Abstract
Study Objectives:
Previous studies have demonstrated pronounced reduction of REM sleep on the first nights following major surgery which may influence pain, analgesic use, and recovery. This placebo-controlled, randomized, double-blind study set out to evaluate the effect of zolpidem on sleep architecture in an elderly population undergoing fast-track total hip and knee arthroplasty (THA/TKA) with length of stay < 3 days.
Methods:
Twenty patients (≥ 60 years) undergoing THA or TKA in a standardized setup with spinal anesthesia and multimodal opioid-sparing postoperative analgesia were included. Polysomnography measures were performed for 2 nights, 1 night at home prior to surgery and on the first night after surgery, when the patient received placebo or zolpidem 10 mg. Analgesic use, pain levels, and subjective measures of fatigue and sleep quality were recorded. Analysis of sleep data was performed according to the American Academy of Sleep Medicine manual.
Results:
Objective sleep data did not show a significant difference between groups in any of the sleep stages. However, subjective data on sleep quality and fatigue showed significantly less fatigue and better sleep quality in the zolpidem group (p < 0.05), and reduced objectively recorded number of arousals (p = 0.004). Levels of pain and opioid use were similar in the 2 groups.
Conclusions:
Our objective data did not support the primary hypothesis that one night's treatment with zolpidem would significantly improve sleep architecture following major surgery, although there was improved feeling of sleep quality and fatigue associated with fewer postoperative arousals.
Citation:
Krenk L; Jennum P; Kehlet H. Postoperative sleep disturbances after zolpidem treatment in fast-track hip and knee replacement. J Clin Sleep Med 2014;10(3):321-326.
Keywords: Postoperative sleep, cognition, sleep deprivation, recovery, zolpidem
Following major surgery most patients experience a pronounced change in their sleep architecture and sleep quality.1–3 Typically, the amount of deep sleep (slow wave sleep [SWS]) and REM sleep is markedly reduced or even lacking immediately after surgery .3,4
Sleep in the postoperative period is affected by many factors, and postoperative pain has a major negative impact.5,6 In addition an intricate relationship exists between sleep, opioids, and pain, where reduced sleep can lead to hyperalgesia and increased opioid demand, and opioid per se has a negative impact on sleep architectur e.7–10 Finally, postoperative sleep disturbances may contribute to delirium and cognitive dysfuncti on.11,12
Since previous studies have shown that sleep, especially REM sleep, is severely disturbed immediately after major surge ry,1–4 we hypothesized that a short-acting hypnotic might play a role in preserving sleep architecture immediately after surgery,13–15 and possibly affect pain and overall well-being following major joint replacement surgery in an elderly population. This randomized, placebo-controlled, clinical trial set out to evaluate the effect of zolpidem treatment on sleep the first night following major arthroplasty surgery in elderly patients using polysomnographic recordings. The primary endpoint was change in REM sleep duration on the first postoperative night compared to preoperative measures in a paired setup.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Immediately postoperatively sleep is severely reduced possibly compromising recovery. The study was performed to clarify a possible role of a short-acting hypnotic medicine in the treatment of postoperative sleep.
Study Impact: The results did not confirm the primary hypothesis since Zolpidem did not show a significant impact on objective sleep measures. As such, the study results cannot fully support an implementation of hypnotics in the standardized fast-track setup to further improve recovery.
METHODS
This study was carried out as a double-blind randomized, placebo-controlled, clinical trial with 20 patients. The study was approved by the regional Ethics Committee (reg. no. H-3-2011-022), and the patients gave written and oral informed consent prior to participating. The study was registered on ClinicalTrial.gov (ID NCT01551485) and also approved by the Danish Medicines Agency (EUDRACT No. 2011-000698-30). The study was conducted according to Good Clinical Practice (GCP) guidelines and monitored accordingly during the study period.
Inclusion began on February 27, 2012, and was concluded on August 2, 2012. We studied 20 patients, all ≥ 60 years, undergoing either total hip (THA) or total knee arthroplasty (TKA) in a standardized fast-track setup with well-defined discharge criteria and estimated length of stay (LOS) less than 3 days.3,12 Exclusion criteria were neurological disease including Parkinson disease, use of sedatives or hypnotics, alcohol abuse (> 35 units per week), inability to comply with polysomnographic measurements, and known allergy to zolpidem. Patients were included according to operation date, as there was equipment for only 2 sleep studies per night. Furthermore, home address more than 40 km from the hospital also excluded patients, due to the logistics of the preoperative measures performed in their own home.
All patients received spinal anesthesia with local anesthetic with no opioid added. On request, supplemental propofol was administered during surgery, but all patients maintained unassisted secure airways during the procedure. All patients received standardized opioid-sparing postoperative pain management, with oral celecoxib 200 mg and slow-releasing acetaminophen 2 g twice daily and gabapentin 300 mg in the morning and 600 mg in the evening. This regime started on the morning of surgery, and no opioid or benzodiazepines were administered on the morning of surgery. The standard regime was combined with tramadol 50 mg or ketobemidone 5-10 mg as supplemental analgesia on request up to 6 times daily. All medication administered during hospitalization was registered. Patients were discharged to their own homes when they fulfilled the standardized discharge criteria.16
Sleep monitoring was performed for 1 night preoperatively in the patient's own home at least 3 days prior to surgery. Electrode placement was performed by the primary investigator (LK). The patients were then instructed to follow their usual evening and nighttime routines and slept in their usual environment without the investigator present. Sleep monitoring was also performed on the first night postoperatively in the hospital; electrodes were placed when the patient returned to the ward after surgery.
Sleep monitoring was performed by Trackit Ambulatory EEG (Lifelines Ltd, UK) continuously recording EEG (F3, F4 and A1, A2 from the frontal and mastoid regions, respectively), submental electromyogram (EMG), electrooculography ([EOG] recorded from the left upper eyelid and just below the right oculomotorius muscle), and 3-lead electrocardiography (ECG). Sleep stage analysis was performed according to the American Academy of Sleep Medicine Manual for the Scoring of Sleep and Associated Events.17 Total sleep time (TST) was defined as minutes spent asleep during the night; sleep period time (SPT) was defined as minutes from initial sleep onset until final morning awakening; and awake time (AT) was defined as time awake during the night after initial sleep onset. Sleep evaluation was performed by an experienced polysomnographic technician (trained neurophysiologist) and supervised by PJ, both of whom were blinded to the treatment regime.
Patients were asked to fill out lights-out (time they went to bed) and lights-on (time of awakening in the morning), as well as score their level of fatigue and subjective sleep quality on a numeric scale (0 = no fatigue/fantastic sleep and 10 = total exhaustion/very poor sleep). In addition, all patients filled in pain scores on a visual analogue scoring scale (VAS) in the morning at rest and as recall of pain level during the night. All medication was registered preoperatively and during hospitalization. Throughout the monitoring period, patients were not allowed to receive any sedatives or hypnotics except propofol during the surgical procedure.
Study medication consisted of oral zolpidem 10 mg blinded in a gelatinous capsule or placebo (a gelatinous capsule without an active ingredient). The capsule was administered about 1 h prior to lights-out (around 22:00), and nurses in the ward verified that the medication had been taken properly. All study personnel and patients were blinded until the conclusion of the study and data handling had been completed. Randomization was computer generated by the pharmacy that also supplied the blinded study medication and supplied a randomization key in sealed envelopes. The pharmacy had no other role in the study.
Estimated sample size for the primary endpoint—the difference in REM duration from preoperative to postoperative measurements—was calculated based on normative material for sleep stages and total sleep time. A previous study in a comparable patient population showed a reduction of REM sleep > 90% postoperatively compared to preoperative levels; REM sleep typically takes up about 20% of total sleep time before surgery.3
Our hypothesis was that the group receiving active treatment (zolpidem 10 mg) would have more REM sleep than the placebo group postoperatively and estimated the difference had to be > 50% to be clinically relevant. With a power of 0.80 and a 2-sided significance level of 5%, we estimated that 10 subjects needed to be evaluated in each group.
For statistical analysis of sleep measures, Student t-test was used, and the Wilcoxon rank sum test was used to compare the subjective data on sleep and fatigue.
RESULTS
Thirty-four patients were approached to participate in the study; 12 declined and 22 patients were enrolled in the study. The patients who did not wish to participate stated 2 main reasons: lack of energy to participate in a study was the most common, and a few did not wish to take extra medication. Of the 22 enrolled in the study, 2 patients were excluded during the study period, one due to technical failure of the sleep equipment on the preoperative night and the second due to failure of the sleep equipment during postoperative measurement. The patient excluded after the postoperative night had already been given study medication when the problem was discovered, but the blinding was not broken at this stage since no adverse effects had occurred.
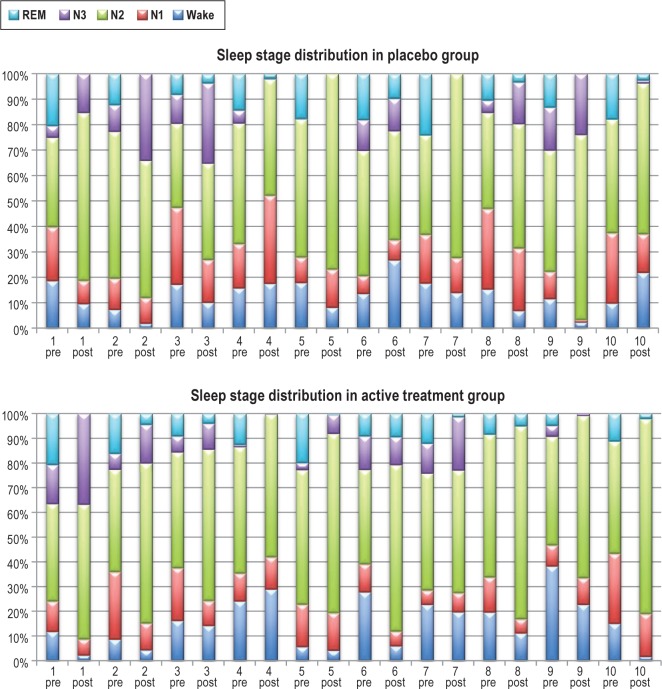
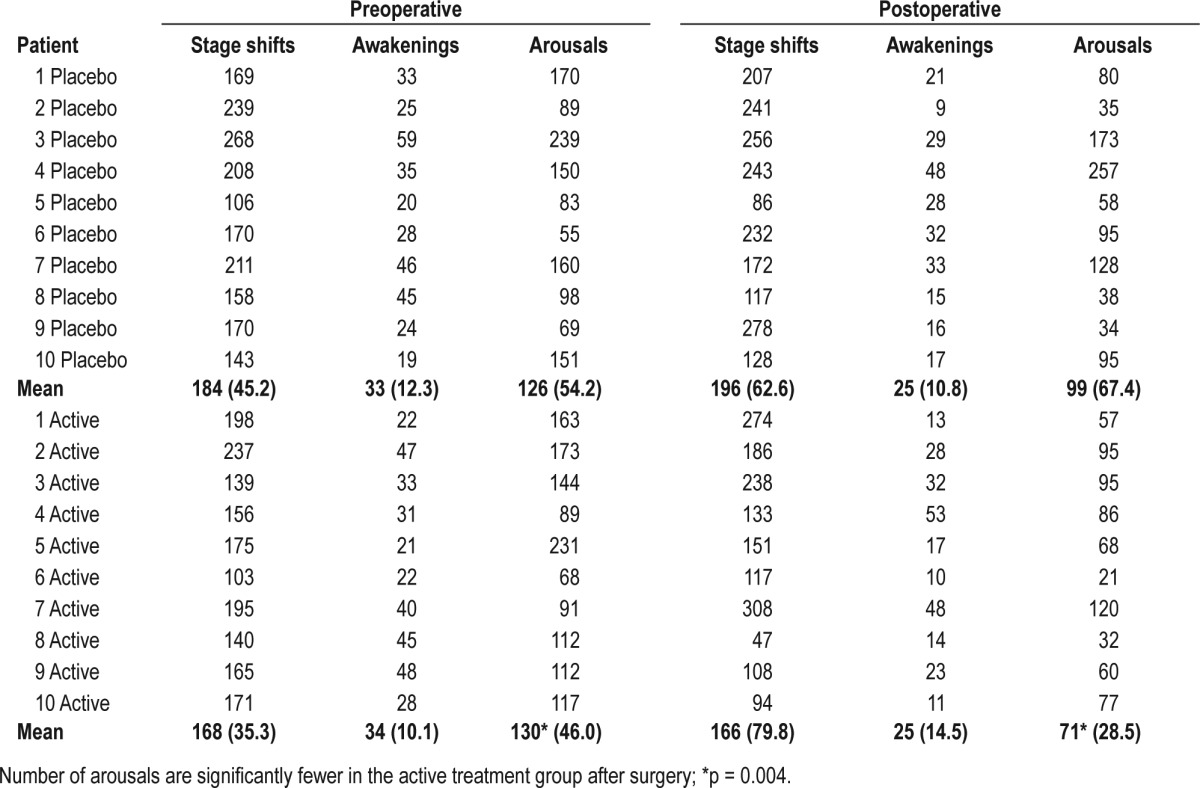
Patient characteristics are illustrated in Table 1. Sleep data for the 2 groups (zolpidem treatment and placebo) are presented in Figure 1 and Tables 2A, 2B. There was a marked tendency towards lighter sleep stages postoperatively, and REM sleep was markedly diminished in both groups at the postoperative measurement but without difference between groups (p = 0.3). This was true for all sleep stages, awake time, and total sleep time, as well as the number of sleep stage shifts. However, there were significantly fewer arousals in the group receiving zolpidem on their postoperative night compared to preoperative levels (p = 0.004) than in the placebo group, (99 arousals in the placebo group and 71 arousals in the zolpidem group).
Table 1.
Demographic data on patients in the zolpidem and placebo groups
Figure 1. Sleep stage distribution in the 2 groups.
In the diagram each patient is illustrated by a preoperative and postoperative column. N1, stage 1 sleep; N2, stage 2 sleep; N3, stage 3 sleep; REM, rapid eye movement sleep.
Table 2A.
Sleep duration for the 2 groups on the preoperative and postoperative nights
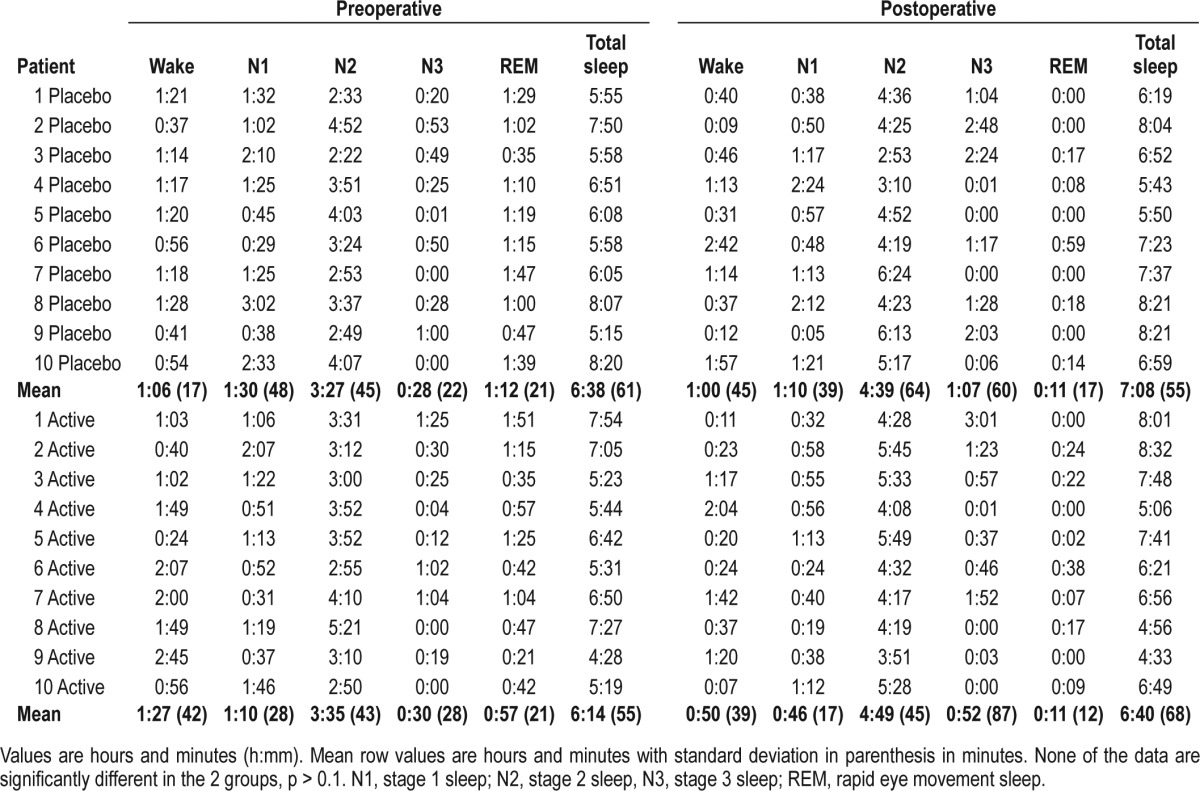
Table 2B.
Stage shifts, arousals, and awakenings for preoperative and postoperative measures, given as mean (SD)
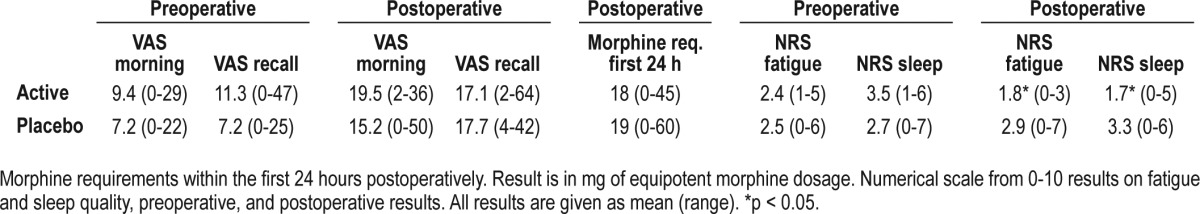
The pain data showed no statistical differences between the groups, as was also the case in use of pain medication (Table 3). The subjective data on fatigue and sleep quality showed less fatigue and a better sleep quality in the zolpidem group than the placebo group (p < 0.05; Table 3).
Table 3.
Visual analogue scoring ([VAS] 0-100 mm) of pain in the morning and as a recall of the nighttime: preoperative, and postoperative results
DISCUSSION
This study aimed to illustrate whether a single night's treatment with zolpidem 10 mg could improve sleep architecture in elderly patients on the first night following major joint replacement surgery. Previous studies have shown that deep sleep and REM sleep are severely reduced immediately after major surgery.1,3,4 However, our objective measures of sleep by polysomnography did not show any effect of zolpidem on sleep duration or distribution in this randomized double-blind, placebo-controlled trial, although there were fewer arousals and better subjective reports of fatigue and sleep quality in the group receiving zolpidem.
There are some limitations to our study. We studied a rather small group—only 10 patients in each group—and the duration of treatment was only a single night. Furthermore, it could be argued that our preoperative measure, which was also only one night, would be affected by the “first-night” effect. Studies in favor of discarding the first night of measurement due to the first-night effect have found anxiety and change in environment to be the two most important factors affecting sleep measures.18,19 However, our set-up took this into account, since the preoperative sleep monitoring was performed in the patient's home and at least three nights prior to surgery to lessen the influence of nervousness and agitation immediately prior to major surgery and hospitalization.3,18,20 We therefore believe our data are valid in that regard and correspond to the changes found previously without use of hypnotics.3
We sought to improve sleep in the immediate postoperative period because a previous study by our group showed very poor sleep on the first night after this type of surgery,3 and poor sleep have many unwanted effects, such as loss of energy, hyperalgesia, and mood instability. Furthermore, an especially important aspect in elderly patients is the detrimental effect on cognition by sleep deprivation.5,6,9,11,21–23
We also aimed to evaluate the patients' well-being and level of fatigue to illustrate their energy levels, since active participation in physical rehabilitation and training is a cornerstone in the fast-track setup with length of stay less than 3 days. Though we cannot explain the significantly better scores on fatigue and sleep quality in the zolpidem group by any of our objective findings from the PSG data on sleep duration, it does correlate well with the fact that the zolpidem group also experienced significantly fewer arousals on the postoperative night. This probably due to zolpidem being a GABA-A agonist, which may generally reduce arousals,24 likely due to a suppression of the reticular activating system. The effect seen on subjective sleep quality and fatigue measures, as well as number of arousals, may also be the result of the ability of the hypnotic to change the threshold for arousal. In a previous study of similar patients, the most common reason given for waking up at night after surgery was the hassle of changing position with the cumbersome newly operated limb.3 Thus, a higher threshold for arousal when the hypnotic was administered may explain the reduction in number of arousals in the zolpidem group. However, we did not find a difference in either pain or use of opioids for the first 24 hours after surgery to explain the differences in arousals and fatigue, despite the fact that pain, inflammation, and opioids may all affect sleep in a negative way.6–8,10,22 Although we used a multimodal opioid-sparing analgesic regimen, the patients received morphine (< 20 mg/24h), which may have influenced sleep.8 Total sleep time and distribution of sleep stages were not modified by zolpidem after surgery.
It may be argued that Gabapentin modified sleep disturbances, but Gabapentin was used in both groups, and apparently did not modify the usual postoperative sleep disturbances,1,2 and also apparent after fast-track THA and TKA.3
In conclusion, this study showed no improvement in postoperative REM or SWS with zolpidem on the first night after hip and knee replacement, although there was improved subjective sleep quality and less fatigue. Consequently, our study does not support a routine use of zolpidem in the postoperative period for improvement of sleep. Future studies are required to elucidate the pathogenesis and preventive measures for the pronounced postoperative sleep disturbances in order to enhance recovery. Such studies should focus on pain, opioid use, and the inflammatory response, all of which may be favorably reduced by glucocorticoids.25
DISCLOSURE STATEMENT
The study was funded by the Lundbeck Foundation with a research grant. The authors have indicated no financial conflicts of interest. The work was performed at Gentofte Hospital, Niels Andersens Vej, 2900 Hellerup, Denmark. Analysis was performed at Glostrup Hospital at the Danish Centre for Sleep Medicine, Denmark.
REFERENCES
- 1.Rosenberg-Adamsen S, Kehlet H, Dodds C, Rosenberg J. Postoperative sleep disturbances: mechanisms and clinical implications. Br J Anaesth. 1996;76:552–9. doi: 10.1093/bja/76.4.552. [DOI] [PubMed] [Google Scholar]
- 2.Gogenur I, Wildschiotz G, Rosenberg J. Circadian distribution of sleep phases after major abdominal surgery. Br J Anaesth. 2008;100:45–9. doi: 10.1093/bja/aem340. [DOI] [PubMed] [Google Scholar]
- 3.Krenk L, Jennum P, Kehlet H. Sleep disturbances after fast-track hip and knee arthroplasty. Br J Anaesth. 2012;109:769–75. doi: 10.1093/bja/aes252. [DOI] [PubMed] [Google Scholar]
- 4.Rosenberg J. Sleep disturbances after non-cardiac surgery. Sleep Med Rev. 2001;5:129–37. doi: 10.1053/smrv.2000.0121. [DOI] [PubMed] [Google Scholar]
- 5.Lavigne GJ, McMillan D, Zucconi M. Pain and sleep. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. Philadelphia: Elsevier Saunders; 2005. pp. 1246–55. [Google Scholar]
- 6.Onen SH, Onen F, Courpron P, Dubray C. How pain and analgesics disturb sleep. Clin J Pain. 2005;21:422–31. doi: 10.1097/01.ajp.0000129757.31856.f7. [DOI] [PubMed] [Google Scholar]
- 7.Lavigne GJ. Effect of sleep restriction on pain perception: towards greater attention! Pain. 2010;148:6–7. doi: 10.1016/j.pain.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 8.Moore JT, Kelz MB. Opiates, sleep, and pain: the adenosinergic link. Anesthesiology. 2009;111:1175–6. doi: 10.1097/ALN.0b013e3181bdfa2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Obermeyer WH, Benca RM. Effects of drugs on sleep. Otolaryngol Clin North Am. 1999;32:289–302. doi: 10.1016/s0030-6665(05)70131-6. [DOI] [PubMed] [Google Scholar]
- 10.Cronin AJ, Keifer JC, Davies MF, King TS, Bixler EO. Postoperative sleep disturbance: influences of opioids and pain in humans. Sleep. 2001;24:39–44. doi: 10.1093/sleep/24.1.39. [DOI] [PubMed] [Google Scholar]
- 11.Alhola P, Polo-Kantola P. Sleep deprivation: Impact on cognitive performance. Neuropsychiatr Dis Treat. 2007;3:553–67. [PMC free article] [PubMed] [Google Scholar]
- 12.Krenk L, Rasmussen LS, Hansen TB, Bogo S, Soballe K, Kehlet H. Delirium after fast-track hip and knee arthroplasty. Br J Anaesth. 2012;108:607–11. doi: 10.1093/bja/aer493. [DOI] [PubMed] [Google Scholar]
- 13.Copinschi G, Akseki E, Moreno-Reyes R, et al. Effects of bedtime administration of zolpidem on circadian and sleep-related hormonal profiles in normal women. Sleep. 1995;18:417–24. doi: 10.1093/sleep/18.6.417. [DOI] [PubMed] [Google Scholar]
- 14.Wagner J, Wagner ML. Non-benzodiazepines for the treatment of insomnia. Sleep Med Rev. 2000;4:551–81. doi: 10.1053/smrv.2000.0126. [DOI] [PubMed] [Google Scholar]
- 15.Monti JM. Effect of zolpidem on sleep in insomniac patients. Eur J Clin Pharmacol. 1989;36:461–6. doi: 10.1007/BF00558070. [DOI] [PubMed] [Google Scholar]
- 16.Husted H, Solgaard S, Hansen TB, Soballe K, Kehlet H. Care principles at four fast-track arthroplasty departments in Denmark. Dan Med Bull. 2010;57:A4166. [PubMed] [Google Scholar]
- 17.Iber C, Ancoli-Israel S, Chesson A, Quan SF. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. [Google Scholar]
- 18.Riedel BW, Winfield CF, Lichstein KL. First night effect and reverse first night effect in older adults with primary insomnia: does anxiety play a role? Sleep Med. 2001;2:125–33. doi: 10.1016/s1389-9457(00)00054-x. [DOI] [PubMed] [Google Scholar]
- 19.Carskadon MA, Dement WC, Mitler MM, Guilleminault C, Zarcone VP, Spiegel R. Self-reports versus sleep laboratory findings in 122 drug-free subjects with complaints of chronic insomnia. Am J Psychiatry. 1976;133:1382–8. doi: 10.1176/ajp.133.12.1382. [DOI] [PubMed] [Google Scholar]
- 20.Edinger JD, Fins AI, Sullivan RJ, Jr., et al. Sleep in the laboratory and sleep at home: comparisons of older insomniacs and normal sleepers. Sleep. 1997;20:1119–26. doi: 10.1093/sleep/20.12.1119. [DOI] [PubMed] [Google Scholar]
- 21.Shapiro CM, Flanigan MJ. ABC of sleep disorders. Function of sleep. BMJ. 1993;306:383–5. doi: 10.1136/bmj.306.6874.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Closs SJ. Patients' night-time pain, analgesic provision and sleep after surgery. Int J Nurs Stud. 1992;29:381–92. doi: 10.1016/0020-7489(92)90016-a. [DOI] [PubMed] [Google Scholar]
- 23.Krenk L, Jennum P, Kehlet H. Activity, sleep and cognition after fast-track hip or knee arthroplasty. J Arthroplasty. 2013;28:1265–9. doi: 10.1016/j.arth.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 24.Parrino L, Smerieri A, Giglia F, Milioli G, De PF, Terzano MG. Polysomnographic study of intermittent zolpidem treatment in primary sleep maintenance insomnia. Clin Neuropharmacol. 2008;31:40–50. doi: 10.1097/wnf.0b013e3180674e0e. [DOI] [PubMed] [Google Scholar]
- 25.Lunn TH, Kehlet H. Perioperative glucocorticoids in hip and knee surgery -benefit vs. harm? A review of randomized clinical trials. Acta Anaesthesiol Scand. 2013;57:823–34. doi: 10.1111/aas.12115. [DOI] [PubMed] [Google Scholar]