Abstract
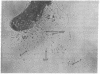
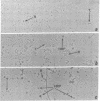
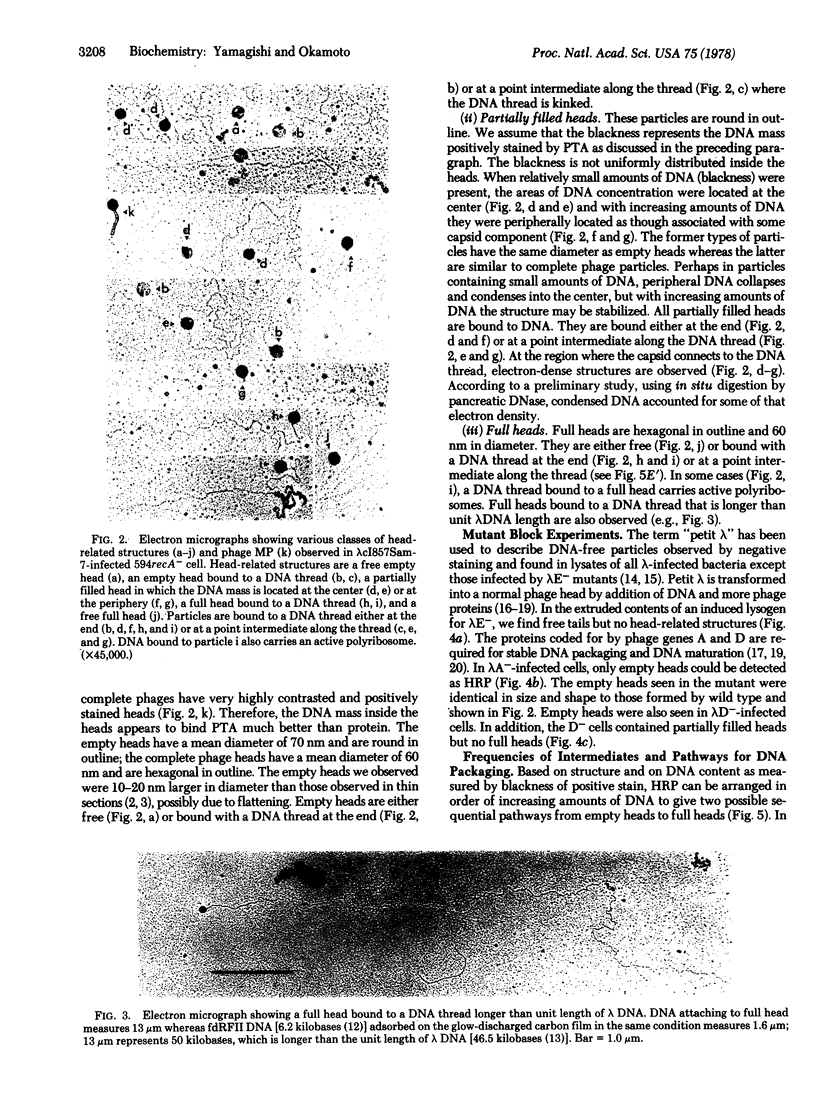
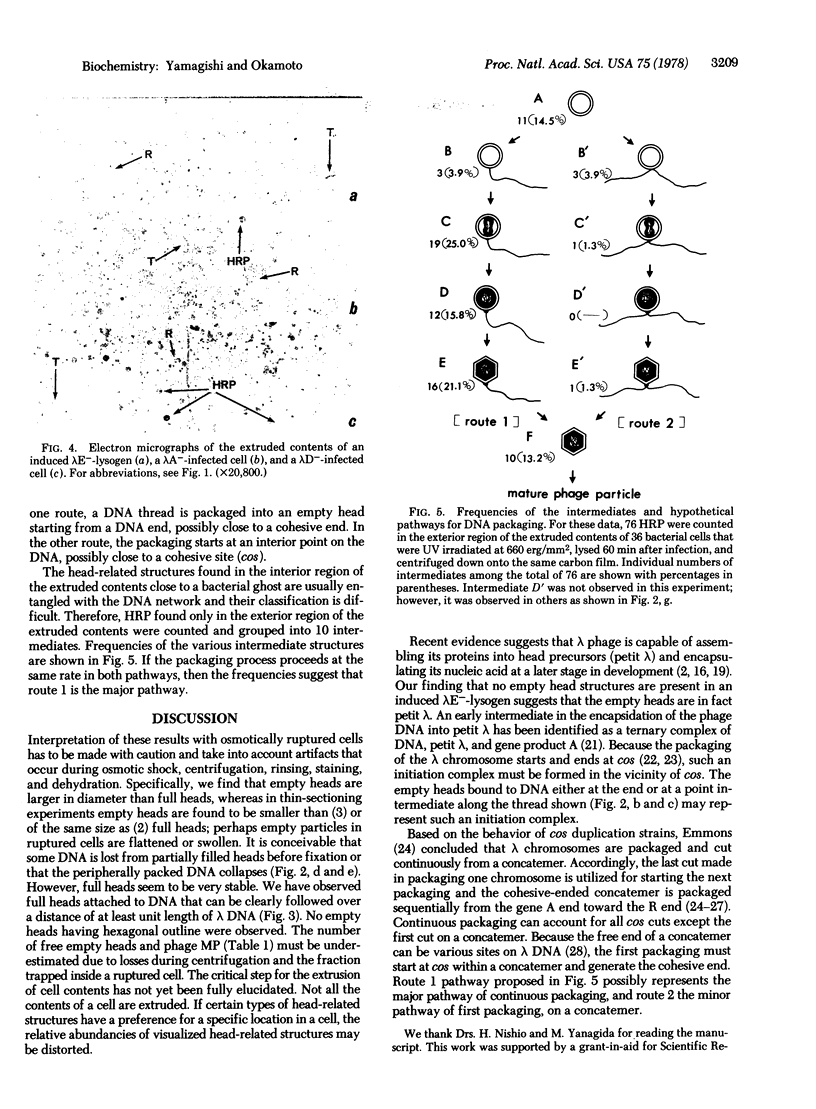
To reveal intermediates in lambda DNA packaging, infected cells were osmotically ruptured and the cell lysates were deposited on electron microscope grids by sedimentation through a sucrose/formalin cushion. A fixation procedure that crosslinks head-related structures to DNA allowed us to study successive stages in the process of head filling. Three types of head-related structures can be distinguished: (i) empty heads (petit lambda), less angular in outline than complete lambda heads; (ii) heads partially filled with DNA (partially filled heads), having a roundish outline; and (iii) particles tightly packed with DNA (full heads), having a hexagonal outline. DNA-head complexes were bound either at the terminal end of a DNA thread or at a point intermediate along the thread. The terminal complexes were more abundant. No head-related structures could be found in an induced lambda mutant lysogen blocked in the synthesis of petit lambda (amber in lambda gene E). One type of mutant blocked in DNA packaging (amber in gene A) produces empty heads and free tails, whereas another (amber in gene D) produces partially filled heads in addition. Our data suggest that a DNA-petit lambda complex may be an early intermediate in packaging and that the lambda DNA substrate can be a cohesive-ended concatemer or a concatemer with double-stranded cohesive site sequences.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker A., Marko M., Gold M. Early events in the in vitro packaging of bacteriophage lambda DNA. Virology. 1977 May 1;78(1):291–305. doi: 10.1016/0042-6822(77)90100-3. [DOI] [PubMed] [Google Scholar]
- Casjens S., King J. Virus assembly. Annu Rev Biochem. 1975;44:555–611. doi: 10.1146/annurev.bi.44.070175.003011. [DOI] [PubMed] [Google Scholar]
- Dawson P., Skalka A. Bacteriophage lambda head morphogenesis: studies on the role of DNA. J Mol Biol. 1975 Apr 5;93(2):167–180. doi: 10.1016/0022-2836(75)90126-6. [DOI] [PubMed] [Google Scholar]
- Emmons S. W. Bacteriophage lambda derivatives carrying two copies of the cohesive end site. J Mol Biol. 1974 Mar 15;83(4):511–525. doi: 10.1016/0022-2836(74)90511-7. [DOI] [PubMed] [Google Scholar]
- Enquist L. W., Skalka A. Replication of bacteriophage lambda DNA dependent on the function of host and viral genes. I. Interaction of red, gam and rec. J Mol Biol. 1973 Apr 5;75(2):185–212. doi: 10.1016/0022-2836(73)90016-8. [DOI] [PubMed] [Google Scholar]
- Feiss M., Bublitz A. Polarized packaging of bacteriophage lambda chromosomes. J Mol Biol. 1975 Jun 5;94(4):583–594. doi: 10.1016/0022-2836(75)90323-x. [DOI] [PubMed] [Google Scholar]
- Freifelder D., Chud L., Levine E. E. Requirement for maturation of Escherichia coli bacteriophage lambda. J Mol Biol. 1974 Mar 15;83(4):503–509. doi: 10.1016/0022-2836(74)90510-5. [DOI] [PubMed] [Google Scholar]
- Goldberg A. R., Howe M. New mutations in the S cistron of bacteriophage lambda affecting host cell lysis. Virology. 1969 May;38(1):200–202. doi: 10.1016/0042-6822(69)90148-2. [DOI] [PubMed] [Google Scholar]
- Hohn B. DNA as substrate for packaging into bacteriophage lambda, in vitro. J Mol Biol. 1975 Oct 15;98(1):93–106. doi: 10.1016/s0022-2836(75)80103-3. [DOI] [PubMed] [Google Scholar]
- Hohn B., Hohn T. Activity of empty, headlike particles for packaging of DNA of bacteriophage lambda in vitro. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2372–2376. doi: 10.1073/pnas.71.6.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn B., Wurtz M., Klein B., Lustig A., Hohn T. Phage lambda DNA packaging, in vitro. J Supramol Struct. 1974;2(2-4):302–317. doi: 10.1002/jss.400020220. [DOI] [PubMed] [Google Scholar]
- Hohn T., Flick H., Hohn B. Petit lambda, a family of particles from coliphage lambda infected cells. J Mol Biol. 1975 Oct 15;98(1):107–120. doi: 10.1016/s0022-2836(75)80104-5. [DOI] [PubMed] [Google Scholar]
- Kaiser D., Syvanen M., Masuda T. DNA packaging steps in bacteriophage lambda head assembly. J Mol Biol. 1975 Jan 15;91(2):175–186. doi: 10.1016/0022-2836(75)90158-8. [DOI] [PubMed] [Google Scholar]
- Kaiser D., Syvanen M., Masuda T. Processing and assembly of the head of bacteriophage lambda. J Supramol Struct. 1974;2(2-4):318–328. doi: 10.1002/jss.400020221. [DOI] [PubMed] [Google Scholar]
- Lickfeld K. G., Menge B. Morphogenesis of bacteriophage lambda: electron microscopy of thin sections. J Mol Biol. 1976 May 15;103(2):299–318. doi: 10.1016/0022-2836(76)90314-4. [DOI] [PubMed] [Google Scholar]
- Miller O. L., Jr, Bakken A. H. Morphological studies of transcription. Acta Endocrinol Suppl (Copenh) 1972;168:155–177. doi: 10.1530/acta.0.071s155. [DOI] [PubMed] [Google Scholar]
- Miller O. L., Jr, Beatty B. R. Visualization of nucleolar genes. Science. 1969 May 23;164(3882):955–957. doi: 10.1126/science.164.3882.955. [DOI] [PubMed] [Google Scholar]
- Miller O. L., Jr, Hamkalo B. A. Visualization of RNA synthesis on chromosomes. Int Rev Cytol. 1972;33:1–25. doi: 10.1016/s0074-7696(08)61446-1. [DOI] [PubMed] [Google Scholar]
- Murialdo H., Siminovitch L. The morphogenesis of phage lambda. V. Form-determining function of the genes required for the assembly of the head. Virology. 1972 Jun;48(3):824–835. doi: 10.1016/0042-6822(72)90163-8. [DOI] [PubMed] [Google Scholar]
- Sternberg N., Weisberg R. Packaging of prophage and host DNA by coliphage lambda. Nature. 1975 Jul 10;256(5513):97–103. doi: 10.1038/256097a0. [DOI] [PubMed] [Google Scholar]
- Syvanen M. Processing of bacteriophage lambda DNA during its assembly into heads. J Mol Biol. 1975 Jan 15;91(2):165–174. doi: 10.1016/0022-2836(75)90157-6. [DOI] [PubMed] [Google Scholar]
- Szpirer J., Brachet P. Relations physiologiques entre les phages tempérés lambda et phi80. Mol Gen Genet. 1970;108(1):78–92. doi: 10.1007/BF00343187. [DOI] [PubMed] [Google Scholar]
- Takahashi S. The starting point and direction of rolling-circle replicative intermediates of coliphage lambda DNA. Mol Gen Genet. 1975 Dec 29;142(2):137–153. doi: 10.1007/BF00266095. [DOI] [PubMed] [Google Scholar]
- Willetts N. S., Clark A. J. Characteristics of some multiply recombination-deficient strains of Escherichia coli. J Bacteriol. 1969 Oct;100(1):231–239. doi: 10.1128/jb.100.1.231-239.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi H., Inokuchi H., Ozeki H. Excision and duplication of su3+-transducing fragments carried by bacteriophage phi 80. I. Novel structure of phi 80sus2psu3+ DNA molecule. J Virol. 1976 Jun;18(3):1016–1023. doi: 10.1128/jvi.18.3.1016-1023.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi H., Ozeki H. Comparative study of thermal inactivation of phage phi 80 and lambda. Virology. 1972 May;48(2):316–322. doi: 10.1016/0042-6822(72)90042-6. [DOI] [PubMed] [Google Scholar]
- Zachary A., Simon L. D., Litwin S. Lambda head morphogenesis as seen in the electron microscope. Virology. 1976 Jul 15;72(2):429–442. doi: 10.1016/0042-6822(76)90172-0. [DOI] [PubMed] [Google Scholar]