Abstract
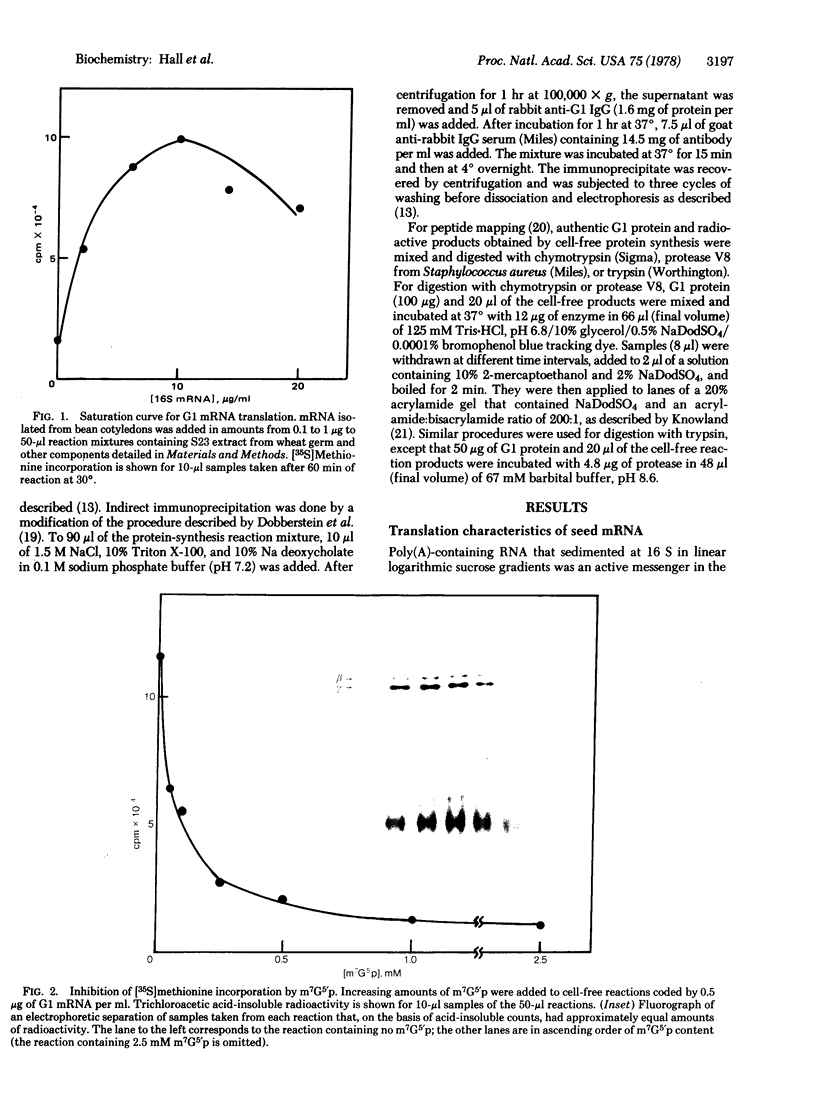
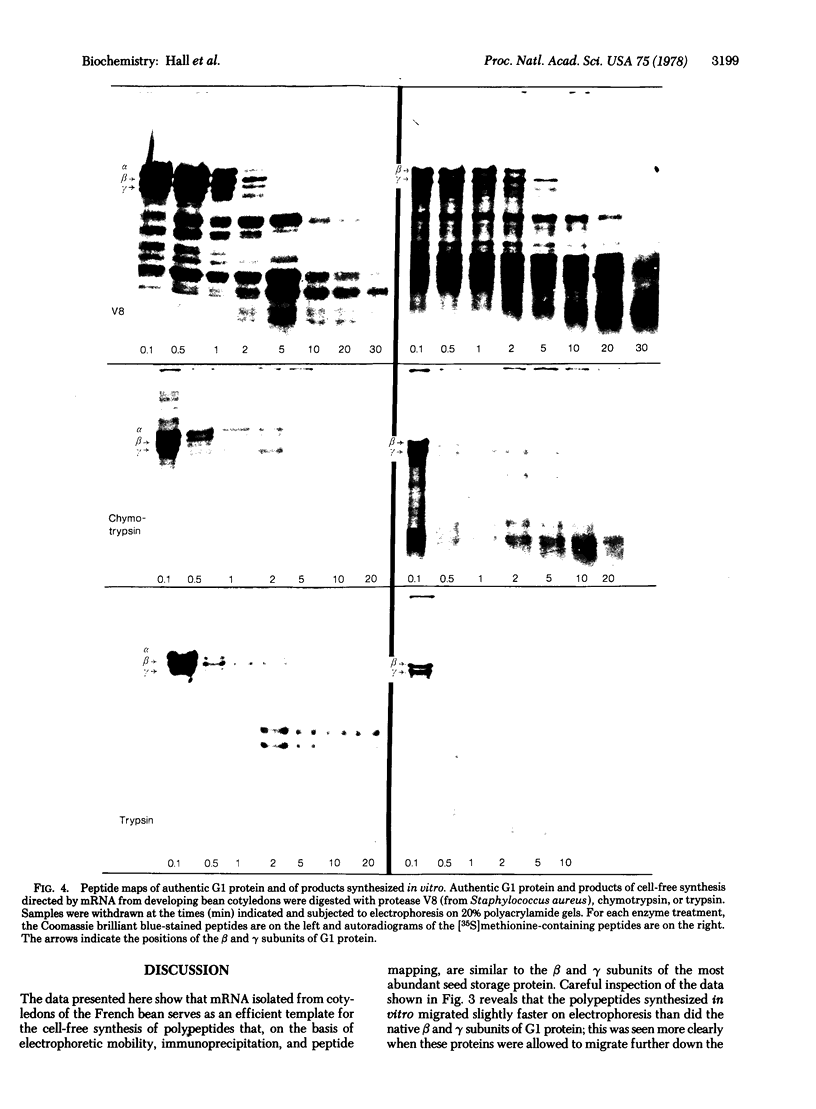
The fraction of poly(A)-containing RNA isolated from ripening bean (Phaseolus vulgaris) cotyledons that sedimented at 16 S in linear logarithmic sucrose gradients was at least as active a messenger as viral RNA when added to a cell-free protein-synthesizing system from wheat germ. The major products synthesized in vitro were polypeptides of about 47,000 and 43,000 daltons, corresponding to two of the three subunits of G1 protein, the most abundant bean seed storage protein. No trace of the largest (53,000 daltons) subunit was found among the polypeptides synthesized in vitro. Proof that the 47,000- and 43,000-dalton polypeptides coded by the 16S RNA were indeed subunits of G1 protein was obtained by immunoprecipitation with monovalent antibody to G1 protein and by electrophoretic mapping of peptides on acrylamide gels after digestion of mixtures of authentic protein and radioactive translation products with protease V8, chymotrypsin, and trypsin. The subunits synthesized in vitro were slightly smaller than the native subunits, probably because they lacked the sugar residues present on the holoprotein.
Keywords: storage protein, peptide mapping in denaturing gels, protein synthesis, modified 5′ termini
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Brakke M. K., Van Pelt N. Linear-log sucrose gradients for estimating sedimentation coefficients of plant viruses and nucleic acids. Anal Biochem. 1970 Nov;38(1):56–64. doi: 10.1016/0003-2697(70)90155-7. [DOI] [PubMed] [Google Scholar]
- Burr B., Burr F. A. Zein synthesis in maize endosperm by polyribosomes attached to protein bodies. Proc Natl Acad Sci U S A. 1976 Feb;73(2):515–519. doi: 10.1073/pnas.73.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Davies J. W., Kaesberg P. Translation of virus mRNA: protein synthesis directed by several virus RNAs in a cell-free extract from wheat germ. J Gen Virol. 1974 Oct;25(1):11–20. doi: 10.1099/0022-1317-25-1-11. [DOI] [PubMed] [Google Scholar]
- Davies J. W., Kaesberg P. Translation of virus mRNA: synthesis of bacteriophage Q beta proteins in a cell-free extract from wheat embryo. J Virol. 1973 Dec;12(6):1434–1441. doi: 10.1128/jvi.12.6.1434-1441.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobberstein B., Blobel G., Chua N. H. In vitro synthesis and processing of a putative precursor for the small subunit of ribulose-1,5-bisphosphate carboxylase of Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1082–1085. doi: 10.1073/pnas.74.3.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall T. C., McLeester R. C., Bliss F. A. Equal Expression of the Maternal and Paternal Alleles for the Polypeptide Subunits of the Major Storage Protein of the Bean Phaseolus vulgaris L. Plant Physiol. 1977 Jun;59(6):1122–1124. doi: 10.1104/pp.59.6.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey E. D., Weber L. A., Baglioni C. Inhibition of initiation of protein synthesis by 7-methylguanosine-5'-monophosphate. Proc Natl Acad Sci U S A. 1976 Jan;73(1):19–23. doi: 10.1073/pnas.73.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins T. J., Spencer D. Cell-free Synthesis of Pea Seed Proteins. Plant Physiol. 1977 Nov;60(5):655–661. doi: 10.1104/pp.60.5.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowland J. Protein synthesis directed by the RNA from a plant virus in a normal animal cell. Genetics. 1974 Sep;78(1):383–394. doi: 10.1093/genetics/78.1.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkins B. A., Jones R. A., Tsai C. Y. Isolation and in vitro translation of zein messenger ribonucleic acid. Biochemistry. 1976 Dec 14;15(25):5506–5511. doi: 10.1021/bi00670a014. [DOI] [PubMed] [Google Scholar]
- Luthe D. S., Peterson D. M. Cell-free Synthesis of Globulin by Developing Oat (Avena sativa L.) Seeds. Plant Physiol. 1977 May;59(5):836–841. doi: 10.1104/pp.59.5.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeester R. C., Hall T. C. Simplification of amino acid incorporation and other assays using filter paper techniques. Anal Biochem. 1977 May 1;79(1-2):627–630. doi: 10.1016/0003-2697(77)90447-x. [DOI] [PubMed] [Google Scholar]
- Shatkin A. J. Capping of eucaryotic mRNAs. Cell. 1976 Dec;9(4 Pt 2):645–653. doi: 10.1016/0092-8674(76)90128-8. [DOI] [PubMed] [Google Scholar]
- Sun S. M., Buchbinder B. U., Hall T. C. Cell-free Synthesis of the Major Storage Protein of the Bean, Phaseolus vulgaris L. Plant Physiol. 1975 Dec;56(6):780–785. doi: 10.1104/pp.56.6.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]