Abstract
Objectives
To determine the prevalence and relationship of frailty and health-related quality of life (HRQOL) among residents of long-term care [nursing homes (NH) and assisted living (AL)] facilities.
Methods
Residents of NH and AL facilities in La Crosse County, Wisconsin, were recruited 1/2009–6/2010 and assessed for frailty (gait speed, unintended weight loss, grip strength), comorbidity (Charlson index), and HRQOL [Short Form (SF)-36].
Results
Among 137 participants, 85% were frail. Frail residents were older, had more comorbidities (2.0 vs. 0, p<0.001) and lower mean SF-36 Physical Component Score (PCS) (32 vs. 48, p<0.001). Following adjustments for age, sex, and comorbidities, compared to non-frail residents, frail residents had lower SF-36 PCS (mean difference −14.7, 95% CI. −19.3,−10.1, p <0.001). Frailty, comorbidity, and HRQOL did not differ between NH and AL facilities.
Discussion
Frail residents had lower HRQOL, suggesting that preventing frailty may lead to better HRQOL among residents of long-term care facilities.
Keywords: frailty, quality of life, assisted living facilities, nursing homes, long-term care
Introduction
Frailty is a dynamic construct and is defined as an intermediate syndrome in the continuum from normal aging to a final stage of disability, institutionalization, and death (Fried et al., 1998; Fried et al., 2001). Frailty is not routinely measured and thus data on its prevalence in many relevant conditions is lacking. A frailty assessment tool which is easy, inexpensive, and reliable could help clinicians in their practice. In a recent European, Canadian, and American Geriatric Advisory Panel (GAP), gait speed at a usual pace was proposed as a reliable measure of frailty (Abellan van Kan et al., 2008).
Older patients have unique healthcare issues that impact not only morbidity and mortality, but also health status including symptoms, functional status, and health-related quality of life (HRQOL). Older patients may be equally or even more concerned with the quality of their life as compared with its longevity. Previous studies have highlighted the overlap between frailty with comorbid conditions and disability, but lacked data on patients' self-reported health status (Fried, Ferrucci, Darer, Williamson, & Anderson, 2004). Health status evaluation is important to patients and also aligns with the Institute of Medicine's goals for a patient-centered healthcare delivery system (America, 2001).
Frailty is more prevalent in residents of long-term care facilities (LTCF) as compared to a healthy cohort (Fried & Mor, 1997). The 2010 US Census estimates that 13.1% of people are above 65 years, and 6.0% over the age of 75 years (Bureau, 2010), and it is expected that by the year 2030 every fifth person will be over 65 years. With the aging of the population, it will likely increase the utilization of LTCF, as an increasing number of elders will reside in these facilities. We currently lack contemporary data on frailty among residents of LTCF and it is unknown whether frailty is associated with HRQOL. To assess these gaps in knowledge, we conducted a cross-sectional study to examine the relationship between frailty and HRQOL among LTCF residents.
Subjects and Methods
Residents (65 years or older) of two nursing homes and one assisted living facility affiliated to Franciscan Skemp Healthcare in La Crosse, Wisconsin, were prospectively enrolled in the study from January 2009 to June 2010. One of the investigators (AK) visited these facilities for enrollment. After informed consent, standardized questionnaires and tests were administered at the facilities to the participating residents to ascertain frailty, comorbid conditions, and HRQOL. Residents who were non-ambulatory or had a clinical history of stroke with neurological deficit, severe Parkinson's disease, dementia, or cognitive impairment were excluded from the study. The Franciscan Skemp Healthcare Institutional Review Board approved the study and all participants gave their informed consent prior to enrollment.
Frailty Assessment
Due to simplicity and ease of application, we chose walking speed <1.0 m/s as the primary criterion of frailty. Walking speed was calculated from the time to walk 15 feet. Gait speed was recently endorsed by the Geriatric Advisory Panel as the most suitable instrument for quick, inexpensive, and reliable instrument to measure frailty in older people (Cesari et al., 2005; Rolland et al., 2004). We also measured unintended weight loss (>10 lb in the preceding year), and grip strength by Jamar® handgrip dynamometer as other criteria for frailty.
Quality of Life Assessment
HRQOL was assessed with the Short Form 36 (SF-36) (McHorney, Ware, Lu, & Sherbourne, 1994; McHorney, Ware, & Raczek, 1993). The SF-36 is a generic instrument widely used to measure health status with the physical component score (PCS) and mental component score (MCS). Scores range from 0–100, with higher scores indicating better HRQOL. This instrument has well-established validity, reproducibility, and prognostic value (McHorney, et al., 1994; McHorney, et al., 1993). The overall internal-consistency reliability (Cronbach's alpha) is 0.93 for the physical functioning scale and 0.90 for the mental health scale. Importantly, the reliability is similar among older adults aged 75 or older; the Cranbach's alpha is 0.92 for the physical functioning scale and 0.86 for the mental health scale (McHorney, et al., 1994).
Statistical Methods
Continuous variables are summarized by mean ± standard deviation, unless otherwise specified. Categorical variables are presented as group frequencies and percentages. Student's t-test was used to test differences in age, body mass index, and SF-36 scores between groups. The Wilcoxon rank sum test was used to test the difference in the Charlson comorbidity index between groups. Group differences in categorical variables were tested using Pearson's chi-squared test. To obtain age and sex-adjusted p-values for group differences in continuous variables, the variable of interest was modeled using linear regression with age, sex and frailty and the p-value for the frailty parameter estimate is reported. To obtain age and sex-adjusted p-values for categorical variables, a logistic regression model for frailty was calculated with age, sex and the categorical variable as covariates. A likelihood ratio test for the significance of the categorical variable in the model was reported. Linear regression was used to model the SF-36 standardized physical and mental component scores as functions of age, sex, comorbid burden (Charlson Index) and frailty.
Results
Of 201 potential participants, 64 were further screened out because they were unable to walk without assistance, thus we enrolled 137 participants. (50 from assisted living and 87 from the nursing homes). One hundred sixteen participants (85%) were frail according to our definition of gait speed <1.0 m/s. Table 1 shows the baseline characteristics of residents with and without frailty. As compared to those without frailty, frail residents were older and had a greater number of comorbid conditions, including diabetes mellitus, hypertension, heart failure and chronic kidney disease. Residents of nursing homes and assisted living were similar with regards to the baseline characteristics, prevalence of frailty and comorbid conditions.
Table 1.
Baseline Characteristics of Residents Living in Long Term Care Facilities, Stratified by Frailty Statusa
| Characteristic | Total | Not Frail (n = 21) | Frail (n = 116) | P-Value | Adjusted p Valueb |
|---|---|---|---|---|---|
| Age, mean ± SD | 84.6 ± 7.6 | 82.1 ± 6.5 | 85.0 ± 7.7 | 0.11 | |
| Men, n (%) | 25 (18%) | 0 (0%) | 25 (22%) | 0.019 | |
| Body mass index, mean ± SD | 26.9 ± 6.6 | 26.0 ± 4.9 | 27.1 ± 6.8 | 0.50 | 0.20 |
| Hypertension, n (%) | 103 (75%) | 11 (52%) | 92 (79%) | 0.009 | 0.003 |
| Current smoker, n (%) | 2 (1%) | 0 (0%) | 2 (2%) | 0.54 | 0.30 |
| Diabetes mellitus, n (%) | 23 (17%) | 0 (0%) | 23 (21%) | 0.025 | <0.001 |
| Chronic kidney disease, n (%) | 25 (18%) | 2 (10%) | 23 (20%) | 0.24 | 0.46 |
| Peripheral Arterial Disease, n (%) | 13 (9%) | 1 (5%) | 12 (11%) | 0.40 | 0.16 |
| Congestive Heart Failure, n (%) | 23 (17%) | 0 (0%) | 23 (20%) | 0.025 | 0.034 |
| Atrial Fibrillation, n (%) | 29 (21%) | 1 (5%) | 28 (24%) | 0.045 | 0.086 |
| History of MI, n (%) | 13 (9%) | 1 (5%) | 12 (10%) | 0.42 | 0.48 |
| Chronic lung disease/COPD, n (%) | 36 (26%) | 3 (14%) | 33 (29%) | 0.17 | 0.15 |
| Rheumatologic disease, n (%) | 44 (32%) | 5 (24%) | 39 (34%) | 0.36 | 0.31 |
| Any tumor, n (%) | 27 (20%) | 2 (10%) | 25 (22%) | 0.20 | 0.19 |
| Unexpected fall within 6 months, n (%) | 22 (16%) | 2 (10%) | 20 (17%) | 0.37 | 0.44 |
| Charlson comorbidity Index, Median (Q1, Q3) | 2.0(1.0,3.0) | 0.0 (0.0, 1.0) | 2.0 (1.0,3.0) | <0.001 | 0.012 |
All comparisons between assisted living and nursing home residents were not significant
Adjusted for age and sex
COPD= chronic obstructive pulmonary disease
MI= myocardial infarction
Table 2 shows the prevalence of frailty among all residents, as measured by different criterion. Even after reducing the gait speed cut-off to 0.6 m/s, the prevalence of frailty was high (n = 90, 66%). Poor grip strength was present among 66 residents (43%) and unintended weight loss >10 lb was present among 20 (15%).
Table 2.
Prevalence of Frailty According to Different Measures
| Frailty measure | n (%) |
|---|---|
| Gait speed <1.0 m/s | 116 (85) |
| Gait speed <0.6 m/s | 90 (66) |
| Low grip strength | 63 (46) |
| Unintentional weight loss >10 lbs in the last year | 20 (15) |
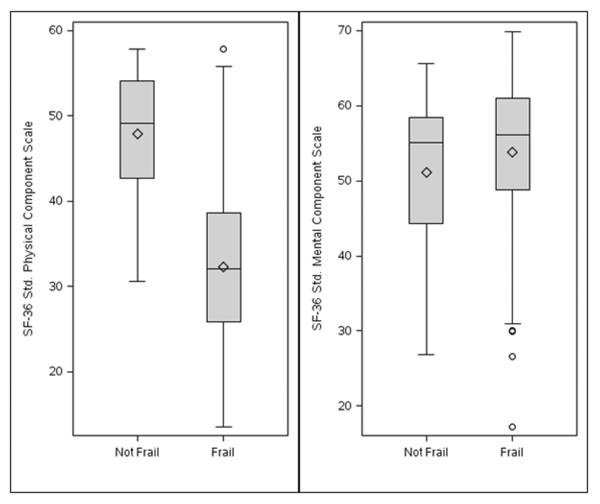
The SF-36 PCS was significantly and inversely related to frailty status (Figure 1). The mean SF-36 PCS was lower among frail residents (32 vs. 48, p <0.001). After adjustment for age, sex and comorbidity, the mean SF-36 PCS was 15 points lower among frail residents (−14.7, 95% CI: −19.3, −10.1, p <0.001). There was no association between the SF-36 MCS and frailty. A 1-point increase in the comorbidity index was associated with significantly lower expected SF-36 MCS score (−0.86 per point, 95% CI −1.70,−0.02, p = 0.048). Gender was not associated with either HRQOL measure. Older age was significantly associated with higher MCS scores (2.85 per decade, 95% CI 0.49, 5.22, p = 0.02).
Figure 1.
Box-and-whisker plots of SF-36 Physical and Mental Component scores grouped by frailty.
Discussion
Frailty was prevalent among residents of long-term care facilities. Residents of both nursing homes and assisted living had similar prevalence of frailty and comorbid conditions. Furthermore, frail residents had higher prevalence of comorbid conditions and poor HRQOL.
Frailty and long-term care facilities
There was a high and similar prevalence of frailty and comorbid conditions among residents of either nursing homes or assisted living facilities. HRQOL as measured by the SF-36 questionnaire was similar between the two groups as well. Prior studies have demonstrated higher mortality, disability, increased hospitalization, and health services utilization in older adults (Fried, et al., 1998; Singh et al., 2002; Singh et al., 2007). These relationships, however, have not been well studied in residents living in long-term care facilities. In a study of 3,782 nursing home residents, severe functional impairment and worsening activities of daily living were found to be associated with an increased risk of subsequent hospitalization (Fried & Mor, 1997). Almost a third (30.2%) of 11,113 community dwelling and nursing home residents were found to be frail according to one of three frailty models tested by the Health and Retirement Study (Cigolle, Ofstedal, Tian, & Blaum, 2009). Frailty in older adults living in the community was a predictor for nursing home admissions following discharge from emergency department (Hastings, Purser, Johnson, Sloane, & Whitson, 2008).
As noted however, these studies used differing methods to measure frailty. Recently, gait speed has been proposed as a quick, inexpensive and reliable measure of frailty (Abellan van Kan, et al., 2008). Indeed, in a recent study, data was pooled from 9 cohorts of community-dwelling older adults to assess whether gait speed was associated with survival. Within these cohorts, the prevalence of gait speed <1.0 m/s ranged from 20%-96% and the prevalence of gait speed <0.6 ranged from 1%–60%. (Studenski et al., 2011). The study concluded that gait speed was indeed associated with survival in older adults.
Similarly, in the present study, we used gait speed to measure frailty, and found that a large proportion of LTCF residents were frail. Even after redefining frailty as walking speed less than 0.6 meter/second, over 60% of the residents were frail (Guralnik et al., 2000). This high prevalence was in stark contrast to 7% in community-dwelling elders (Fried, et al., 2001), 27% in residents with coronary heart disease (Purser et al., 2006), and 30% in the Health and Retirement Study (Cigolle, et al., 2009). This suggests we may need to define frailty differently in this population. Recently, definitions for frailty that may be more applicable to nursing home residents have been proposed (Abellan van Kan, Rolland, Morley, & Vellas, 2008).
Quality of life measures and frailty
There was an association between HRQOL measures and frailty among LTCF residents. Frailty was not only associated with a lower SF-36 PCS score, but also with comorbidity. Each component of age-associated impairment, including frailty, comorbidity, and disability, is known to influence long-term prognosis, and a significant overlap between them was previously demonstrated by Fried and colleagues (Fried, et al., 2004). Information on self-perceived health status may further our understanding of the association with frailty and comorbid conditions.
Our finding of poor HRQOL in frail LTCF residents is important for several reasons. First, data on the cross-sectional association between poor QOL and frailty are scant. Similar to our results, Masel and colleagues found that frailty status was significantly associated with lower scores on all physical and cognitive health related QOL scales in older Mexican American individuals (Masel, Graham, Reistetter, Markides, & Ottenbacher, 2009). In another small study, 24 residents with frailty were noted to have lower QOL, as well as mental well-being, independent of age, diabetes, macrovascular complication, kidney dysfunction, and depressed mood (Kanauchi, Kubo, Kanauchi, & Saito, 2008). Recently, another cross-sectional study also found an inverse association between frailty and dimensions of QOL among 239 community dwelling outpatients aged 65 and over (Bilotta et al., 2010).
Secondly, measuring HRQOL will likely improve our understanding of its complex interplay with already established age-associated variables. It will also lay the foundation for developing effective and targeted strategies for prevention and management of the frail older adult population with poor health status. It is important to note the clustering of risk factors in these vulnerable LTCF residents who not only have higher preponderance of traditional risk factors but also have higher burden of comorbid conditions, frailty, and now poor HRQOL. The study raises the possibility for potential interventions in the frail older adults living in LCTFs that can arrest or reverse frailty in order to improve HRQOL. Recently a prognostic index, incorporating age, sex, self-reported comorbid conditions, and functional measures, accurately stratified community-dwelling older adults into varying risks of death and those with poor functional status had worse long-term outcomes (Lee, Lindquist, Segal, & Covinsky, 2006). As the sample size is small and the study presents cross-sectional associations, additional studies are needed with longitudinal follow-up to define the prognostic value of these constructs.
Conclusions
Frailty was highly prevalent among residents of nursing homes and assisted living facilities. Furthermore frailty was associated with poor HRQOL. Understanding this health dimension in conjunction with the already multifaceted environment of at-risk older adults may help improve the evaluation and management of these residents. Future studies are needed to assess if interventions to improve frailty, lead to improvement in HRQOL.
Acknowledgements
The authors greatly acknowledge the support of Franciscan Skemp Healthcare in La Crosse, Wisconsin and Deborah S. Russell for secretarial assistance.
Funding
This work was supported in part by a grant from the National Institutes of Health [R01 HL72435]. The funding source played no role in the design, conduct, or reporting of this study.
Footnotes
Conflict of Interest Statement
None.
Publisher's Disclaimer: The final manuscript has been seen and approved by all authors. All authors meet criteria for authorship, and have no financial or other relationships that could lead to a conflict of interest. The paper is original research that has not been published and is not under consideration elsewhere. The IRB numbers associated with this manuscript are Mayo Clinic Health System-Franciscan IRB: FWA00004023; OHRP IRB 00003093.
References
- Abellan van Kan G, Rolland Y, Bergman H, Morley JE, Kritchevsky SB, Vellas B. The I.A.N.A Task Force on frailty assessment of older people in clinical practice. J Nutr Health Aging. 2008;12(1):29–37. doi: 10.1007/BF02982161. [DOI] [PubMed] [Google Scholar]
- Abellan van Kan G, Rolland YM, Morley JE, Vellas B. Frailty: toward a clinical definition. J Am Med Dir Assoc. 2008;9(2):71–72. doi: 10.1016/j.jamda.2007.11.005. [DOI] [PubMed] [Google Scholar]
- America, C. o. Q. o. H. C. i. In: Crossing the quality chasm: a new health system for the 21st century. Institute of Medicine., editor. National Academy Press; Washington DC: 2001. [Google Scholar]
- Bilotta C, Bowling A, Casè A, Nicolini P, Mauri S, Castelli M, et al. Dimensions and correlates of quality of life according to frailty status: a cross-sectional study on community-dwelling older adults referred to an outpatient geriatric service in Italy. Health and Quality of Life Outcomes. 2010;8(1):56. doi: 10.1186/1477-7525-8-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bureau UC. AmericanFactFinder. 2010 http://factfinder.census.gov/home/saff/main.html?_lang=en.
- Cesari M, Kritchevsky SB, Penninx BW, Nicklas BJ, Simonsick EM, Newman AB, et al. Prognostic value of usual gait speed in well-functioning older people--results from the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2005;53(10):1675–1680. doi: 10.1111/j.1532-5415.2005.53501.x. [DOI] [PubMed] [Google Scholar]
- Cigolle CT, Ofstedal MB, Tian Z, Blaum CS. Comparing models of frailty: the Health and Retirement Study. Journal of the American Geriatrics Society. 2009;57(5):830–839. doi: 10.1111/j.1532-5415.2009.02225.x. [DOI] [PubMed] [Google Scholar]
- Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59(3):255–263. doi: 10.1093/gerona/59.3.m255. [DOI] [PubMed] [Google Scholar]
- Fried LP, Kronmal RA, Newman AB, Bild DE, Mittelmark MB, Polak JF, et al. Risk factors for 5-year mortality in older adults: the Cardiovascular Health Study. JAMA. 1998;279(8):585–592. doi: 10.1001/jama.279.8.585. [DOI] [PubMed] [Google Scholar]
- Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- Fried TR, Mor V. Frailty and hospitalization of long-term stay nursing home residents. J Am Geriatr Soc. 1997;45(3):265–269. doi: 10.1111/j.1532-5415.1997.tb00938.x. [DOI] [PubMed] [Google Scholar]
- Guralnik JM, Ferrucci L, Pieper CF, Leveille SG, Markides KS, Ostir GV, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55(4):M221–231. doi: 10.1093/gerona/55.4.m221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings SN, Purser JL, Johnson KS, Sloane RJ, Whitson HE. Frailty predicts some but not all adverse outcomes in older adults discharged from the emergency department. J Am Geriatr Soc. 2008;56(9):1651–1657. doi: 10.1111/j.1532-5415.2008.01840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanauchi M, Kubo A, Kanauchi K, Saito Y. Frailty, health-related quality of life and mental well-being in older adults with cardiometabolic risk factors. Int J Clin Pract. 2008;62(9):1447–1451. doi: 10.1111/j.1742-1241.2008.01830.x. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Lindquist K, Segal MR, Covinsky KE. Development and validation of a prognostic index for 4-year mortality in older adults. Jama. 2006;295(7):801–808. doi: 10.1001/jama.295.7.801. [DOI] [PubMed] [Google Scholar]
- Masel MC, Graham JE, Reistetter TA, Markides KS, Ottenbacher KJ. Frailty and health related quality of life in older Mexican Americans. Health Qual Life Outcomes. 2009;7:70. doi: 10.1186/1477-7525-7-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHorney CA, Ware JE, Jr., Lu JF, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S.] Medical care. 1994;32(1):40–66. doi: 10.1097/00005650-199401000-00004. [DOI] [PubMed] [Google Scholar]
- McHorney CA, Ware JE, Jr., Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S.] Medical care. 1993;31(3):247–263. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- Purser JL, Kuchibhatla MN, Fillenbaum GG, Harding T, Peterson ED, Alexander KP. Identifying frailty in hospitalized older adults with significant coronary artery disease. J Am Geriatr Soc. 2006;54(11):1674–1681. doi: 10.1111/j.1532-5415.2006.00914.x. [DOI] [PubMed] [Google Scholar]
- Rolland YM, Cesari M, Miller ME, Penninx BW, Atkinson HH, Pahor M. Reliability of the 400-m usual-pace walk test as an assessment of mobility limitation in older adults. J Am Geriatr Soc. 2004;52(6):972–976. doi: 10.1111/j.1532-5415.2004.52267.x. [DOI] [PubMed] [Google Scholar]
- Singh M, Reeder GS, Jacobsen SJ, Weston S, Killian J, Roger VL. Scores for post-myocardial infarction risk stratification in the community. Circulation. 2002;106(18):2309–2314. doi: 10.1161/01.cir.0000036598.12888.de. [DOI] [PubMed] [Google Scholar]
- Singh M, Rihal C, Roger VL, Lennon R, Spertus J, Jahangir A, et al. Comorbid Conditions and Outcomes After Percutaneous Coronary Intervention. Heart. 2007 [Google Scholar]
- Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, et al. Gait speed and survival in older adults. [Research Support, N.I.H., Extramural Research Support, N.I.H., Intramural Research Support, Non-U.S. Gov't] JAMA : the journal of the American Medical Association. 2011;305(1):50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]