Abstract
Study Objectives:
To investigate the effects of botulinum toxin type A (BoNT-A) injection on jaw motor episodes during sleep in patients with or without orofacial pain who did not respond to oral splint treatment.
Methods:
Twenty subjects with a clinical diagnosis of SB completed this study. Ten subjects received bilateral BoNT-A injections (25 U per muscle) into the masseter muscles only (group A), and the other 10 received the injections into both the masseter and temporalis muscles (group B). Video-polysomnographic (vPSG) recordings were made before and at 4 weeks after injection. Rhythmic masticatory muscle activity (RMMA) and orofacial activity (OFA) were scored and analyzed for several parameters (e.g., frequency of episodes, bursts per episode, episode duration). The peak amplitude of electromyographic (EMG) activity in the two muscles was also measured.
Results:
BoNT-A injection did not reduce the frequency, number of bursts, or duration for RMMA episodes in the two groups. The injection decreased the peak amplitude of EMG burst of RMMA episodes in the injected muscles (p < 0.001, repeated measure ANOVA) in both groups. At 4 weeks after injection, 9 subjects self-reported reduction of tooth grinding and 18 subjects self-reported reduction of morning jaw stiffness.
Conclusions:
A single BoNT-A injection is an effective strategy for controlling SB for at least a month. It reduces the intensity rather than the generation of the contraction in jaw-closing muscles. Future investigations on the efficacy and safety in larger samples over a longer follow-up period are needed before establishing management strategies for SB with BoNT-A.
Citation:
Shim YJ; Lee MK; Kato T; Park HU; Heo K; Kim ST. Effects of botulinum toxin on jaw motor events during sleep in sleep bruxism patients: a polysomnographic evaluation. J Clin Sleep Med 2014;10(3):291-298.
Keywords: Sleep bruxism, botulinum toxin, polysomnography, rhythmic masticatory muscle activity, jaw motor activity, masticatory muscle pain
Sleep bruxism (SB) is defined as a stereotyped oromandibular activity during sleep characterized by teeth grinding and clenching.1,2 SB is one of the common sleep related movement disorders, with a prevalence of self-reported tooth grinding of 5% to 8% in the adult population.3,4 In the field of dentistry, SB has been recognized to be related to teeth destruction, dental pros-theses/implant failure, pain in the teeth, jaw, masticatory muscle, and temporomandibular joint (TMJ), or headache.1,5,6 These dental problems can be associated with the frequent and intense contractions of the jaw-closing muscles during sleep.7,8 There are various treatment modalities for the management of SB such as oral splint, behavioral approaches, and pharmacological management; oral splint is the most commonly used treatment. The previous studies reported that the EMG activity of masseter muscle during sleep were decreased, increased, or not changed after oral splint usage.9,10 Currently, oral splint is considered as the first choice for protecting teeth and prostheses from damages. Although various treatment modalities, such as behavioral approaches and pharmacological management were tested for SB, there were no effective treatment strategies for controlling intense contractions of masticatory muscles during sleep in SB patients.1
BRIEF SUMMARY
Current Knowledge/Study Rationale: Botulinum toxin type A (BoNT-A) has been used for managing involuntary orofacial movements and secondary bruxism in patients with movement disorders, but its usefulness and objective effects on sleep bruxism (SB) have not been evaluated using objective measures. The aim of this study was to investigate the effects of intramuscular BoNT-A injection on orofacial motor events during sleep in patients with clinical diagnosis of SB.
Study Impact: A single injection of BoNT-A into jaw-closing muscles is an effective strategy for controlling SB for at least a month. It reduces the intensity rather than the generation of the contraction in the jaw-closing muscles. Establishing management of SB with BoNT-A awaits further studies on larger samples over a longer follow-up period.
Many case reports have been reported that botulinum toxin type A (BoNT-A) injections are effective for controlling involuntary orofacial movements and secondary bruxism in patients with movement disorders, and awake bruxism (e.g., clenching habit).11–15 Other studies with only SB patients showed that BoNT-A injections can reduce the frequency of jaw motor events,16 decrease bruxism-induced pain levels,16,17 and is equally effective in pain reduction when compared with oral splint.17,18 The result of these studies and case reports were based on the subjective evaluation of SB, not on the objective assessment using electromyography (EMG) or polysomnography (PSG). A study using portable electromyographic (EMG) device showed that the counts of sleep related masticatory EMG bursts, detected by the pre-defined threshold, were significantly reduced after a single injection of BoNT-A in the masseter muscle.19 However, it remains unclear how BoNT-A injection works specifically for sleep-related masticatory EMG activity, as a variety of jaw motor activities occur in association with transient arousal changes during sleep.20 Video-polysomnography (vPSG) can be a useful tool allowing discrimination of the types of sleep-related masticatory EMG events.2 In previous case reports and studies, some cases injected BoNT-A into the masseter muscle only,13–15,17,18 and others into both the masseter and temporalis muscles.10,11,15 The masseter and temporalis muscles are synergetic muscles and activated during teeth grinding and clenching; however, there have been no studies assessing the difference between the choices or the combination of the injected muscles for motor activity control of the jaw during sleep.
Therefore, the aims of this study were to investigate the effects of intramuscular BoNT-A injection on jaw motor episodes using vPSG. In addition to the use of vPSG, we compared the effects of BoNT-A on jaw motor episodes between the injection sites (i.e., the masseter muscle injection only versus injection of both masseter and temporalis muscles).
METHODS
Subjects
The protocol of this study was undertaken with the approval of Korea Food & Drug Administration and Institutional Review Board of the Yonsei University Dental Hospital. All subjects were informed of the nature of the study, and written consent was obtained from each participant.
Twenty-four subjects (M: 10; F: 14, age: 20.2-38.7 years) were selected from among outpatients at the Department of Orofacial Pain and Oral Medicine, Yonsei University Dental Hospital. All subjects were interviewed by one clinician (YJS), who documented his or her age, gender, medical history, and drug history at the first visit. The subjects had the following clinical signs and symptoms for SB: (1) a history of tooth grinding occurring ≥ 3 nights per week, (2) experience of morning jaw stiffness, and (3) clinical presence of tooth wear.1,2 They had been using an oral splint for SB with orofacial pain or without orofacial pain. Fifteen patients had jaw muscle pain, and 11 patients had a TMJ click sound (evaluated by clinical examination). The subjects had been treated with an oral splint for controlling SB or orofacial pain for periods ranging from 3 months to 10 years. However, they still self-reported bruxism activity and showed moderate to severe wear facets on their oral splint. So, we decided to consider an additional management modality, i.e., BoNT-A injection. The following exclusion criteria were applied: (1) previously received BoNT-A injection into both the masseter and temporalis muscles, (2) taking medications affecting muscle relaxation (e.g., antiepileptic drugs, benzodiazepines), (3) infectious skin lesion at the site of injection, (4) allergy to BoNT-A, (5) neuromuscular disease, and (6) pregnant females. The subjects were randomly assigned into 2 groups; Group A: 12 subjects who received bilateral BoNT-A injections into the masseter muscle only, and Group B: the other 12 who received bilateral BoNT-A injections into both the masseter and temporalis muscles. The subjects were requested not to take any other treatments or medications that would affect the muscles and not to use an oral splint during the time period of this study.
Botulinum Toxin
The BoNT-A (Neuronox, Medytox Inc., Seoul, Korea) was supplied as a freeze-dried powder of 200 U, and was reconstituted with 4 mL of sterile saline to a concentration of 5 U/0.1 mL. A dose of 25 U of BoNT-A was injected into each muscle using a 1-mL syringe with a 29-gauge, 0.5-inch needle. BoNT-A was injected into 3 sites of each subject's masseter and/ or temporalis muscles depending on the experimental group. The BoNT-A injection sites are shown in Figure 1.21–23 In the masseter muscle, the first site was the inferior, prominent part of the masseter muscle that was observed when the subject was asked to clench, and the other 2 sites formed a triangle 10 mm away from the first site (Figure 1A). In the temporalis muscle, the first site was the prominent part of the anterior temporalis muscle that was observed when the subject was asked to clench, which was parallel to eyebrow. The other 2 sites were 10 mm, 20 mm away from the first site posteriorly (Figure 1B).
Figure 1. BoNT-A injection sites.
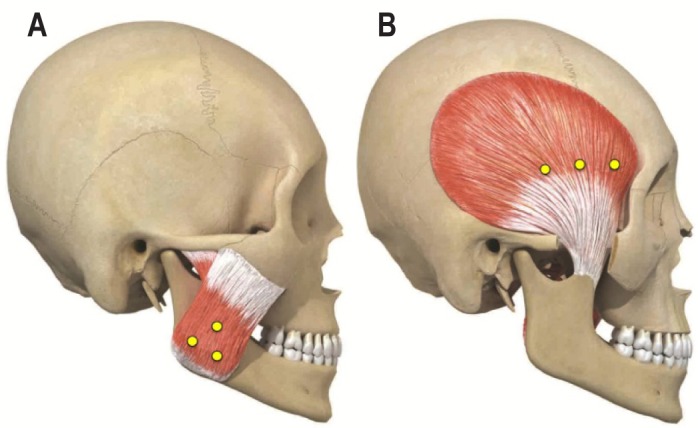
The sites of BoNT-A injections in the masseter (A) and temporalis (B) muscles. BoNT-A was injected into 3 sites of masseter and/or temporalis muscles. (A) In the masseter muscle, the 3 injection sites formed a triangle 10 mm apart with a reference of the inferior, prominent part of the masseter muscle when the subject clenched the teeth. (B) In the temporalis muscle, the 3 injection sites were arranged posteriorly, in parallel to eyebrow, from the site at the prominent part of the anterior temporalis muscle when the subject clenched the teeth.
Video-Polysomnography
All subjects were studied in the sleep laboratory for 2 nights. The first night was before BoNT-A injection (baseline PSG recording) and the second night was 4 weeks after BoNT-A injection (follow-up PSG recording).
Prior to sleep recordings, each patient completed an Epworth Sleepiness Scale questionnaire and a series of oromotor tasks to enable signal recognition and calibration of EMG amplification. Biocalibration for the masseter and temporalis muscles EMG recordings included maximal voluntary clenching (MVC), lateral jaw movement, tooth tapping, and jaw opening. The sleep recordings commenced at 22:30 (± 30 min) and ended upon the subject's spontaneous awakening or at 07:30. PSG montage included electrooculography, electroencephalography (EEG), abdominal and chest bands, pulse oximetry, and EMG recordings from the submental and bilateral tibialis anterior muscles, as well as from the bilateral masseter and temporalis muscles. Video (focused on the head and neck area) and audio recordings were made simultaneously. All signals were amplified and recorded at a sampling rate of 200 Hz and stored for off-line analysis using Twin-PSG software (Grass Technologies, West Warwick, RI, USA).
Data Analysis
Sleep Variables
Sleep stages and microstructures, apnea and hypopnea events and periodic leg movements in sleep were scored according to the standard criteria.1 The following sleep parameters were calculated: total sleep time, sleep stage, sleep efficiency, sleep latency, arousal index, frequency of awakenings per hour of sleep, apneahypopnea index, and periodic limb movement index.
Scoring Jaw and Orofacial Motor Activity (OFA)
A single observer (YJS) scored jaw and orofacial motor events under a guidance of another coauthor (TK). The observer was blinded to the subject and the experimental group to which the subject belonged. Jaw motor activity was scored based on the EMG recordings of the masseter and temporalis muscles and the audio-video recordings. Most episodes of jaw motor activity were shown in the 2 muscles simultaneously, and the types of each episode in the 2 muscles were same. Jaw motor episodes related to the diagnosis of SB were scored as rhythmic masticatory muscle activity (RMMA) that included phasic (3 EMG bursts with durations of 0.25-2.0 sec), mixed types (both phasic and tonic bursts), and isolated tonic bursts (EMG bursts lasting > 2.0 sec).24,25 These episodes were separated by intervals > 3 sec. However, scoring criteria for RMMA episodes were modified because BoNT is known to decrease the amplitude of EMG burst in the injected muscles.26 In fact, it was difficult for us to use MVC as a reference for normalizing the amplitude of EMG bursts in the injected muscles on the follow-up night. Accordingly, phasic and mixed type episodes were scored first based on the observable jaw movements in audio-video records and then confirmed with EMG traces regardless of amplitude criteria. Since it was difficult to detect the isolated tonic bursts by audio-video recordings, we used the threshold criteria for tonic episodes in the baseline and follow-up nights: EMG amplitude with 20% MVC on the baseline night was used.19,24 For RMMA episodes, the number of episodes per hour of sleep (RMMA index), bursts per hour, the number of bursts per episode, the number of episodes with sound, and the mean duration were calculated. In addition to these episodes, OFAs including lip sucking, head movement, chewing-like activity, swallowing, and eye opening were scored using audio-video.27 The number of OFAs per hour of sleep was calculated. In addition to the above counts, the peak amplitude of EMG burst of the masseter and temporalis muscles during RMMA episodes were measured by ruler on the screen and maximum peak value was determined for each episode.
Clinical Symptoms
All subjects were asked to answer questions about subjective symptoms 4 weeks after BoNT-A injection: (1) how has the teeth grinding been changed since the injection? and (2) how has the sensation of morning jaw stiffness been changed after the injection? There were 3 possible responses to the first question: decreased, increased, and no change; the second question was answered with a percentage value.
Statistics
Except where stated otherwise, data are presented as mean ± standard deviation. The Kolmogorov-Smirnov normality test was applied to our data. The data which were not normally distributed (Kolmogorov-Smirnov test: p < 0.05) underwent log-transformation to achieve close to normal distribution. Two-sample t-test and paired t-test were used to determine whether differences existed within the groups. Repeated measure ANOVA was used to determine whether differences existed between the groups. For the evaluation of sleep variables, two-sample t-test and Mann-Whitney U test were used to determine whether differences existed between the groups before injection, and paired t-test and Wilcoxon signed-rank test were used to determine whether differences existed within the groups, depending on the data distribution. The χ2 test was used to compare the differences according to sex, masticatory muscle pain, and clinically subjective symptoms between the groups. The level of statistical significance was set at p < 0.05. The SPSS statistical package (version 18.0, SPSS, Chicago, IL, USA) was used for all statistical analyses.
RESULTS
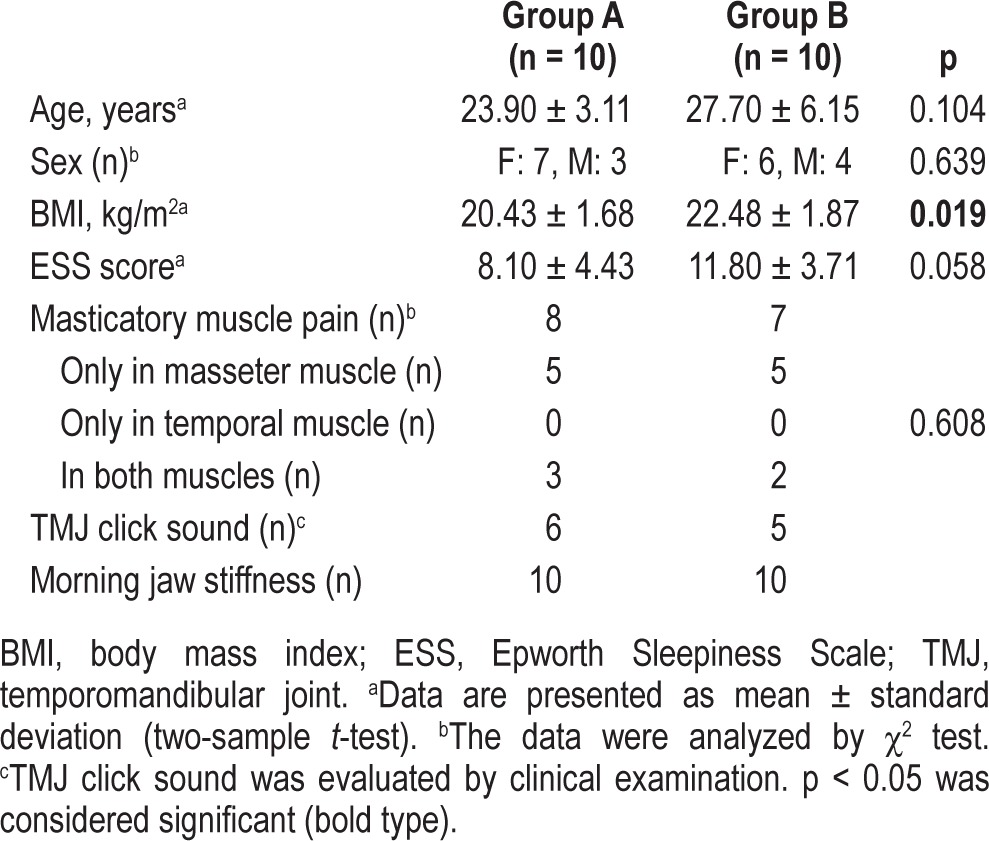
Among 24 subjects, 2 subjects dropped out because they were unavailable for a follow-up PSG recording. Two other subjects did not exhibit a RMMA at the baseline PSG recording. Thus, data of 20 subjects (25.8 ± 5.1 years; range, 20.2-38.7 years; 10 from group A and 10 from group B) were used for the analysis. The baseline characteristics of the subjects are listed in Table 1. Body mass index was the only characteristic that differed significantly between the 2 groups.
Table 1.
Characteristics of the subjects
Sleep Variables
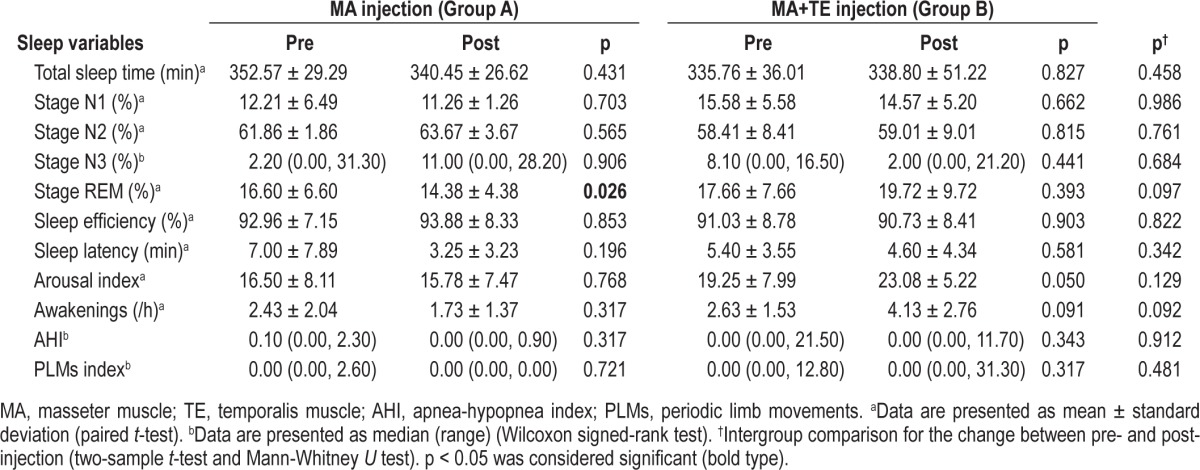
Data for the sleep variables are given in Table 2. Sleep variables did not change significantly between baseline and follow-up in the groups except for the percentage of REM sleep in group A. No group difference was noted for sleep variables (all variables were p > 0.05).
Table 2.
Sleep variables
Jaw Motor Activity Variables
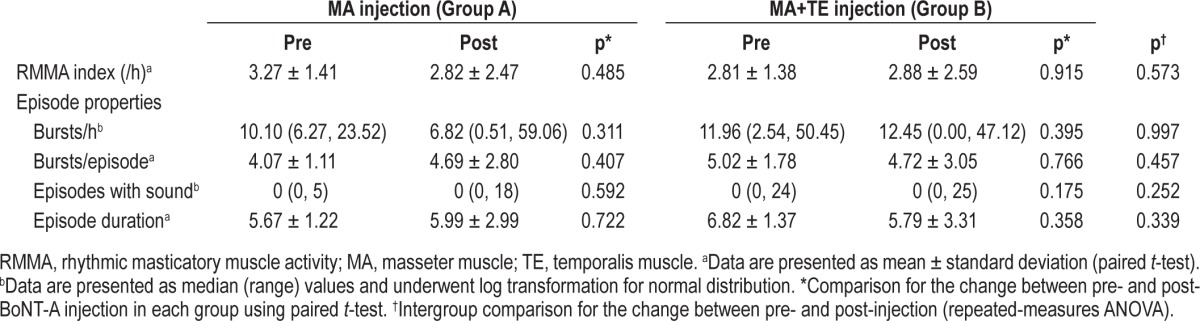
Data for the parameters of RMMA episodes are given in Table 3. None of these parameters differed significantly between groups A and B at baseline (all variables were p > 0.05). Phasic and mixed types were scored for 65.06% and 16.19% of RMMA episodes before BoNT-A injection. None of the parameters for RMMA episodes were significantly decreased after BoNT-A injection in both groups. Intergroup comparison revealed that none of the parameters differed significantly between groups A and B after injection. When both groups were pooled, RMMA index (F = 0.170, p = 0.685), bursts per hour (F = 1.891, p = 0.186), bursts per episode (F = 0.070, p = 0.795), episodes with sound (F = 0.040, p = 0.844), and mean episode duration (F = 0.266, p = 0.613) did not significantly decreased from baseline to follow-up recording.
Table 3.
Variables for RMMA episodes
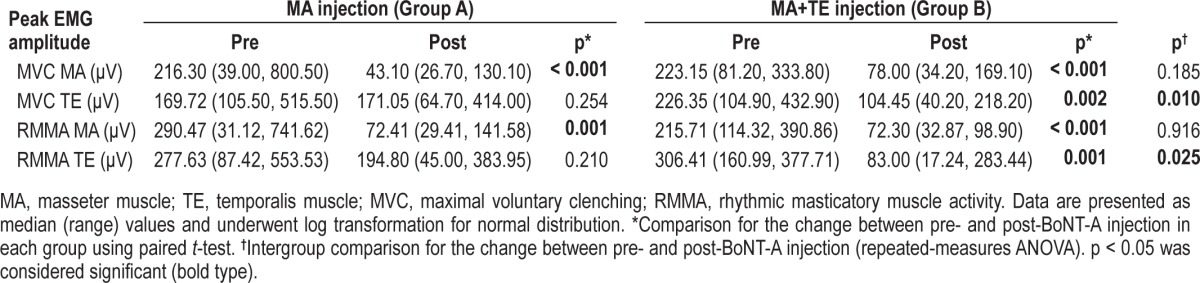
Peak Amplitude of EMG Burst
Data for the change in peak amplitude of EMG burst are given in Table 4. Before BoNT-A injection, the peak amplitude of EMG burst of the masseter and temporalis muscles during MVC and RMMA episodes did not differ significantly between the groups A and B (all variables were p > 0.05). The peak amplitude of EMG burst of BoNT-A injected muscles were significantly decreased in both groups. In group A, the amplitude of EMG bursts of the masseter muscle during MVC (MVC MA) was decreased by 66.9%, and during RMMA episodes (RMMA MA) was decreased by 61.7%, while temporalis muscle during MVC (MVC TE) and RMMA episodes (RMMA TE) did not differ before and after injection. In group B, the amplitude of EMG burst of the 2 muscles during MVC and RMMA episodes was significantly decreased after injection: the MVC MA was decreased by 56.0%, RMMA MA by 68.5%, MVC TE by 44.8%, and RMMA TE by 62.1%. Intergroup comparisons revealed that the MVC TE (F = 8.342, p = 0.010) and RMMA TE (F = 5.998, p = 0.025) differed significantly between groups A and B, whereas the other amplitude parameters did not.
Table 4.
The peak amplitude of EMG burst of the masseter and temporalis muscles during MVC tasks and during RMMA episodes
Orofacial Activity
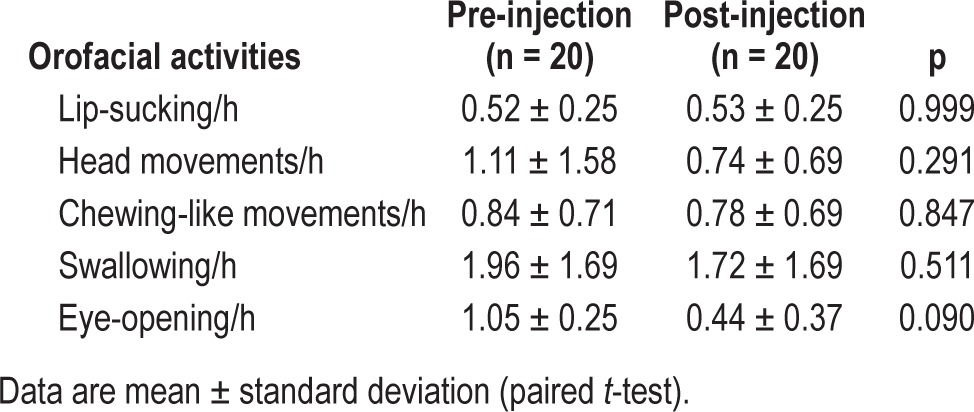
There were no significant differences in the occurrence of OFAs between baseline and follow-up recordings (Table 5).
Table 5.
Orofacial activities
Subjective Symptoms
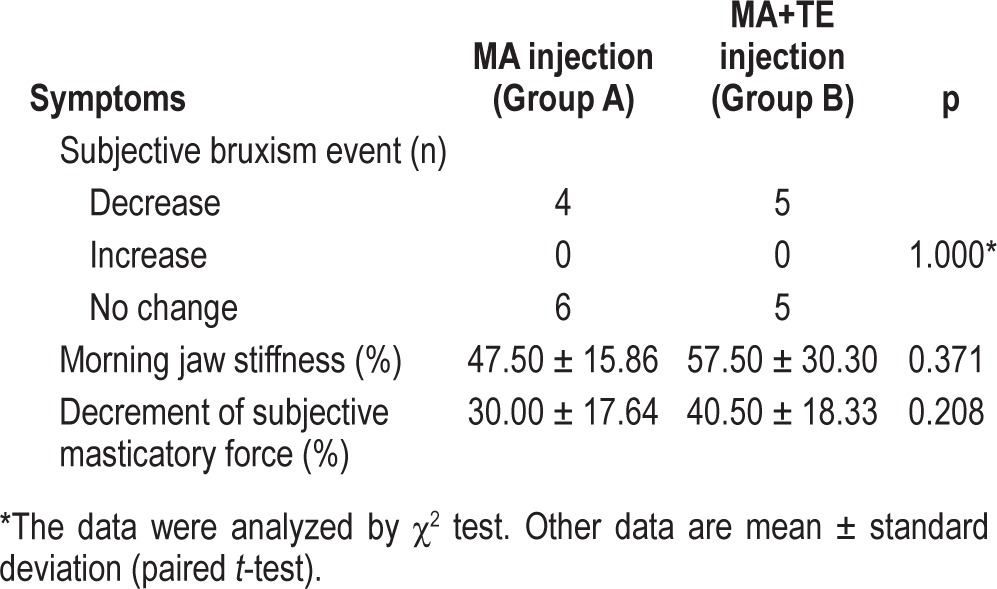
Data for the clinically subjective symptoms at a follow-up recording are given in Table 6. Four of 10 subjects in group A and 5 of 10 in group B self-reported reduction of tooth grinding during sleep. These 9 subjects with a self-reported decrease of tooth grinding had a significantly higher RMMA index (3.83 ± 1.50/h) compared to other 11 subjects who self-reported no change (2.38 ± 0.87/h; p = 0.015). The reduction in the sensation of morning jaw stiffness after BoNT-A injection was 47.50% ± 15.86% in group A and 57.50% ± 30.30% in group B. Fourteen subjects reported discomfort associated with a decrease in masticatory force after BoNT-A injection. The reduction in the sensation of masticatory force after injection was 30.00% ± 17.64% in group A and 40.50% ± 18.33% in group B. The above variables did not differ between groups A and B. Among 14 subjects who reported a decrease in masticatory force, only 3 subjects complained of masticatory difficulties.
Table 6.
Clinically subjective symptoms
DISCUSSION
This is the first study to evaluate the effect of a single BoNT-A injection on SB using vPSG. The results showed that BoNT-A injection to the masseter and temporalis muscles did not change the occurrence of RMMA episodes and OFAs. However, in both groups, the amplitude of EMG burst during RMMA episodes was significantly decreased for the muscles that received BoNT-A injection. Therefore, the findings of this present study confirm that the effect of BoNT-A on jaw motor activity during sleep is characterized by a reduction of the intensity of the contractions in the injected muscles rather than by the reduction of event occurrence.
Effects on Jaw Motor Activity during Sleep
In this study, BoNT-A injections did not decrease the RMMA episodes per hour (e.g., RMMA index). In addition, other variables for RMMA episodes, the number of bursts per hour of sleep, bursts per episode, episodes with sound, and the mean duration did not differ before and after BoNT-A injection. These results can be expected considering the pathophysiology of SB and the pharmacological actions of BoNT-A. RMMA episodes in SB have been shown to be generated by the central commands in relation to transient arousals.28,29 On the other hand, BoNT-A prevents synaptic transmissions at the neuromuscular junctions, which results in a decrease of compound muscle action potentials.27 Thus, the injections of BoNT-A on jaw-closing muscles failed to the prevent the genesis of RMMA episodes and OFAs during sleep. But, this study demonstrated the reducing effects of BoNT-A on the intensity of muscle contractions during sleep: the peak amplitude of EMG bursts during RMMA episodes decreased in the muscles with BoNT-A injection, i.e., masseter in group A and the masseter and temporalis muscles in group B.
Our results are inconsistent with those of a previous study in which the number of masticatory EMG bursts decreased by BoNT-injection.19 The discrepancy is explained by the methodological difference between our study and the previous study; in the previous study, the occurrences of EMG bursts were assessed by ambulatory EMG recording system.19 It is difficult for ambulatory EMG recordings system to detect RMMA EMG bursts with a amplitude lower than the predetermined EMG threshold after injection,20 whereas the use of vPSG in this study allowed us to avoid the underestimation of the jaw motor activity after injections. Nevertheless, the results of our study and the previous study suggest that BoNT-A injections decrease the number of intense jaw motor activities during sleep.
No significant differences were found for RMMA episodes between groups A (only masseter muscle) and B (both masseter and temporalis muscles). A difference was found only at the peak amplitude of EMG burst of the temporalis muscles during RMMA episodes. Although it is not known which muscle (temporalis or masseter) is more engaged with the act of RMMA, no significant difference was found for the peak amplitude of EMG burst during RMMA episodes before injection between the masseter and temporalis muscles (p = 0.433). There is a clinical suggestion that bilateral BoNT injections to the temporalis muscles are the first-line recommendation for the management of SB when single muscle injection is the clinical plan.30 The importance of controlling the temporalis muscle activity for managing SB needs further investigation. However, when compared with single muscle injection, injections into both muscles would have more reducing effects on total occlusal loads during RMMA episodes, as both the masseter and temporalis muscles contract synergistically to produce masticatory force.
BoNT-A Injection
In this study, the injection sites were selected based on previous studies.21–23 In the BoNT injection procedure, the most effective point of injection is the area where the appropriate nerve innervates the affected muscle. The bulky region of the masseter and temporalis muscles has been regarded as an effective injection point clinically as the richest arborized area of the perforating nerve branches.21–23 Previous studies reporting the effect of BoNT-A on bruxism used dose ranging from 15 to 60 U (equivalent to those of Neuronox).11–19 Kim et al. showed that the effects of BoNT-A on the muscular thickness and EMG changes of the masseter muscle did not differ between 25 and 35 U injections.31 Therefore, only 25 U of BoNT-A was chosen for the present study. In addition, the reducing effect on the masticatory force was maximal at 4 weeks after BoNT-A injection,31–33 and the maximum bite force gradually recovered within 12 weeks. So, we evaluated the effect of BoNT-A on RMMA episodes during sleep at the point when the maximum effect was exerted.
Effects of BoNT Injection on Sleep
There were no significant differences in usual sleep variables such as sleep efficiency, arousal index, sleep stages, or awakenings per hour between baseline and follow-up recordings. Similarly to this, oral splint treatment had no influence on the sleep variables in the previous study.9 RMMA is associated with autonomic sympathetic cardiac activity and sleep arousals characterized by increased EEG, muscle and heart activity without return to consciousness.2,8 As BoNT injection cannot modulate the sleep arousals, the only EMG amplitude of injected muscle was decreased after BoNT injection in this study. However, it is not known whether a decreased intensity of muscle contraction modifies the accompanying cardiac and cortical activities related to arousals during sleep. To clarify this, we need to quantitatively analyze the change of cortical EEG and heart rates related to RMMA episodes before and after BoNT injection in the further study.
Subjective Symptoms
Although BoNT-A reduced the intensity of muscle contractions during sleep, the same effects presented during waking oral functions as shown in the data for the peak amplitude of EMG burst during MVC. It is known that muscle weakness is a medication-related side effect. In this study, fourteen subjects reported discomfort associated with a decrease of the sensation in the masticatory force after injection; among them, three subjects complained of masticatory difficulties. This side effect was well tolerated by subjects probably because of the compensation mechanisms by other jaw muscles (e.g., medial pterygoid muscle) in jaw movements. Masticatory muscle weakness can recover gradually from 4 to 12 weeks after BoNT injection in association with the changes of masticatory force.32,33
In association with a decrease in the peak amplitude of EMG burst of RMMA episodes, the BoNT-A injection improved the subjective symptoms for morning jaw stiffness and tooth grinding during sleep. Morning jaw stiffness is commonly reported by the subjects with frequent RMMA episodes.34,35 Morning symptoms are thought to be a form of post-exercise muscle soreness caused by intense jaw muscle contractions during sleep.36 Thus the results might suggest that the subjects perceived less muscle fatigue in the morning in relation to the decreased motor activities during sleep. The most frequently cited theory is that the masticatory muscle pain and dysfunction are caused by muscle hyperactivity. But it is questionable because EMG studies of the masticatory muscles in patients with myofascial pain (MFP) do not always show an increase in resting muscle activity. So, there was an opinion that BoNT should not be used for the treatment of MFP involving the masticatory muscles.37 However, there was a report that MFP symptoms in bruxers were reduced after BoNT-A injection.18,38 Although there are insufficient systematic evaluation of the effect of BoNT-A on masticatory muscle pain and the subjective symptoms are probably overevaluated, it is helpful in relieving subjective symptoms of morning jaw stiffness in our subjects who still reported morning jaw stiffness after the oral splint treatment. Further controlled studies with placebo injections are needed to investigate whether BoNT-A injection reduces the risk of muscle pain symptoms by decreasing masseter and temporalis muscle forces during sleep with systematic evaluations of pain.
Study Limitations
There were several limitations to our study. First, most of the subjects in our study had a low frequency of RMMA episodes (e.g., mild level according to the previous publication34), although subjects who failed usual oral splint treatment for SB were selected on the clinical signs and symptoms of SB. Among 20 subjects, 15 complained of pain and fatigue of masticatory muscles, and all reported morning jaw stiffness. SB patients with low frequency of RMMA episodes were more at risk of reporting pain in the masticatory muscles.34,39 Thus, the treatment responses to BoNT-A injections may be influenced by the severity of SB and concomitant pain conditions. Second, a lack of control group (i.e., placebo injection) and systematic assessment of masticatory muscle pain at a follow-up period would obscure the effects of BoNT-A on subjective symptoms in association with PSG data. Third, the sample size in the present study was small, due to the high cost and difficulties of patient recruitment. Fourth, PSG evaluation was done for one night at baseline. There might be first-night effects on the occurrence of RMMA, and night-to-night variability of RMMA is also known.2,40 However, the influences of such factors seem to be minimal, since no major difference was found for sleep variables between the two recordings. Assessment of long-term effects also should be considered. The effects of single BoNT-A injection to the masticatory force lasted from 2 weeks to up to 24 weeks,33 and the effects started to decline 4 weeks after injection. The previous study reported that the effects of a single BoNT-A injection on jaw muscle EMG activity persisted up to 12 weeks.2 It would therefore be better for future studies to include both 2-week and longer-term follow-ups (e.g., 8, 12, and 16 weeks). At last, in this study, the BoNT-A injection did not cause a significant side effect for at least a month. However, safety of the injections needs to be assessed on long term. Future studies should address the limitations of the present study.
In summary, this study is significant in being the first to evaluate the short-term effect of BoNT-A on SB using vPSG. We confirmed that BoNT-A did not change the occurrence but reduced the intensity of contractions for the masseter and temporalis muscles during sleep. Future studies are needed before BoNT-A will be safe and effective management option for controlling jaw motor activities during sleep in patients with SB and protecting the orofacial structures from the excessive forces.
DISCLOSURE STATEMENT
This was an investigator-initiated (CLM) study fully supported by Medytox, Inc. of Korea. Medytox provided botulinum toxin (Neuronox) which was used in this clinical trial and supported a research grant of this study. All data collection, statistical analyses and manuscript writing were performed by the investigators independent of Medytox. All authors have indicated no financial conflicts of interest. This study includes off-label use of botulinum toxin type A for management of sleep bruxism.
ACKNOWLEDGMENTS
Dr. Kyoung Heo and Dr. Seong Taek Kim contributed equally to this study. The authors thank the patients who participated in this study, and all the sleep technologists at the Sleep Disorder Clinic of Severance Hospital for their help with this study. This work was performed at Department of Orofacial Pain and Oral Medicine, Yonsei University Dental Hospital, Seoul, Korea.
REFERENCES
- 1.American Academy of Sleep Medicine. Diagnostic and Coding Manual. 2nd ed. Weschester, IL: American Academy of Sleep Medicine; 2005. International Classification of Sleep Disorders. [Google Scholar]
- 2.Lavigne GJ, Manzini C, Huynh NT. Sleep bruxism. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 5th ed. St Louis, MO: Elsevier Saunders; 2011. pp. 1129–39. [Google Scholar]
- 3.Carra MC, Huynh N, Morton P, et al. Prevalence and risk factors of sleep bruxism and wake-time tooth clenching in a 7- to 17-yr-old population. Eur J Oral Sci. 2011;119:386–94. doi: 10.1111/j.1600-0722.2011.00846.x. [DOI] [PubMed] [Google Scholar]
- 4.Kato T, Velly AM, Nakane T, Masuda Y, Maki S. Age is associated with self-reported sleep bruxism, independently of tooth loss. Sleep Breath. 2012;16:1159–65. doi: 10.1007/s11325-011-0625-7. [DOI] [PubMed] [Google Scholar]
- 5.Carra MC, Huynh N, Lavigne GJ. Sleep bruxism: A comprehensive overview for the dental clinician interested in sleep medicine. Dent Clin North Am. 2012;56:387–413. doi: 10.1016/j.cden.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Okeson JP. Management of Temporomandibular Disorders and Occlusion. 7th ed. Missouri: Mosby; 2011. Etiology of functional disturbances in the masticatory system. [Google Scholar]
- 7.Clarke NG, Townsend GC, Carey SE. Bruxing patterns in man during sleep. J Oral Rehabil. 1984;11:123–7. doi: 10.1111/j.1365-2842.1984.tb00561.x. [DOI] [PubMed] [Google Scholar]
- 8.Lavigne GJ, Rompré PH, Poirier G, Huard H, Kato T, Montplaisir JY. Rhythmic masticatory muscle activity during sleep in humans. J Dent Res. 2001;80:443–8. doi: 10.1177/00220345010800020801. [DOI] [PubMed] [Google Scholar]
- 9.van der Zaag J, Lobbezoo F, Wicks DJ, Visscher CM, Hamburger HL, Naeije M. Controlled assessment of the efficacy of occlusal stabilization splints on sleep bruxism. J Orofac Pain. 2005;19:151–8. [PubMed] [Google Scholar]
- 10.Clark GT, Beemsterboer PL, Solberg WK, Rugh JD. Nocturnal electromyographic evaluation of myofascial pain dysfunction in patients undergoing occlusal splint therapy. J Am Dent Assoc. 1979;99:607–11. doi: 10.14219/jada.archive.1979.0348. [DOI] [PubMed] [Google Scholar]
- 11.Van Zandijcke M, Marchau MM. Treatment of bruxism with botulinum toxin injections. J Neurol Neurosurg Psychiatry. 1990;53:530. doi: 10.1136/jnnp.53.6.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ivanhoe CB, Lai JM, Francisco GE. Bruxism after brain injury: successful treatment with botulinum toxin-A. Arch Phys Med Rehabil. 1997;78:1272–3. doi: 10.1016/s0003-9993(97)90343-9. [DOI] [PubMed] [Google Scholar]
- 13.Pidcock FS, Wise JM, Christensen JR. Treatment of severe post-traumatic bruxism with botulinum toxin-A: case report. J Oral Maxillofac Surg. 2002;60:115–7. doi: 10.1053/joms.2002.29127. [DOI] [PubMed] [Google Scholar]
- 14.See SJ, Tan EK. Severe amphethamine-induced bruxism: treatment with botulinum toxin. Acta Neurol Scand. 2003;107:161–3. doi: 10.1034/j.1600-0404.2003.02086.x. [DOI] [PubMed] [Google Scholar]
- 15.Tan EK, Jankovic J. Treating severe bruxism with botulinum toxin. J Am Dent Assoc. 2000;131:211–16. doi: 10.14219/jada.archive.2000.0149. [DOI] [PubMed] [Google Scholar]
- 16.Guarda-Nardini L, Manfredini D, Salamone M, Salmaso L, Tonello S, Ferronato G. Efficacy of botulinum toxin in treating myofascial pain in bruxers: a controlled placebo pilot study. Cranio. 2008;26:126–35. doi: 10.1179/crn.2008.017. [DOI] [PubMed] [Google Scholar]
- 17.Bolayir G, Bolayir E, Coskun A, et al. Botulinum toxin type-A practice in bruxism cases. Neurol Psychiat Br. 2005;12:43–5. [Google Scholar]
- 18.Long H, Liao Z, Wang Y, Liao L, Lai W. Efficacy of botulinum toxins on bruxism: an evidence-based review. Int Dent J. 2012;62:1–5. doi: 10.1111/j.1875-595X.2011.00085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee SJ, McCall WD, Jr., Kim YK, Chung SC, Chung JW. Effect of botulinum toxin injection on nocturnal bruxism: a randomized controlled trial. Am J Phys Med Rehabil. 2010;89:16–23. doi: 10.1097/PHM.0b013e3181bc0c78. [DOI] [PubMed] [Google Scholar]
- 20.Kato T, Yamaguchi T, Okura K, Abe S, Lavigne GJ. Sleep less and bite more: Sleep disorders associated with occlusal loads during sleep. J Prosthodont Res. 2013;57:69–81. doi: 10.1016/j.jpor.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 21.Hu KS, Kim ST, Hur MS, Park JH, Song WC, Koh KS, Kim HJ. Topography of the masseter muscle in relation to treatment with botulinum toxin type A. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;110:167–71. doi: 10.1016/j.tripleo.2009.12.036. [DOI] [PubMed] [Google Scholar]
- 22.Ziccardi VB, Mu L, Schneider RE, Sanders I. Innervation pattern of the temporalis muscle. J Craniofac Surg. 1998;9:185–89. doi: 10.1097/00001665-199803000-00019. [DOI] [PubMed] [Google Scholar]
- 23.Hwang K, Cho HJ, Chung IH. Innervation of the temporalis muscle for selective electrical denervation. J Craniofac Surg. 2004;15:352–57. doi: 10.1097/00001665-200403000-00036. [DOI] [PubMed] [Google Scholar]
- 24.Lavine GJ, Rompré PH, Montplaisir JY. Sleep bruxism: Validity of clinical research diagnositc criteria in a controlled polysomnographic study. J Dent Res. 1996;75:546–52. doi: 10.1177/00220345960750010601. [DOI] [PubMed] [Google Scholar]
- 25.Franco L, Rompré PH, de Grandmont P, Abe S, Lavigne GJ. A mandibular advancement appliance reduces pain and rhythmic masticatory muscle activity in patients with morning headache. J Orofac Pain. 2011;25:240–9. [PubMed] [Google Scholar]
- 26.Montecucco C, Molgo J. Botulinal neurotoxins: revival of an old killer. Curr Opin Pharmacol. 2005;5:274–9. doi: 10.1016/j.coph.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 27.Dutra KM, Pereira FJ, Jr., Rompré PH, Huynh N, Fleming N, Lavigne GJ. Oro-facial activities in sleep bruxism patients and in normal subjects: a controlled polygraphic and audio-video study. J Oral Rehabil. 2009;36:86–92. doi: 10.1111/j.1365-2842.2008.01912.x. [DOI] [PubMed] [Google Scholar]
- 28.Kato T, Montplaisir JY, Guitard F, Sessle BJ, Lund JP, Lavigne GJ. 1. Evidence that experimentally induced sleep bruxism is a consequence of transient arousal. J Dent Res. 2003;82:284–8. doi: 10.1177/154405910308200408. [DOI] [PubMed] [Google Scholar]
- 29.Lavigne GJ, Kato T, Kolta A, Sessle BJ. Neurobiological mechanisms involved in sleep bruxism. Crit Rev Oral Biol Med. 2003;14:30–46. doi: 10.1177/154411130301400104. [DOI] [PubMed] [Google Scholar]
- 30.Clark GT. The management of oromandibular motor disorders and facial spasms with injections of botulinum toxin. Phys Med Rehabil Clin N Am. 2003;14:727–48. doi: 10.1016/s1047-9651(03)00044-5. [DOI] [PubMed] [Google Scholar]
- 31.Kim JH, Shin JH, Kim ST, Kim CY. Effects of two different units of botulinum toxin type a evaluated by computed tomography and electromyographic measurements of human masseter muscle. Plast Reconstr Surg. 2007;119:711–7. doi: 10.1097/01.prs.0000239453.67423.99. [DOI] [PubMed] [Google Scholar]
- 32.Kim KS, Byun YS, Kim YJ, Kim ST. Muscle weakness after repeated injection of botulinum toxin type A evaluated according to bite force measurement of human masseter muscle. Dermatol Surg. 2009;35:1902–6. doi: 10.1111/j.1524-4725.2009.01319.x. [DOI] [PubMed] [Google Scholar]
- 33.Ahn KY, Kim ST. The change of maximum bite force after botulinum toxin type a injection for treating masseteric hypertrophy. Plast Reconstr Surg. 2007;120:1662–6. doi: 10.1097/01.prs.0000282309.94147.22. [DOI] [PubMed] [Google Scholar]
- 34.Rompré PH, Daigle-Landry D, Guitard F, Montplaisir JY, Lavinge GJ. Identification of a sleep bruxism subgroup with a higher risk of pain. J Dent Res. 2007;86:837–42. doi: 10.1177/154405910708600906. [DOI] [PubMed] [Google Scholar]
- 35.Yoshizawa S, Suganuma T, Takaba M, et al. Phasic jaw motor episodes in healthy subjects with or without clinical signs and symptoms of sleep bruxism: a pilot study. Sleep Breath. 2013 Jun 18; doi: 10.1007/s11325-013-0868-6. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 36.Svensson P, Jadidi F, Arima T, Baad-Hansen L, Sessle BJ. Relationships between craniofacial pain and bruxism. J Oral Rehabil. 2008;35:524–47. doi: 10.1111/j.1365-2842.2008.01852.x. [DOI] [PubMed] [Google Scholar]
- 37.Laskin DM. Botulinum toxin A in the treatment of myofascial pain and dysfunction: the case against its use. J Oral Maxillofac Surg. 2012;70:1240–2. doi: 10.1016/j.joms.2011.05.030. [DOI] [PubMed] [Google Scholar]
- 38.Manfredini D, Winocur E, Guarda-Nardini L, Lobbezoo F. Self-reported bruxism and temporomandibular disorders: findings from two specialised centres. J Oral Rehabil. 2012;39:319–25. doi: 10.1111/j.1365-2842.2011.02281.x. [DOI] [PubMed] [Google Scholar]
- 39.Raphael KG, Sirois DA, Janal MN, et al. Sleep bruxism and myofascial temporomandibular disorders: a laboratory-based polysomnographic investigation. J Am Dent Assoc. 2012;143:1223–31. doi: 10.14219/jada.archive.2012.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lavigne GJ, Guitard F, Rompré PH, Montplaisir JY. Variability in sleep bruxism activity over time. J Sleep Res. 2001;10:237–44. doi: 10.1046/j.1365-2869.2001.00261.x. [DOI] [PubMed] [Google Scholar]


