 2.61 | .009 |
| Age (5-year increments) | 0.87 | 0.51 | 0.78, 0.98 | −2.35 | .019 | |
| SCQ score | 1.14 | 0.06 | 1.02, 1.26 | 2.41 | .016 | |
| KPS score (10-point increments) | 0.77 | 0.11 | 0.58, 1.02 | −1.84 | .066 | |
| Overall model fit: χ2 = 31.00, p < .001, R2 = 0.075 | ||||||
GMM = growth mixture model, CI = confidence interval, SCQ = Self-administered Comorbidity Questionnaire, KPS = Karnofsky Performance Status.
Self-reported race/ethnicity and the first three principle components identified in the analysis of ancestry informative markers were retained in the model to adjust for potential confounding due to population stratification (data not shown). The genotypic predictor evaluated in the model was IL1R1 rs949963 genotype (GG versus GA+AA).
Figure 2.
Differences among the attentional function (AF) latent classes in the percentages of patients who were homozygous for the common “G” allele versus heterozygous or homozygous for the rare “A” allele for rs949963 in interleukin 1 receptor, type I (IL1R1) (p=.016).
The final model explained 7.5% of variance in class membership (p<.001). Controlling for covariates, carrying the rare “A” allele (i.e., GA or AA genotype) was associated with a two-fold increase in the odds of belonging to a lower attentional function class (OR: 1.98; 95% CI: 1.18, 3.30; p=.009).
4. Discussion
This study is the first to use GMM to identify subgroups of women with breast cancer who reported distinct trajectories of attentional function prior to and after surgery and to evaluate for phenotypic and genotypic differences among these classes. Differences in mean AFI scores among the classes prior to surgery represent clinically meaningful differences [44] in self-reported attentional function (d=0.68 for High versus Moderate classes and d=0.89 for Moderate versus Low-moderate classes). The GMM solution found in this study partially confirms findings from our previous study [21]. Both studies found that a three-class solution best fit the data. However, AFI scores in the current study for each of the classes were lower than in the previous study. In addition, the trajectories of attentional function in each of the three classes varied between the studies.
These differences in AFI scores and trajectories may be due to the inclusion of male patients and male family caregivers in the previous sample. Since gender differences in the severity of other symptoms have been reported [40, 45–49], additional research is warranted to evaluate for gender differences in self-reported attentional function. Future studies of patients with cancer diagnoses that affect both men and women (e.g., colorectal cancer) may provide insights into these relationships.
An alternative explanation is that the different treatments that patients underwent in the two studies may have differentially impacted attentional function. However, treatment was not associated with attentional function class membership in either study. While none of the clinical characteristics differentiated among the classes, visual inspection of the class trajectories (Figure 1) suggests that the Moderate class had a significant decrease in attentional function after surgery followed by a significant increase in attentional function approximately three months later. This trajectory was possibly influenced by treatment. While larger sample sizes may identify treatment-related predictors of attentional function, our findings suggest that several patient characteristics (i.e., younger age, higher number and/or severity of comorbidities, lower functional status) are risk factors for poorer attentional function after diagnosis of breast cancer and during treatment.
The phenotypic predictors of class membership that remained significant in multivariable models were age, comorbidities, and functional status. Consistent with previous reports [1, 50, 51], younger patients were more likely to belong to a lower attentional function class. It is hypothesized that younger patients may notice changes in their attentional function in response to the diagnosis and treatment of cancer more than do older adults, who may have adjusted to previous age-related alterations in attentional function [1].
Consistent with our previous study [21], functional status was a phenotypic predictor of attentional function class membership. The Low-moderate class reported a pre-treatment mean KPS score of 88.83 (±12.77), which is a clinically meaningful difference from 95.74 (±8.62) for the High class and 94.90 (±6.89) for the Moderate class (d=0.62 and d=0.55, respectively). One possible explanation is that the higher comorbidity score reported by the Low-moderate class in the present study influenced this relationship. Managing multiple comorbidities may decrease a patient’s capacity to direct and sustain attention before the diagnosis of cancer [51], or cognitive changes may be associated with specific comorbidities [8, 52].
The most commonly reported comorbidities regardless of class membership were high blood pressure (30.9%), back pain (28.1%), and depression (21.9%). While the Low-moderate class reported the same top three comorbidities, the proportions of patients who reported back pain (35.1%) and depression (29.0%) were higher. It is possible that the greater proportions of patients with pain and depression in this class at enrollment accounts for its lack of improvement in attentional function during cancer therapy. Future studies should evaluate the effects of these symptoms on attentional function class membership.
In a previous study by our group [50], higher BMI before radiation therapy was associated with improvement in attentional function over time in women with breast cancer. Although BMI was associated with differences in latent class membership in the current study, no significant relationships were apparent between higher versus lower BMI and class membership. Future studies may clarify these relationships.
Income was significantly different among the classes. The lowest annual household income level (i.e., <$30,000) was associated with membership in the lowest attentional function class. Although income level did not remain a significant predictor of class membership in multivariable models, it is possible that stress associated with lower income [53] in the context of the cost of breast cancer treatment contributed to these class differences. Chronic stress negatively impacts immune system function [54], which may contribute to cognitive changes in these patients [9]. This finding warrants more research in terms of social and environmental characteristics associated with socioeconomic status that may influence attention.
In the bivariate analyses, genotype distributions differed significantly among classes for three SNPs and one haplotype in NFKB1. NFKB1 encodes for a transcription factor involved in inflammatory processes through regulation of inflammatory cytokine production [55, 56]. The transcription factor is thought to be involved in chronic inflammation [57], which may negatively impact cognition [20]. Therefore, variations in NFKB1 could explain some of the cognitive changes that cancer patients experience. While these associations did not remain significant in the multivariate analyses, studies with independent samples may identify an association between variations in NFKB1 and attentional function.
One SNP in IL1R1 (rs949963) significantly predicted class membership after controlling for covariates. Genotype uniquely explained 1.5% of variance in class membership. Carrying the rare “A” allele was associated with an increased odds of belonging to a lower attentional function class. Although significant pairwise post hoc class comparisons were not found after correction for multiple testing, examination of these relationships is warranted in future studies.
IL1R1 rs949963 is located in the promoter region for IL1R1, 616 base pairs upstream of the transcription start site [58]. Although no studies have demonstrated a link between transcription factors involved in regulation of gene expression and this SNP, the “A” allele is predicted to have decreased affinity for two transcription factors (i.e., Yin Yang 1 [YY1], upstream stimulatory factor 1 [USF1]), as compared to the “G” allele [59]. YY1 is a pleiotropic human transcription factor involved in the regulation of inflammation [60] and neural plasticity [61]. USF1 is involved in regulation of inflammation [62] and lipid metabolism genes involved in cognition (e.g., APOE) [63]. Given the predicted differential binding sites for these two mechanistically plausible transcription factors at IL1R1 rs949963, it is reasonable to hypothesize that variation in this SNP may influence the regulation of IL1R1 in a manner that is associated with differences in attentional function. Functional studies to determine if either of these theoretical binding sites are active and influenced by rs949963 are warranted.
No studies were found that described a relationship between IL1R1 rs949963 and clinical outcomes. However, in mouse models, the inhibition of interleukin 1 receptor, type I production decreased joint inflammation [64] and reduced the behavioral outcome of despair (i.e., immobility during tail suspension and forced swim tests) [65]. In addition, inhibiting the receptor blocked the development of stress-related glucocorticoid resistance, which is a possible mechanism for chronic inflammation [66]. The relationships of this interleukin 1 receptor to inflammation [67] and cognition [68] are hypothesized to extend to humans.
Given the numerous mechanisms by which inflammation may negatively impact attentional function [10–20], it is reasonable to suggest that carriers of the rare “A” allele for IL1R1 rs949963 have increased production of this interleukin 1 receptor. Therefore, interleukin-1 production at the time of diagnosis and treatment for breast cancer would be more efficient in producing an inflammatory state that could impact the CNS. However, future studies must evaluate for differences in expression of IL1R1 in carriers of the rare “A” allele to determine whether this hypothesis is tenable.
In our previous study [21], this SNP was not associated with attentional function class membership. The MAFs for the SNP in the two studies were similar, which suggests that the lack of an association in the previous study was not due to differences in allele frequency. Moreover, in the previous study no significant associations were found between IL1R1 SNPs and class membership in bivariate analyses. The lack of a significant finding may be due to sample variation or to different composition of the GMM groups. Also, AFI scores for the three classes identified in the present study were lower than in the previous study, which may have contributed to differences in SNP associations.
The present study did not confirm the finding of our previous study that variation in IL6 rs1800795 predicted attentional function class membership [21]. Moreover, no significant associations were found between IL6 SNPs and class membership in bivariate analyses. This difference in findings could be due in part to the fact that the MAF for this SNP in the present study was 19.7% lower than in the previous study, which may be due to sampling variability. An alternative hypothesis is that the classes derived from the two samples are phenotypically distinct.
Because it is possible that the revised AFI used in the present study [1] is not directly comparable to the original AFI used in the previous study [21], analyses were run with the original instrument. These analyses showed no differences in results for these two SNPs (data not shown). For both IL1R1 rs949963 and IL6 rs1800795, larger samples could resolve whether their relationships to attentional function can be replicated.
Study limitations should be acknowledged. Larger samples may identify additional genetic associations. Because of the exploratory nature of the study, adjustments were not made for multiple testing in the analyses of the genetic data. The relationship between IL1R1 rs949963 and attentional function class membership warrants replication and functional studies before clinical implications are evaluated. Measuring serum cytokine levels could support the hypothesized relationship between cytokine levels and cognitive function [10–20]. Studies of genes that encode for other physiological pathways (e.g., dopaminergic, serotonergic) [69] may clarify the etiology of reduced attentional function in women with breast cancer. Because of sample size limitations, gene by treatment effects were not evaluated. However, no differences in treatment characteristics were found among the latent classes.
While neuropsychological tests may not be sensitive to the changes in attentional function that patients report [70], inclusion of objective tests could improve understanding of subgroups of patients at risk for diminished attentional function. In addition, studies should evaluate for changes in other cognitive domains (e.g., working memory, executive function) that may be associated with genetic variation in IL1R1.
The Low-moderate class was the only class who did not report significantly improved attentional function over the six months of the study. It is possible that acute deficits in attentional function may lead to chronic deficits. An alternative hypothesis is that class trajectories may be influenced by co-occurring symptoms. Future studies may clarify long-term trends.
This study provides evidence for a relationship between IL1R1 rs949963 and distinct trajectories of self-reported attentional function. The finding suggests that cytokine dysregulation negatively impacts attentional function in women with breast cancer at a time when the capacity to direct and sustain attention is important for quality of life during treatment for breast cancer.
Highlights.
Three attentional function classes were identified: High, Moderate, and Low-moderate
Low-moderate class was younger, with more comorbidities and lower functional status
IL1R1 rs949963 is a significant genotypic predictor of class membership
Carrying the rare “A” allele conferred increased odds of lower attentional function
Acknowledgments
This study was funded by grants from the National Cancer Institute (CA107091 and CA118658). Dr. Merriman was supported by a National Institute of Nursing Research (NINR) F31 National Research Service Award (NR012604); an American Cancer Society (ACS) Doctoral Degree Scholarship in Cancer Nursing (DSCNR-10-087); an Oncology Nursing Society (ONS) Foundation Doctoral Scholarship; and a University of California, San Francisco (UCSF) Nursing Alumni Association Scholarship. He is currently supported as a Postdoctoral Scholar by an NINR T32 NRSA (NR011972). Dr. Aouizerat was funded through a National Institutes of Health (NIH) Roadmap for Medical Research Grant (KL2 RR624130). Dr. Cataldo is partially supported by an ONS Genetic Fellowship Award. Dr. Dunn received funding from the Mount Zion Health Fund. Dr. Baggott is supported by an ACS Mentored Research Scholar Grant (MRSG-12-01-PCSM). Dr. Dhruva is funded through an NIH Mentored Patient-Oriented Research Career Development Award (K23 AT005340). Dr. Langford is supported by a Breast Cancer Research Program Department of Defense Postdoctoral Fellowship. Dr. Leutwyler is supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number KL2TR000143. Dr. Ritchie is funded through an NIH Geriatric Academic Leadership Award (1K07AG31779) and the Harris Fishbon Professorship for Clinical Translational Research in Aging. Dr. Miaskowski is funded by the ACS as a Clinical Research Professor. This project is supported by NIH/NCRR UCSF-CTSI Grant Number UL1 RR024131. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Abbreviations: AIMs, ancestry informative markers; AFI, Attentional Function Index; BIC, Bayesian information criterion; BMI, body mass index; BLRT, bootstrapped likelihood ratio test; CI, confidence interval; CNS, central nervous system; DNA, deoxyribonucleic acid; GMM, growth mixture modeling; KPS, Karnofsky Performance Status; LD, linkage disequilibrium; MAF, minor allele frequency; OR, odds ratio; SCQ, Self-administered Comorbidity Questionnaire; SNP, single nucleotide polymorphism; VLMR, Vuong-Lo-Mendell-Rubin likelihood ratio test.
Financial disclosures: none.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
John D. Merriman, Email: jdm150@pitt.edu.
Bradley E. Aouizerat, Email: bradley.aouizerat@nursing.ucsf.edu.
Janine K. Cataldo, Email: janine.cataldo@nursing.ucsf.edu.
Laura Dunn, Email: laura.dunn@ucsf.edu.
Bruce A. Cooper, Email: bruce.cooper@nursing.ucsf.edu.
Claudia West, Email: claudia.west@nursing.ucsf.edu.
Steven M. Paul, Email: steven.paul@nursing.ucsf.edu.
Christina R. Baggott, Email: christina.baggott@ucsf.edu.
Anand Dhruva, Email: adhruva@hemeonc.ucsf.edu.
Kord Kober, Email: kord.kober@nursing.ucsf.edu.
Dale J. Langford, Email: dale.langford@nursing.ucsf.edu.
Heather Leutwyler, Email: heather.leutwyler@nursing.ucsf.edu.
Christine S. Ritchie, Email: christine.ritchie@ucsf.edu.
Gary Abrams, Email: gary.abrams@ucsf.edu.
Marylin Dodd, Email: marylin.dodd@nursing.ucsf.edu.
Charles Elboim, Email: celboim@rrmg.com.
Deborah Hamolsky, Email: debby.hamolsky@ucsfmedctr.org.
Michelle Melisko, Email: mmelisko@medicine.ucsf.edu.
References
- 1.Cimprich B, Visovatti M, Ronis DL. The Attentional Function Index--a self-report cognitive measure. Psychooncology. 2011;20:194–202. doi: 10.1002/pon.1729. [DOI] [PubMed] [Google Scholar]
- 2.Myers JS. Chemotherapy-related cognitive impairment: the breast cancer experience. Oncol Nurs Forum. 2012;39:E31–40. doi: 10.1188/12.ONF.E31-E40. [DOI] [PubMed] [Google Scholar]
- 3.Boykoff N, Moieni M, Subramanian SK. Confronting chemobrain: an in-depth look at survivors’ reports of impact on work, social networks, and health care response. J Cancer Surviv. 2009;3:223–32. doi: 10.1007/s11764-009-0098-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gazzaley A, Nobre AC. Top-down modulation: bridging selective attention and working memory. Trends Cogn Sci. 2012;16:129–35. doi: 10.1016/j.tics.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lezak MD. Neuropsychological assessment. 5. New York: Oxford University Press; 2012. [Google Scholar]
- 6.Ferguson RJ, McDonald BC, Saykin AJ, Ahles TA. Brain structure and function differences in monozygotic twins: possible effects of breast cancer chemotherapy. J Clin Oncol. 2007;25:3866–70. doi: 10.1200/JCO.2007.10.8639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reuter-Lorenz PA, Cimprich B. Cognitive function and breast cancer: promise and potential insights from functional brain imaging. Breast Cancer Res Treat. 2013;137:33–43. doi: 10.1007/s10549-012-2266-3. [DOI] [PubMed] [Google Scholar]
- 8.Wefel JS, Vardy J, Ahles T, Schagen SB. International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol. 2011;12:703–8. doi: 10.1016/S1470-2045(10)70294-1. [DOI] [PubMed] [Google Scholar]
- 9.Seruga B, Zhang HB, Bernstein LJ, Tannock IF. Cytokines and their relationship to the symptoms and outcome of cancer. Nat Rev Cancer. 2008;8:887–99. doi: 10.1038/nrc2507. [DOI] [PubMed] [Google Scholar]
- 10.Capuron L, Miller AH. Immune system to brain signaling: neuropsychopharmacological implications. Pharmacol Ther. 2011;130:226–38. doi: 10.1016/j.pharmthera.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watkins LR, Goehler LE, Relton JK, Tartaglia N, Silbert L, Martin D, et al. Blockade of interleukin-1 induced hyperthermia by subdiaphragmatic vagotomy: evidence for vagal mediation of immune-brain communication. Neurosci Lett. 1995;183:27–31. doi: 10.1016/0304-3940(94)11105-r. [DOI] [PubMed] [Google Scholar]
- 12.Banks WA, Erickson MA. The blood-brain barrier and immune function and dysfunction. Neurobiol Dis. 2010;37:26–32. doi: 10.1016/j.nbd.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 13.Plotkin SR, Banks WA, Kastin AJ. Comparison of saturable transport and extracellular pathways in the passage of interleukin-1 alpha across the blood-brain barrier. J Neuroimmunol. 1996;67:41–7. doi: 10.1016/0165-5728(96)00036-7. [DOI] [PubMed] [Google Scholar]
- 14.Konsman JP, Vigues S, Mackerlova L, Bristow A, Blomqvist A. Rat brain vascular distribution of interleukin-1 type-1 receptor immunoreactivity: relationship to patterns of inducible cyclooxygenase expression by peripheral inflammatory stimuli. J Comp Neurol. 2004;472:113–29. doi: 10.1002/cne.20052. [DOI] [PubMed] [Google Scholar]
- 15.D’Mello C, Le T, Swain MG. Cerebral microglia recruit monocytes into the brain in response to tumor necrosis factoralpha signaling during peripheral organ inflammation. J Neurosci. 2009;29:2089–102. doi: 10.1523/JNEUROSCI.3567-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joshi G, Sultana R, Tangpong J, Cole MP, St Clair DK, Vore M, et al. Free radical mediated oxidative stress and toxic side effects in brain induced by the anti cancer drug adriamycin: insight into chemobrain. Free Radic Res. 2005;39:1147–54. doi: 10.1080/10715760500143478. [DOI] [PubMed] [Google Scholar]
- 17.Raison CL, Borisov AS, Woolwine BJ, Massung B, Vogt G, Miller AH. Interferon-alpha effects on diurnal hypothalamic-pituitary-adrenal axis activity: relationship with proinflammatory cytokines and behavior. Mol Psychiatry. 2010;15:535–47. doi: 10.1038/mp.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tong L, Balazs R, Soiampornkul R, Thangnipon W, Cotman CW. Interleukin-1 beta impairs brain derived neurotrophic factor-induced signal transduction. Neurobiol Aging. 2008;29:1380–93. doi: 10.1016/j.neurobiolaging.2007.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilson CJ, Finch CE, Cohen HJ. Cytokines and cognition--the case for a head-to-toe inflammatory paradigm. J Am Geriatr Soc. 2002;50:2041–56. doi: 10.1046/j.1532-5415.2002.50619.x. [DOI] [PubMed] [Google Scholar]
- 20.Ahles TA, Saykin AJ. Candidate mechanisms for chemotherapy-induced cognitive changes. Nat Rev Cancer. 2007;7:192–201. doi: 10.1038/nrc2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Merriman JD, Aouizerat BE, Langford DJ, Cooper BA, Baggott CR, Cataldo JK, et al. Preliminary evidence of an association between an interleukin 6 promoter polymorphism and self-reported attentional function in oncology patients and their family caregivers. Biol Res Nurs. 2013 doi: 10.1177/1099800413479441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCann B, Miaskowski C, Koetters T, Baggott C, West C, Levine JD, et al. Associations between pro- and anti-inflammatory cytokine genes and breast pain in women prior to breast cancer surgery. J Pain. 2012;13:425–37. doi: 10.1016/j.jpain.2011.02.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karnofsky D. Performance scale. New York: Plenum Press; 1977. [Google Scholar]
- 24.Sangha O, Stucki G, Liang MH, Fossel AH, Katz JN. The Self-administered Comorbidity Questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis Rheum. 2003;49:156–63. doi: 10.1002/art.10993. [DOI] [PubMed] [Google Scholar]
- 25.Cimprich B, So H, Ronis DL, Trask C. Pre-treatment factors related to cognitive functioning in women newly diagnosed with breast cancer. Psychooncology. 2005;14:70–8. doi: 10.1002/pon.821. [DOI] [PubMed] [Google Scholar]
- 26.Cimprich B. Pretreatment symptom distress in women newly diagnosed with breast cancer. Cancer Nurs. 1999;22:185–94. doi: 10.1097/00002820-199906000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Lehto RH, Cimprich B. Anxiety and directed attention in women awaiting breast cancer surgery. Oncol Nurs Forum. 1999;26:767–72. [PubMed] [Google Scholar]
- 28.Cimprich B. Attentional fatigue following breast cancer surgery. Res Nurs Health. 1992;15:199–207. doi: 10.1002/nur.4770150306. [DOI] [PubMed] [Google Scholar]
- 29.Jansen CE, Cooper BA, Dodd MJ, Miaskowski CA. A prospective longitudinal study of chemotherapy-induced cognitive changes in breast cancer patients. Support Care Cancer. 2011;19:1647–56. doi: 10.1007/s00520-010-0997-4. [DOI] [PubMed] [Google Scholar]
- 30.Chen ML, Miaskowski C, Liu LN, Chen SC. Changes in perceived attentional function in women following breast cancer surgery. Breast Cancer Res Treat. 2012;131:599–606. doi: 10.1007/s10549-011-1760-3. [DOI] [PubMed] [Google Scholar]
- 31.Von Ah D, Russell KM, Storniolo AM, Carpenter JS. Cognitive dysfunction and its relationship to quality of life in breast cancer survivors. Oncol Nurs Forum. 2009;36:326–36. doi: 10.1188/09.ONF.326-334. [DOI] [PubMed] [Google Scholar]
- 32.Dunn LB, Aouizerat BE, Cooper BA, Dodd M, Lee K, West C, et al. Trajectories of anxiety in oncology patients and family caregivers during and after radiation therapy. Eur J Oncol Nurs. 2012;16:1–9. doi: 10.1016/j.ejon.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, N.J: L. Erlbaum Associates; 1988. [Google Scholar]
- 34.Gabriel SB, Schaffner SF, Nguyen H, Moore JM, Roy J, Blumenstiel B, et al. The structure of haplotype blocks in the human genome. Science. 2002;296:2225–9. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
- 35.Stephens M, Smith NJ, Donnelly P. A new statistical method for haplotype reconstruction from population data. Am J Hum Genet. 2001;68:978–89. doi: 10.1086/319501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miaskowski C, Cooper BA, Dhruva A, Dunn LB, Langford DJ, Cataldo JK, et al. Evidence of associations between cytokine genes and subjective reports of sleep disturbance in oncology patients and their family caregivers. PloS One. 2012;7:e40560. doi: 10.1371/journal.pone.0040560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Halder I, Shriver M, Thomas M, Fernandez JR, Frudakis T. A panel of ancestry informative markers for estimating individual biogeographical ancestry and admixture from four continents: utility and applications. Hum Mutat. 2008;29:648–58. doi: 10.1002/humu.20695. [DOI] [PubMed] [Google Scholar]
- 38.Hoggart CJ, Parra EJ, Shriver MD, Bonilla C, Kittles RA, Clayton DG, et al. Control of confounding of genetic associations in stratified populations. Am J Hum Genet. 2003;72:1492–504. doi: 10.1086/375613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tian C, Gregersen PK, Seldin MF. Accounting for ancestry: population substructure and genome-wide association studies. Hum Mol Genet. 2008;17:R143–50. doi: 10.1093/hmg/ddn268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dunn LB, Aouizerat BE, Langford DJ, Cooper BA, Dhruva A, Cataldo JK, et al. Cytokine gene variation is associated with depressive symptom trajectories in oncology patients and family caregivers. Eur J Oncol Nurs. 2013;17:346–53. doi: 10.1016/j.ejon.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–6. [PubMed] [Google Scholar]
- 42.Hattersley AT, McCarthy MI. What makes a good genetic association study? Lancet. 2005;366:1315–23. doi: 10.1016/S0140-6736(05)67531-9. [DOI] [PubMed] [Google Scholar]
- 43.Jung T, Wickrama KAS. An introduction to latent class growth analysis and growth mixture modeling. Soc Personal Psychol Compass. 2008;2:302–17. [Google Scholar]
- 44.Wyrwich KW, Bullinger M, Aaronson N, Hays RD, Patrick DL, Symonds T, et al. Estimating clinically significant differences in quality of life outcomes. Qual Life Res. 2005;14:285–95. doi: 10.1007/s11136-004-0705-2. [DOI] [PubMed] [Google Scholar]
- 45.Hagedoorn M, Buunk BP, Kuijer RG, Wobbes T, Sanderman R. Couples dealing with cancer: role and gender differences regarding psychological distress and quality of life. Psychooncology. 2000;9:232–42. doi: 10.1002/1099-1611(200005/06)9:3<232::aid-pon458>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 46.Walsh D, Donnelly S, Rybicki L. The symptoms of advanced cancer: relationship to age, gender, and performance status in 1,000 patients. Support Care Cancer. 2000;8:175–9. doi: 10.1007/s005200050281. [DOI] [PubMed] [Google Scholar]
- 47.Reyes-Gibby CC, Aday LA, Anderson KO, Mendoza TR, Cleeland CS. Pain, depression, and fatigue in community-dwelling adults with and without a history of cancer. J Pain Symptom Manage. 2006;32:118–28. doi: 10.1016/j.jpainsymman.2006.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Illi J, Miaskowski C, Cooper B, Levine JD, Dunn L, West C, et al. Association between pro- and anti-inflammatory cytokine genes and a symptom cluster of pain, fatigue, sleep disturbance, and depression. Cytokine. 2012;58:437–47. doi: 10.1016/j.cyto.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hagedoorn M, Sanderman R, Bolks HN, Tuinstra J, Coyne JC. Distress in couples coping with cancer: a meta-analysis and critical review of role and gender effects. Psychol Bull. 2008;134:1–30. doi: 10.1037/0033-2909.134.1.1. [DOI] [PubMed] [Google Scholar]
- 50.Merriman JD, Jansen C, Koetters T, West C, Dodd M, Lee K, et al. Predictors of the trajectories of self-reported attentional fatigue in women with breast cancer undergoing radiation therapy. Oncol Nurs Forum. 2010;37:423–32. doi: 10.1188/10.ONF.423-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Merriman JD, Dodd M, Lee K, Paul SM, Cooper BA, Aouizerat BE, et al. Differences in self-reported attentional fatigue between patients with breast and prostate cancer at the initiation of radiation therapy. Cancer Nurs. 2011;34:345–53. doi: 10.1097/NCC.0b013e318202520a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bauer LC, Johnson JK, Pozehl BJ. Cognition in heart failure: an overview of the concepts and their measures. J Am Acad Nurse Pract. 2011;23:577–85. doi: 10.1111/j.1745-7599.2011.00668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hauksdottir A, McClure C, Jonsson SH, Olafsson O, Valdimarsdottir UA. Increased stress among women following an economic collapse--a prospective cohort study. Am J Epidemiol. 2013 doi: 10.1093/aje/kws347. [DOI] [PubMed] [Google Scholar]
- 54.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–41. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Libermann TA, Baltimore D. Activation of interleukin-6 gene expression through the NF-kappa B transcription factor. Mol Cell Biol. 1990;10:2327–34. doi: 10.1128/mcb.10.5.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Napetschnig J, Wu H. Molecular basis of NF-kappaB signaling. Annu Rev Biophys. 2013;42:443–68. doi: 10.1146/annurev-biophys-083012-130338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Courtois G, Gilmore TD. Mutations in the NF-kappaB signaling pathway: implications for human disease. Oncogene. 2006;25:6831–43. doi: 10.1038/sj.onc.1209939. [DOI] [PubMed] [Google Scholar]
- 58.Database of Single Nucleotide Polymorphisms (dbSNP), dbSNP accession: rs949963 (dbSNP Build ID: 86/137) Bethesda, MD: National Center for Biotechnology Information, National Library of Medicine; [Google Scholar]
- 59.Grabe N. AliBaba2.1. TRANSFAC; 2000. Retrieved January 19, 2013, from http://www.gene-regulation.com/pub/programs/alibaba2/index.html. [Google Scholar]
- 60.Landvik NE, Tekpli X, Anmarkrud KH, Haugen A, Zienolddiny S. Molecular characterization of a cancer-related single nucleotide polymorphism in the pro-inflammatory interleukin-1B gene. Mol Carcinog. 2012;51 (Suppl 1):E168–75. doi: 10.1002/mc.21910. [DOI] [PubMed] [Google Scholar]
- 61.Gao J, Wang WY, Mao YW, Graff J, Guan JS, Pan L, et al. A novel pathway regulates memory and plasticity via SIRT1 and miR-134. Nature. 2010;466:1105–9. doi: 10.1038/nature09271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ratajewski M, Walczak-Drzewiecka A, Salkowska A, Dastych J. Upstream stimulating factors regulate the expression of RORgammaT in human lymphocytes. J Immunol. 2012;189:3034–42. doi: 10.4049/jimmunol.1200519. [DOI] [PubMed] [Google Scholar]
- 63.Isotalo K, Kok EH, Luoto TM, Haikonen S, Haapasalo H, Lehtimaki T, et al. Upstream transcription factor 1 (USF1) polymorphisms associate with Alzheimer’s disease-related neuropathological lesions: Tampere Autopsy Study. Brain Pathol. 2012;22:765–75. doi: 10.1111/j.1750-3639.2012.00586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Torres R, Macdonald L, Croll SD, Reinhardt J, Dore A, Stevens S, et al. Hyperalgesia, synovitis and multiple biomarkers of inflammation are suppressed by interleukin 1 inhibition in a novel animal model of gouty arthritis. Ann Rheum Dis. 2009;68:1602–8. doi: 10.1136/ard.2009.109355. [DOI] [PubMed] [Google Scholar]
- 65.Zhu CB, Lindler KM, Owens AW, Daws LC, Blakely RD, Hewlett WA. Interleukin-1 receptor activation by systemic lipopolysaccharide induces behavioral despair linked to MAPK regulation of CNS serotonin transporters. Neuropsychopharmacology. 2010;35:2510–20. doi: 10.1038/npp.2010.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Engler H, Bailey MT, Engler A, Stiner-Jones LM, Quan N, Sheridan JF. Interleukin-1 receptor type 1-deficient mice fail to develop social stress-associated glucocorticoid resistance in the spleen. Psychoneuroendocrinology. 2008;33:108–17. doi: 10.1016/j.psyneuen.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cibelli M, Fidalgo AR, Terrando N, Ma D, Monaco C, Feldmann M, et al. Role of interleukin-1beta in postoperative cognitive dysfunction. Ann Neurol. 2010;68:360–8. doi: 10.1002/ana.22082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Posner MI, Rothbart MK, Sheese BE. Attention genes. Dev Sci. 2007;10:24–9. doi: 10.1111/j.1467-7687.2007.00559.x. [DOI] [PubMed] [Google Scholar]
- 70.Schagen SB, Das E, van Dam FS. The influence of priming and pre-existing knowledge of chemotherapy-associated cognitive complaints on the reporting of such complaints in breast cancer patients. Psychooncology. 2009;18:674–8. doi: 10.1002/pon.1454. [DOI] [PubMed] [Google Scholar]