Abstract
Background
Diabetes mellitus is a major risk factor for coronary heart disease (CHD), renal failure, retinopathy, and neuropathy. Lowering glycosylated hemoglobin (HbA1c) as well as low-density lipoprotein-cholesterol (LDL-C) has been associated with a decreased risk of these complications. We evaluated the utility of glycated albumin (GA) and direct LDL-C, 2 novel assays, as compared to HbA1c and calculated LDL-C, in evaluating diabetes control and lipid in a heterogeneous population and in specific subgroups of patients with type 2 diabetes mellitus.
Methods
We obtained fasting blood samples and measured HbA1c, GA, and direct LDL-C, as well as other parameters, in a multi-ethnic population of 616 male and female patients with type 2 diabetes and 895 non-diabetic controls.
Results
HbA1c and GA levels, which measure different periods of glycemia, had a correlation of r=0.70 (p<0.001), and mean values in patients were 38.7% and 43.4% higher, respectively, than controls in men, and 41.1% and 40.1% higher, respectively, than controls, in women (both p<0.001). Calculated and direct LDL-C values correlated very highly (r=0.96, p<0.001). The correlations between HbA1c and GA, and between calculated and direct LDL-C were similar for subgroups defined by gender, race, age, and other factors.
Conclusions
Calculated LDL-C provides an accurate assessment of fasting LDL-C compared with a direct measurement in most subjects, except for those with hypertriglyceridemia, and GA correlates with HbA1c in diabetic and non-diabetic subjects and may serve as a reasonable marker of short term diabetic control.
Keywords: glycated albumin, low density lipoprotein cholesterol, type 2 diabetes
INTRODUCTION
Diabetes mellitus is a condition characterized by hyperglycemia and a markedly increased risk of cardiovascular disease, a major cause of death and disability in our society. The Third Adult Treat Panel of the National Cholesterol Education Program of the National Institutes of Health in the United States concluded that the presence of diabetes was a coronary heart disease (CHD) risk equivalent (i.e., that diabetic patients without prior CHD had the same risk of having a future CHD event as non-diabetic patients with prior CHD), and recommended that all patients with diabetes have their low-density lipoprotein (LDL) -cholesterol (LDL-C) level lowered to <100 mg/dl or 2.6 mmol/l (1). A more aggressive LDL-C target of <70 mg/dl or 1.8 mmol/l has been suggested by this same panel as optional in diabetic patients with established CHD. Lowering LDL-C levels with statins in diabetic patients in both the Heart Protection Study and the Collaborative Atorvastatin Diabetes Study, as well as in diabetic patients with CHD, has been associated with significant reductions in CHD risk vs placebo (2-4). A meta-analysis has indicated that for every 1.0 mmol/l or 38.5 mg/dl decrease in LDL-C there is in average a 23% reduction in CHD death, and that diabetic subjects get at least as much benefit as non-diabetic subjects (5).
The presence of diabetes mellitus also markedly increases the risk of retinopathy and blindness, nephropathy and renal failure, and neuropathy. In a number of diabetes treatment trials, lowering levels of glycosylated hemoglobin (HbA1c) has resulted in significant reductions in the risk of the above mentioned complications, as well as CHD (6-8). For these reasons, the American Diabetes Association has recommended that treatment of diabetic patients should aim to lower HbA1c levels to <7% (9). Control of HbA1c, LDL-C, and blood pressure are all critical in preventing the complications of diabetes, and having accurate values for these parameters becomes important for adequate treatment.
HbA1c analysis requires the use of whole blood and is often measured by HPLC, which lacks high through-put, and is more labor-intensive than automated assays. LDL-C is a value that is usually not measured directly, but is generally calculated from fasting values of total cholesterol, triglyceride, and high-density lipoprotein-cholesterol (HDL-C). The purpose of this study was to evaluate novel assays for direct LDL-C and glycated albumin (GA) in stored samples from a multi-ethnic male and female population of diabetic subjects and controls and to compare these results with calculated LDL-C and HbA1c values. We also determined whether the correlations between assay methods applied across specific subgroups defined by gender, race, age and other factors.
METHODS
Study Subjects
Patients with diabetes mellitus and non-diabetic control subjects, with no personal history or family history of diabetes in first degree relatives and with normal (< 6.1 mmol/l or 110 mg/dl) fasting glucose levels, were recruited and evaluated by the Diabetes Center, Massachusetts General Hospital and the Division of Endocrinology and Metabolism, Brigham and Women's Hospital as part of an observational study of diabetic and pre-diabetic subjects. Subjects were evaluated after an overnight fast of at least 8 h, and underwent a standard history including personal and family history, and medication use, and a physical examination including height, weight, waist circumference, and blood pressure. Blood samples were drawn for the measurement of HbA1c and plasma and serum biomarkers, and cell aliquots were frozen at −80° C for subsequent biochemical and genetic analyses.
Biochemical Measurements
HbA1c levels were measured on fresh whole blood samples with an HPLC assay (10) that serves as a primary reference method for the National Glycohemoglobin Standardization Program (11). It has intra- and inter-assay CVs < 2.5% for high and low standards, and utilizes long-term standards to prevent assay drift over time. Using plasma frozen at −80° C, total cholesterol, triglyceride, and high-density lipoprotein-cholesterol (HDL-C) levels in plasma were measured by automated standardized enzymatic analysis on a Hitachi 911 Analyzer (Roche Diagnostics, Indianapolis, IN). This laboratory maintains lipid standardization with the Centers for Disease Control, Atlanta, GA for these assays, and intra-assay and inter-assay CVs were <2% (12,13).
The direct LDL-C kit, obtained from the Kyowa Medex Corp. (Tokyo, Japan). The characteristics and development of this assay have been previously described (14). The assay is a homogeneous on-line assay that utilizes a triblock copolymer as surfactant and α cyclo-dextrin (14). In our laboratory at Tufts University, the direct LDL-C assay had intra-assay and inter-assay CVs of 0.77% and 1.30%, respectively. LDL-C was also calculated (calculated LDL-C) from the plasma total cholesterol, triglyceride, and HDL-C values using the Friedewald formula (LDL-C = total cholesterol – HDL-C – TG/5) (15). The original recommendation was that this formula could be used to estimate LDL-C provided the patient had fasted overnight and the plasma or serum triglyceride levels were <4.50 mmol/l. In our own studies of dyslipidemic patients and participants in the Framingham Offspring Study, we have found that calculated LDL-C levels using this formula are highly correlated (r= 0.93, P<0.001) with LDL-C measured by ultracentrifugation when plasma triglyceride levels are <2.80 mmol/l (16).
The GA assay was performed using kits obtained from the Asahi Kasei Pharma Corporation (Tokyo, Japan). This assay uses a glycated amino acid elimination reaction for an improved enzymatic GA measurement assay, which has correlated very highly (r=0.99) with values for GA obtained by HPLC (17,18). In our laboratory, the assay had intra-assay and inter-assay CVs of 1.1% and 1.6%, respectively.
For the population based study, GA analysis was performed on frozen plasma stored at –80 ° C and never thawed. In all analyses, all laboratory personnel were masked as to subject status. Informed consent was obtained from all subjects under a protocol approved by the Human Investigation Review Committee of the Massachusetts General Hospital and Brigham and Womens Hospital.
Statistical Analysis
The primary objective of this investigation was to compare the results of the 2 new assays with established assays in patients with diabetes mellitus and in controls subjects. A secondary objective was to determine whether the correlations in the entire sample applied similarly to subgroups, based on gender or ethnicity. Comparison between patients with diabetes mellitus and control subjects within gender were made using Student's t-test for continuous variables and χ2 tests for proportions. Spearman correlation coefficients were produced, using the entire sample. A nominal p< 0.05 were considered statistically significant. All statistical analyses were performed in the General Medicine Division at Massachusetts General Hospital using Statistical Analysis Software, (SAS Institute, Cary, NC).
RESULTS
Subject characteristics for the male patients with diabetes (n=297), male control subjects (n=401), female patients with diabetes (n=319) and female control subjects (n=494) are shown in Table 1. Both male and female patients with diabetes were significantly (p <0.001) older than controls, significantly (p < 0.001) more likely than controls to have a body mass index >30 kg/m2 and to have a waist circumference (> 102 cm in men and > 88 cm in women), and significantly more likely to be taking aspirin and medication for blood pressure and lipid control. Further, 17.1% of the men with diabetes (vs 2.6% of the controls) and 5.8% of the women with diabetes (vs 0.6% of the controls) had a history of heart disease, underlying the importance of aggressively treating risk factors in this population. The percentage of diabetic patients with nephropathy or severe proteinuria was <2%. Ethnic background was similar in diabetes patients compared with controls: in men and women with diabetes, 77.0% and 61.7%, respectively, were white, 19.4% and 30.2% were African American, 5.8% and 3.6% were Hispanic, and 2.4% and 3.2% were Asian. For male and female controls these percentages were: 74.4% and 77.2% white, 16.3% and 12.7% African American, 4.3% and 5.8% Hispanic, and 5.2% and 4.4% Asian.
Table 1.
Study Subjects
| Variable | Male |
Female |
||
|---|---|---|---|---|
| Diabetes (n = 297) |
Controls (n = 401) |
Diabetes (n = 319) |
Controls (n = 494) |
|
| Age (y) | 57.9 (11.4) | 51.2 (13.0)* | 56.6 (12.0) | 52.0 (12.4)* |
| BMI (kg/m2) | 34.1 (9.5) | 27.3 (4.6)* | 36.0 (11.1) | 27.7 (7.6)* |
| % BMI > 30 kg/m2 | 64.3 | 23.8* | 69.2 | 25.5* |
| Waist Circumference (cm) | 109.6 (15.1) | 95.9 (12.1)* | 105.4 (19.5) | 86.2 (15.9)* |
| % Elevated Waist Circumference | 69.2 | 27.0* | 82.7 | 39.5* |
| Systolic Blood Pressure (mmHg) | 130.4 (15.7) | 122.7 (16.0)* | 130.7 (16.4) | 118.3 (18.3)* |
| Diastolic Blood Pressure (mmHg) | 78.0 (11.3) | 77.2 (10.1) | 76.7 (9.7) | 73.1 (9.8)* |
| % Hypertensive | 52.0 | 36.2* | 51.9 | 27.0* |
| % on Blood Pressure Medication | 63.3 | 15.2* | 60.5 | 14.0* |
| % on Lipid Lowering Medication | 55.6 | 11.5* | 47.0 | 10.1* |
| % on Aspirin | 60.6 | 25.2* | 47.7 | 22.5* |
| % on Oral Hypoglycemic Agents | 72.1 | 0.2* | 69.6 | 0.2* |
| % on Insulin | 32.7 | 0.0* | 34.2 | 0.0* |
Data provided as mean value (standard deviation) or percentages
p<0.001 for differences between diabetes and controls within each gender, elevated waist circumference defined as > 102 cm in men and > 90 cm in women.
Abbreviations: body mass index (BMI), millimeters of mercury (mmHg).
Data on biochemical variables for the patients with diabetes and respective controls are presented in Table 2. Men and women with diabetes had significantly lower total cholesterol, direct LDL-C, calculated LDL-C, and HDL-C and significantly higher triglyceride, HbA1c, and GA levels in plasma than controls. Mean values for direct LDL-C and calculated LDL-C were similar and highly correlated with one another (r=0.956, p<0.001). In 8.5% of men with diabetes, 3.6% of women with diabetes, 2.1% of male controls, and 0.4% of female controls, LDL-C could not be calculated because of fasting triglyceride levels of >4.5 mmol/l and these subjects were excluded from this comparison analysis.
Table 2.
Biochemical Values in Diabetes and Control Subjects
| Variable | Male+ |
Female+ |
||
|---|---|---|---|---|
| Diabetes (n = 297) |
Controls (n = 401) |
Diabetes (n = 319) |
Controls (n = 494) |
|
| Total Cholesterol (mmol/l) | 4.58 (0.98) | 4.99 (40.0)* | 5.05 (1.09) | 5.23 (1.05)* |
| Triglycerides (mmol/l) | 2.13 (1.60) | 1.50 (105.2)* | 1.66 (0.87) | 1.15 (0.64)* |
| HDL-C (mmol/l) | 1.15 (0.34) | 1.32 (15.5)* | 1.38 (0.44) | 1.64 (0.42)* |
| TC/HDL-C Ratio | 4.2 (1.6) | 3.9 (1.4)* | 3.8 (1.5) | 3.2 (1.7)* |
| Non-HDL-C (mmol/l) | 3.51 (0.66) | 3.82 (31.5)* | 3.74 (0.65) | 3.59 (0.84) |
| Calculated LDL-C (mmol/l)+ | 2.64 (0.86) | 3.05 (34.5)* | 2.95 (0.97) | 3.08 (0.96) |
| Direct LDL-C (mmol/l) | 2.67 (0.83) | 3.11 (33.8)* | 2.98 (0.93) | 3.16 (0.93)** |
| Insulin (pmol/l) | 180 (170) | 85 (60)* | 200 (290) | 90 (90)* |
| Glycosylated Hemoglobin (%) | 7.6 (1.7) | 5.5 (0.5)* | 7.8 (1.6) | 5.5 (0.5)* |
| Glycated Albumin (%) | 19.9 (5.4) | 13.2 (1.4)* | 18.9 (5.5) | 13.5 (1.5)* |
30 male patients with diabetes (8.5%) and 9 male controls (2.2%) of the original sample could not have their LDL-C calculated because of fasting triglycerides > 4.5 mmol/l (400mg/dl), and these were excluded from this analysis. For female this percentage was 3.8% in diabetes versus 0.4% in controls. Data are provided as mean values (standard deviations).
p<0.001 for the difference between diabetes and controls within each gender
p<0.01 for the difference between diabetes and controls within each gender
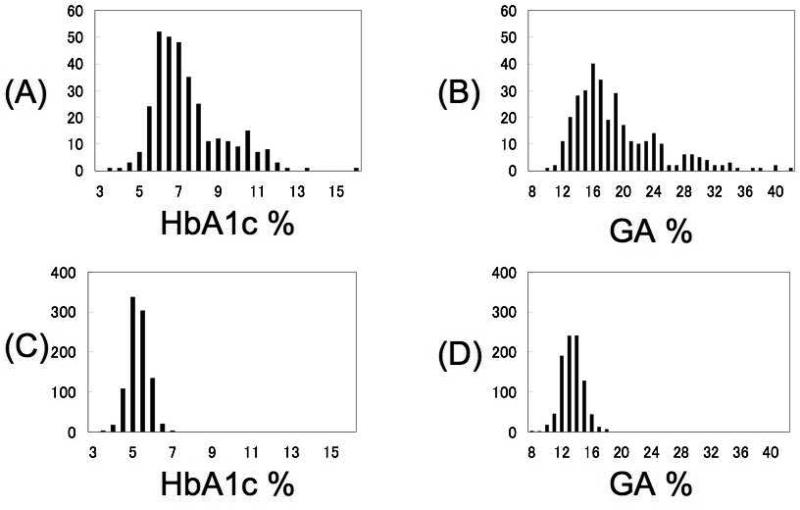
In both patients with diabetes and in controls, HbA1c and GA had a correlation of 0.70 (p<0.001). The distributions of HbA1c and GA in patients with diabetes and control subjects are shown in Figure 1. It is clear from these data the GA has a wider range of distribution than HbA1c, but both parameters distinguish cases from controls. Mean levels of HbA1c and GA were 38.7% and 43.4% higher in men with diabetes than controls and 41.1% and 40.1% higher in women with diabetes than controls, respectively (both p<0.001). In control subjects, the distribution of HbA1c and GA were assumed to be normal, and their values 2 SD above the mean were 6.5% and 16.3%, respectively. These data would suggest that such values would be reasonable targets for glycemic control in the population with diabetes. The same relationships and differences between diabetes cases and controls for HbA1c, GA, direct LDL-C and calculated LDL-C were observed in different ethnic and racial groups.
Figure 1.
Distributions of glycosylated hemoglobin (HbA1c %) and glycated albumin (GA %) in patients with diabetes (A, B) and in control subjects (C, D).
Despite being much more likely to be on lipid lowering medication, patients with diabetes still had significant elevations in the total cholesterol/HDL-C ratio versus controls, and the women with diabetes also had significantly higher non-HDL-C than controls, in contrast to the men.
A subset of men with diabetes (n=115) and controls (n=395), as well women with diabetes (n=153) and controls (n=482) had fasting glucose levels done. The mean values were 8.2 mmol/l (standard deviation or SD, 3.4) and 8.4 mmol/l (SD, 3.7), respectively, in the men and women with diabetes, significantly different (p<0.001) than values in the controls, which were 5.0 mmol/l (SD, 0.5) in the men and 4.9 mmol/l (SD, 0.5) in the women. Fasting glucose levels were significantly correlated with both HbA1c (r=0.56, p<0.001) and GA (r=0.43, p<0.001) levels.
DISCUSSION
Patients with diabetes frequently have hypertriglyceridemia, which can sometimes make the calculation of LDL-C with the Friedewald equation problematic. In our population, 8.5% of the male and 3.8% of the female diabetic population had a triglyceride level that precluded calculating LDL-C. For these reasons efforts have been made to develop direct assays for LDL-C (14,19). Patients with diabetes often have elevations in plasma triglycerides, remnant lipoprotein cholesterol, and small dense LDL, as well as decreased HDL-C as compared to control subjects (20,21). Values obtained with direct LDL-C assays correlate very highly with those obtained by ultracentrifugation (14). In addition to the requirement for triglyceride levels <4.5 mmol/l, conventional lipid measurements require an overnight fast, which is problematic for many patients with diabetes, especially those who are insulin-treated. We have previously documented that this direct LDL-C assay can readily be used in the fasting and non-fasting state to monitor LDL-C values, which change very little in these two states (12). Moreover this assay is an excellent way to monitor effects of statin therapy in CHD patients either in the fasted or fed state (22,23). We have documented that the direct LDL-C assay performs well compared with the calculated LDL-C with the Friedewald formula in both diabetic patients and non-diabetic subjects using plasma obtained after an overnight fast. The direct LDL-C offers the physician the option of assessing LDL-C in the non-fasting state, as well as when the patient has significant hypertriglyceridemia (12,22,23). Moreover this assay can be run in any clinical chemistry laboratory and does not require any specialized equipment except for an automated analyzer that is standard equipment in such laboratories. In addition the assay has intraassay and interassay CVs <2%. Conversely our data indicate that for the vast majority of diabetic patients the calculated LDL-C serves as a very reasonable measure of LDL-C control, provided that the sample has been obtained after an overnight fast.
While the diagnosis of diabetes is often based on the finding of a fasting glucose of >6.9 mmol/l, the standard for monitoring glucose control is the HbA1c. Studies have clearly documented the benefit of lowering values to <7% to reduce the complications of diabetes including retinopathy, nephropathy, neuropathy, and macrovascular disease (6-9). However this test is relatively expensive, and can be cumbersome. Moreover, HbA1c cannot be measured using frozen serum or plasma. For these reasons efforts have been made to develop an assay for GA. Such an assay has now been developed, which can be run on an automated analyzer using fresh or frozen serum or plasma. The assay is approved for use in Japan (17). The assay does not provide the same information as the HbA1c as the shorter half-life of albumin compared with red blood cells means that GA reflects mean glycemia over approximately 2-3 weeks, compared with 2-3 months for HbA1c. GA would need to be measured every 2-4 weeks to capture information regarding chronic glycemia, which is a relative disadvantage compared with measuring HbA1c every 3 months. Increased GA appear to serve as excellent markers of diabetes control in patients with diabetes on hemodialysis (24), as well as an excellent predictor of the presence of CHD in patients with type 2 diabetes (25). In hypertensive diabetic rats, GA levels are excellent markers of vascular damage (26), and GA is unable to serve as an acceptor of endproducts of oxidation in contrast to normal albumin (27). In Japan where both HbA1c and GA are approved and in clinical use, many diabetologists prefer GA especially when initiating or changing medication on their patients, some use GA and HbA1c in combination to obtain information of both long and short term control at the same time, and some use GA in non-diabetic subjects with insulin-resistance, while using HbA1c in diabetic subjects. This is the first report of the application of GA in diabetic subjects in a US population. GA assessment is easier and more cost-effective than HBA1c. However, HbA1c remains the gold standard of diabetes control. The limitations of this study include the use of frozen, rather than fresh samples for assessment of direct LDL-C and GA, and no prospective information about outcomes. The overall data indicate that: 1) direct LDL-C and GA are promising new tests, that calculated LDL-C and non HDL-C can be used in most patients with diabetes (direct LDL-C only needs to be used in subjects with triglycerides > 4.5 mmol/l (400mg/dl) or if the patient is not-fasting), and that 2) both HbA1c and GA can be used as markers of diabetes control.
Acknowledgement
Dr. Ai and Ms. Otokozawa were supported by research fellowships from the Denka-Seiken Corporation, and the Kyowa- Medex Corporation, Tokyo, Japan, respectively. Dr. Nakajima is a Distinguished Visiting Scientist at Tufts University. Dr. Schaefer is supported by contract 53-1950-5-003 from the US Department of Agriculture, and grants HL 74753 and HL 60935 and project PO50HL083813 from the National Institutes of Health, Betheda, MD. Dr. Meigs is supported by an American Diabetes Association Career Development Award, Arlington, VA. Dr. Nathan is supported in part by the Ida Charlton Family Trust. The sampling of study subjects was supported in part through a contract with Roche Diagnostics Inc, Nutley, NJ.
Abbreviations
- TC
Total cholesterol
- TG
triglyceride
- HDL-C
high density lipoprotein cholesterol
- TC/HDL-C ratio
total cholesterol/HDL cholesterol ratio
- LDL-C
low density lipoprotein cholesterol
- GA
glycated albumin
- HBA1c
glycosylated hemoglobin
- BMI
body mass index
- mmHg
millimeters mercury
- SD
standard deviation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Expert Panel Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) J Am Med Assoc. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 2.Collins R, Armitage J, Parish S, Sleigh P. Peto. Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 5963 people with diabetes: a randomized placebo controlled trial. Lancet. 2003;362:2005–2016. doi: 10.1016/s0140-6736(03)13636-7. [DOI] [PubMed] [Google Scholar]
- 3.Colhoun HM, Betteridge DJ, Durrington PN, et al. CARDS investigators. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS). Lancet. 2004;364:685–696. doi: 10.1016/S0140-6736(04)16895-5. [DOI] [PubMed] [Google Scholar]
- 4.Shepherd J, Barter P, Carmena R, et al. Effects of lowering low density lipoprotein cholesterol substantially below currently recommended levels in patients with coronary heart disease and diabetes. Diabetes Care. 2006;29:1220–1226. doi: 10.2337/dc05-2465. [DOI] [PubMed] [Google Scholar]
- 5.Baigent C, Keech A, Kearney PM, et al. Cholesterol Treatment Trialists Collaborators. Efficacy and safety of cholesterol-lowering treatment:prospective meta- analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 6.Nathan DM, Crofford OB, Genuth S, et al. DCCT Research Group The effect of intensive diabetes treatment on the development and progression of long-term complications in insulin-dependent diabetes mellitus: The Diabetes Control and Complications Trial. N Engl J Med. 1993;329:978–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 7.Ohkubo Y, Kishikawa H, Araki E, et al. Intensive insulin therapy prevents the progression of diabetic microvascular disease in Japanese patients with non-insulin dependent diabetes: a randomized prospective six year study. Diabetes Res Clin Pract. 1995;928:103–117. doi: 10.1016/0168-8227(95)01064-k. [DOI] [PubMed] [Google Scholar]
- 8.Nathan DM, Cleary PA, Backlund JY, et al. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643–2355. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Diabetes Association Standards of medical care. Diabetes Care. 2007;33(suppl1):13–25. [Google Scholar]
- 10.Nathan DM, Singer DE, Hurxthal K, Goodson JD. The clinical information value of the glycosylated hemoglobin assay. N Engl J Med. 1984;310:341–346. doi: 10.1056/NEJM198402093100602. [DOI] [PubMed] [Google Scholar]
- 11.Little RR, Rohlfing CL, Wiedmeyer H-M, Myers GL, Sacks DB, Goldstein DE. The National Glycohemoglobin Standardization Program (NGSP): a five-year progress report. Clin Chem. 2001;47:1985–92. [PubMed] [Google Scholar]
- 12.Schaefer EJ, Audelin MC, McNamara JR, et al. Comparison of fasting and postprandial plasma lipoproteins in subjects with and without coronary heart disease. Am J Cardiol. 2001;88:1129–1133. doi: 10.1016/s0002-9149(01)02047-1. [DOI] [PubMed] [Google Scholar]
- 13.Sugiuchi H, Uji Y, Okabe H, et al. Direct measurement of high-density lipoprotein cholesterol in serum with polyethylene glycol-modified enzymes and sulfated alpha-cyclodextrin. Clin Chem. 1995;41:717–723. [PubMed] [Google Scholar]
- 14.Sugiuchi H, Irie T, Yoshinori U, et al. Homogeneous assay for measuring low-density lipoprotein cholesterol in serum with triblock copolymer and alpha-cyclodextrin. Clin Chem. 1998;44:522–531. [PubMed] [Google Scholar]
- 15.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. (1972) [PubMed] [Google Scholar]
- 16.McNamara JR, Cohn JS, Wilson PWF, Schaefer EJ. Calculated values for low-density lipoprotein cholesterol in the assessment of lipid abnormalities and coronary disease risk. Clin. Chem. 1990;36:36–42. [PubMed] [Google Scholar]
- 17.Kouzuma T, Uematsu Y, Usami T, Imamura S. Study of glycated amino acid elimination reaction for an improved enzymatic glycated albumin measurement method. Clin Chim Acta. 346:135–143. doi: 10.1016/j.cccn.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 18.Chujo K, Shima K, Tada H, Oohashi T, Minakuchi J, Kawashima S. Indicators for blood glucose control in diabetics with end-stage chronic renal disease: GHb vs. glycated albumin (GA). J Med Invest. 2006;53:223–228. doi: 10.2152/jmi.53.223. [DOI] [PubMed] [Google Scholar]
- 19.McNamara JR, Cole TG, Contois JH, Ferguson CA, Ordovas JM, Schaefer EJ. Immunoseparation method for measuring low-density lipoprotein cholesterol directly from serum evaluated. Clin. Chem. 1995;41:232–240. [PubMed] [Google Scholar]
- 20.Siegel RD, Cupples A, Schaefer EJ, Wilson PWF. Lipoproteins, apolipoproteins, and low density lipoprotein size among diabetics in the Framingham Offspring Study. Metabolism. 1996;45:1267–1272. doi: 10.1016/s0026-0495(96)90246-2. [DOI] [PubMed] [Google Scholar]
- 21.Schaefer EJ, McNamara JR, Shah PK, et al. Elevated remnant-like particle cholesterol and triglyceride levels in diabetic men and women in the Framingham Offspring Study. Diabetes Care. 2002;25:989–994. doi: 10.2337/diacare.25.6.989. [DOI] [PubMed] [Google Scholar]
- 22.Schaefer EJ, McNamara JR, Tayler T, et al. Effects of atorvastatin on fasting and postprandial lipoprotein subclasses in coronary heart disease patients versus control subjects. Am J Cardiol. 2002;90:689–696. doi: 10.1016/s0002-9149(02)02591-2. [DOI] [PubMed] [Google Scholar]
- 23.Schaefer EJ, McNamara JR, Tayler T, et al. Comparisons of effects of statins (atorvastatin, fluvastatin, lovastatin, pravastatin, and simvastatin) on fasting and postprandial lipoproteins in patients with coronary heart disease versus control subjects. Am J Cardiol. 2004;93:31–39. doi: 10.1016/j.amjcard.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 24.Inaba M, Okuno S, Kumeda Y, et al. Osaka CKD Expert Research Group. Glycated albumin is a better glycemic indicator than glycosylated hemoglobin in hemodialysis patients with diabetes: effects of anemia and erythropoietin. J Am Soc Nephrology. 2007;18:896–903. doi: 10.1681/ASN.2006070772. [DOI] [PubMed] [Google Scholar]
- 25.Pu LJ, Lu L, Xu XW, et al. Value of glycated albumin and high sensitivity C reactive protein in predicting the presence of coronary artery disease in patients with type 2 diabetes. Cardiovasc Diabetol. 2006;5:2480–5. doi: 10.1186/1475-2840-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldin A, Beckman JA, Schmidt AM, Creager MD. Advanced glycation endproducts: sparking the development of diabetic vascular injury. Circulation. 2006;114:597–605. doi: 10.1161/CIRCULATIONAHA.106.621854. [DOI] [PubMed] [Google Scholar]
- 27.Wiernsperger NF. Oxidative stress: the special case of diabetes. Biofactors. 2003;19:11–18. doi: 10.1002/biof.5520190103. [DOI] [PubMed] [Google Scholar]