Summary
Equal chromosome segregation requires proper assembly of many proteins, including Bub3, onto kinetochores to promote kinetochore-microtubule interactions. By screening for mitotic regulators in the Spindle envelope and matrix (Spemix), we identify a conserved Bub3 interacting and GLEBS containing ZNF207 (BuGZ) that associates with spindle microtubules and regulates chromosome alignment. Using its conserved GLE-2-Binding Sequence (GLEBS), BuGZ directly binds and stabilizes Bub3. BuGZ also uses its microtubule-binding domain to enhance the loading of Bub3 to kinetochores that have assumed initial interactions with microtubules in prometaphase. This enhanced Bub3 loading is required for proper chromosome alignment and metaphase to anaphase progression. Interestingly, we show that microtubules are required for the highest kinetochore loading of Bub3, BubR1, and CENP-E during prometaphase. These findings suggest that BuGZ not only serves as a molecular chaperone for Bub3 but also enhances its loading onto kinetochores during prometaphase in a microtubule-dependent manner to promote chromosome alignment.
Keywords: Mitosis, microtubules, spindle envelope and matrix (Spemix), kinetochore, Bub3, BuGZ, ZFP207, ZNF207
Introduction
The kinetochores, assembled from over 90 proteins, are essential in mediating the establishment and detection of proper microtubule (MT)-kinetochore interactions, chromosome alignment, and equal chromosome segregation (Akiyoshi and Biggins, 2012; Foley and Kapoor, 2013; Tanaka, 2013). To establish proper MT-kinetochore interaction, MTs must encounter kinetochores and be ‘captured’ by the kinetochore-localized MT-capture machinery (Elowe, 2011; Johnson et al., 2004; Lo et al., 2007; Tanaka, 2013). Kinetochores can promote MT growth, which would allow more efficient interactions between MTs and kinetochores (Kitamura et al., 2010; Mishra et al., 2010). Kinetochore-bound spindle assembly checkpoint (SAC) proteins such as Mad1, Mad2, Bub1, BubR1, Bub3, and Mps1 generate the wait (or SAC) signal to give cells the time to align all chromosomes to the metaphase plate for equal chromosome segregation (Fang and Zhang, 2011; Kim and Yu, 2011; Musacchio, 2011; Musacchio and Salmon, 2007). Upon proper alignment of all chromosomes, the SAC signal has to be silenced for metaphase to anaphase transition. Thus the machinery involved in MT-kinetochore interaction must be linked to the SAC proteins to sense the status of chromosome alignment. Indeed, Bub3, BubR1, and Bub1 are part of the kinetochore MT-capture machinery required for proper MT-kinetochore interactions and chromosome alignment (Elowe, 2011; Elowe et al., 2007; Guo et al., 2012; Huang et al., 2008; Lampson and Kapoor, 2005; Logarinho et al., 2008; Meraldi and Sorger, 2005; Windecker et al., 2009).
Despite extensive analyses of proteins required for the kinetochore capture of MTs, how the capture machinery is assembled onto kinetochores remains poorly understood. Models that explain the assembly have largely focused on how the kinetochore proteins interact with one another for their proper kinetochore binding. Although the regulation of dynamic MT assembly is critical for proper kinetochore capture, MTs have not been considered to promote the assembly of their own capture machinery. One reason that MTs are thought not to be involved in this task is that proteins such as Bub3 and BubR1 exhibit several fold reduction at the aligned kinetochores in metaphase. Since such reduction can be reversed by nocodazole-induced MT depolymerization (Howell et al., 2004; Shah et al., 2004), MTs seem to negatively regulate the loading of at least some components of the kinetochore MT-capture machinery.
The effect of MTs on the assembly of the capture machinery during prometaphase, however, has not been explored. Upon nuclear envelope breakdown (NEBD), proteins such as Bub3 and BubR1 exhibit increased loading onto kinetochores (Howell et al., 2004). It is thus conceivable that MTs could promote the kinetochore loading of some MT capture proteins. It is important to explore whether MTs promote the assembly of the kinetochore MT-capture complexes during prometaphase because it could help to decipher the mechanism by which efficient chromosome alignment is achieved during prometaphase.
To understand how proteins involved in MT capture assemble onto kinetochores and whether MTs facilitate their loading, it may be necessary to take an approach different from those frequently used for mitosis studies. Indeed, large-scale RNA interference (RNAi) screens for mitotic regulators have not revealed obvious candidate regulators that could function with MTs to promote kinetochore assembly of the MT-capture machinery, possibly due to the limitations of the strategy. Additionally, since such regulators might only weakly and transiently interact with kinetochores or spindles, studies focusing on the resident kinetochore proteins or canonical spindle assembly factors that bind to MTs tightly would not lead to their identification. Previously, we have isolated a lamin-B-containing membranous network, which we refer to here as the Spindle envelope and matrix (Spemix) because the network appears to both ensheath and permeate the spindle MTs (Goodman et al., 2010; Ma et al., 2009; Tsai et al., 2006; Zheng, 2010). Since a number of known mitotic regulators, including several SAC proteins, are present in the Spemix based on our proteomic analyses (Ma et al., 2009; Zheng, 2010), the Spemix may contain new regulators of SAC proteins. Through studying the Spemix components, we report a MT-associated Zinc finger protein that regulates Bub3 stability and kinetochore targeting using its GLE-2-Binding Sequence (GLEBS) motif and MT-binding domain, respectively. Our results also imply a role of MTs in promoting their own capture by kinetochores.
Results
BuGZ is a Spemix component that binds along spindle MTs and regulates mitosis
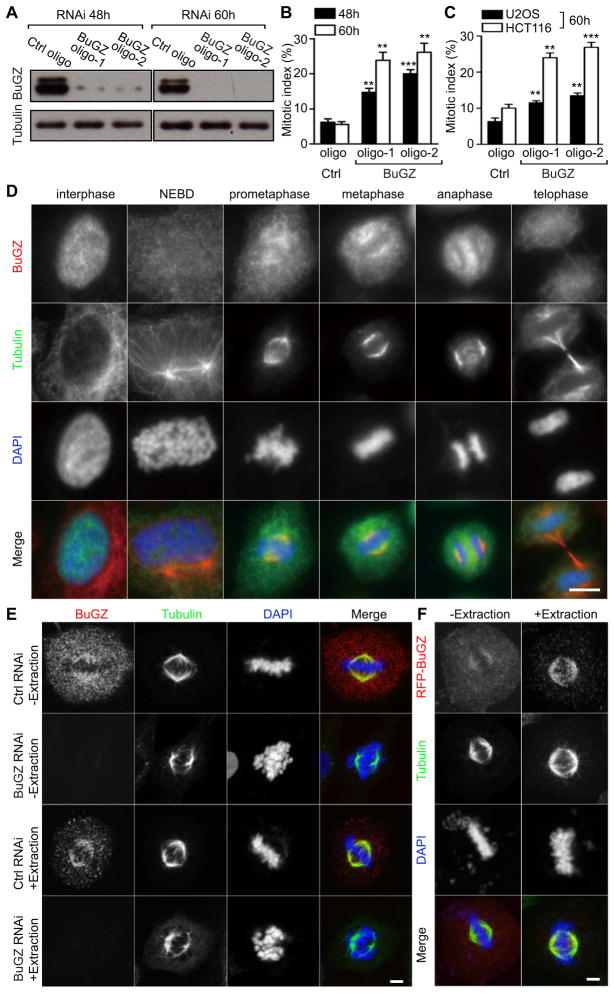
In a pilot RNA-interference (RNAi) screen of the Spemix proteome using mouse embryonic stem cells (mESCs), we found that the knockdown of a poorly characterized nuclear Zinc Finger Protein, ZFP207 or ZNF207, resulted in an increase in the time mESCs spent between NEBD and metaphase-anaphase transition (Figure S1A) followed by cell death (not shown). We named this protein as BuGZ (Bub3 interacting and GLEBS motif containing ZNF207). Similar to lamin-B, BuGZ was present in the isolated Xenopus Spemix (Figure S1B) (Tsai et al., 2003; Tsai and Zheng, 2005). We confirmed the mitotic arrest phenotype by measuring mitotic index and duration upon RNAi in several human cell lines, including HeLa, U2OS, and HCT116 (Figure 1A–C, S1C–E, and Supplementary movies 1 and 2). For example, BuGZ depletion in HeLa cells resulted in prolonged arrest in prometaphase followed by death in most cells (see Figure S1D and E).
Figure 1. BuGZ is a spindle-associated protein critical for mitosis.
A. Two different BuGZ siRNA oligos reduced BuGZ levels in HeLa cells 48 h and 60 h post transfection. Loading controls, tubulin.
B and C. Reduction of BuGZ by RNAi caused a significant increase in the mitotic index in HeLa cells (B), U2OS and HCT116 cells (C). For quantifications, ~500 cells were counted for each experiment and condition. Error bars, standard error of the mean (SEM). Student’s t-test: **p<0.01, ***p<0.001 from triplicates.
D. Epifluorescence imaging of endogenous BuGZ localization in HeLa cells during the cell cycle. Scale bar, 10 μm.
E. Confocal imaging of endogenous BuGZ localization in mitotic HeLa cells treated by control or BuGZ RNAi with or without detergent extraction. Scale bar, 5 μm.
F. RFP-BuGZ localization in HeLa cells with or without extraction as visualized by confocal microscopy. Scale bar, 5 μm.
Using a specific antibody (Figure S1F), we found BuGZ to be concentrated in the nucleus and, at NEBD, the nuclear BuGZ diffused throughout HeLa cells (Figure 1D). During prometaphase through anaphase, a discernable enrichment of BuGZ was observed by epifluorescence microscopy in the spindle region (Figure 1D). A similar localization pattern was observed for BuGZ in U2OS and HCT116 cells (not shown). The Flag-tagged BuGZ (Flag-BuGZ), but not Flag-luciferase, was also enriched in the spindle region as revealed by epifluorescence microscopy (Figure S1G). Using confocal microscopy, we found that a fraction of BuGZ to be localized along spindle MTs and the spindle localization appeared clearer when cells were subjected to a brief detergent extraction under the MT-stabilizing condition (Figure 1E and see the Experimental procedures). The specificity of BuGZ immunostaining was confirmed by BuGZ RNAi, which removed both the cytosolic and spindle-associated BuGZ signals (Figure 1E). The cytosolic and spindle localizations of BuGZ were further confirmed by the localization of RFP-BuGZ in HeLa cells with or without detergent extraction (Figure 1F). The large pool of the cytosolic BuGZ suggests that the protein binds to spindle MTs weakly, which could explain its absence in previously reported proteomes of spindle or MT-associated protein (Gache et al., 2010; Sauer et al., 2005; Torres et al., 2011).
BuGZ regulates kinetochore-MT interaction and chromosome alignment
To further understand why BuGZ RNAi resulted in mitotic block, we asked whether BuGZ reduction caused defects in chromosome alignments since chromosome misalignment can trigger SAC and mitotic block (Rieder et al., 1995). We incubated the siRNA-treated cells with the proteasome inhibitor MG132 for 1.5 h to block metaphase to anaphase transition and then quantified the percentage of misaligned chromosomes. Compared to controls, BuGZ depletion caused a significant increase of mitotic cells with misaligned chromosomes in HeLa, HCT116, and U2OS cells (Figure 2A, S2A and B). To rule out the off-target effects, we generated HeLa cells expressing siRNA-insensitive GFP-BuGZ and found a significant rescue of chromosome misalignment caused by BuGZ RNAi (Figure 2B). Similar rescue was also observed by expressing the siRNA-insensitive Flag-BuGZ (Figure S2C). Consistent with the activation of SAC due to chromosome misalignment, we found that all misaligned chromosomes in BuGZ-depleted mitotic cells had a strong Mad1 signal at their kinetochores (Figure 2C). The mitotic arrest caused by BuGZ could be rescued by inhibiting SAC using the Mps1 inhibitor NMS-P715 (Figure 2D).
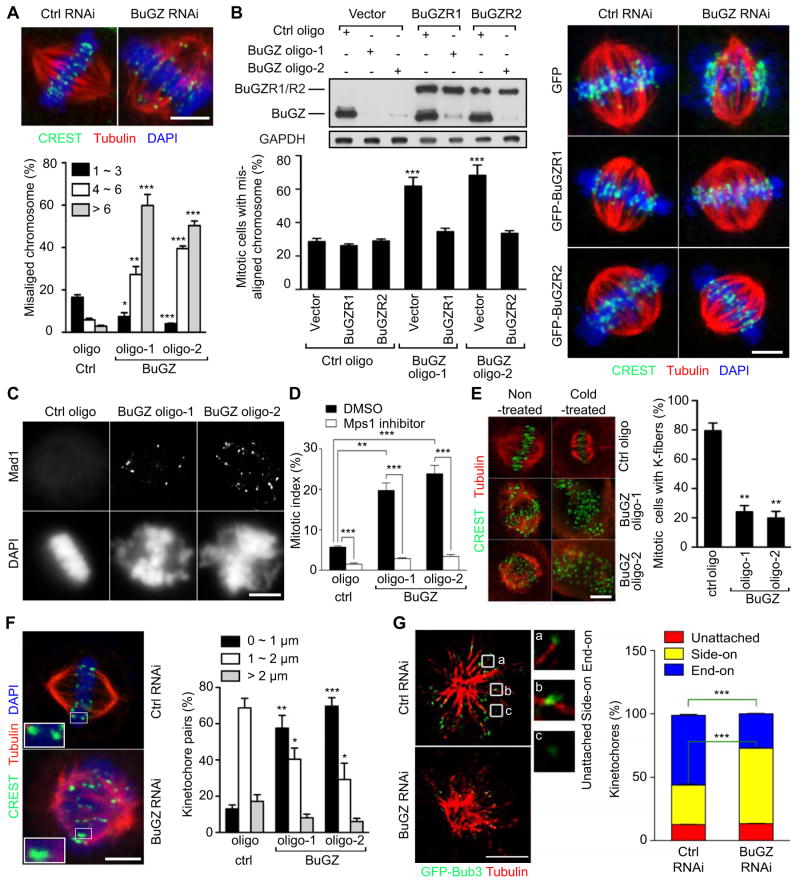
Figure 2. BuGZ regulates MT-kinetochore interaction.
A. Images of mitotic cells with properly aligned chromosomes (control RNAi) or misaligned chromosomes (BuGZ RNAi). BuGZ RNAi caused chromosome misalignment (see the graph below). ~100 mitotic cells were measured for each experiment and condition. Centromeres were detected by the CREST serum.
B. Expression of RNAi-insensitive GFP-BuGZ, but not GFP alone, in siRNA-treated HeLa cells rescued chromosome misalignment caused by BuGZ RNAi. BuGZR1 and BuGZR2 are two GFP-BuGZ constructs insensitive to BuGZ oligo-1 and oligo-2, respectively. GFP-luciferase was used as a negative control. Representative images are shown to the right. ~30 mitotic cells were measured for each experiment and condition.
C. Control or BuGZ siRNA-treated HeLa cells were analyzed for kinetochore localization of Mad1.
D. Inhibiting SAC by Mps1 inhibitor NMS-P715 prevented mitotic block caused by BuGZ depletion. 1 μM of NMS-P715 was added to siRNA-treated cells for 20 h followed by fixation and analyses. ~500 cells were counted for each experiment and condition.
E. BuGZ RNAi caused a reduction of kinetochore MT stability. Mitotic cells with a complete lack of kinetochore MTs were counted as cells without kinetochore fibers. ~100 mitotic cells were analyzed for each experiment and condition.
F. BuGZ RNAi reduced inter-kinetochore distances as quantified by measuring the distance between sister chromatids congressed to the metaphase plate. ~30 kinetochores from 7–12 different cells were measured for each experiment and condition.
G. BuGZ reduction caused an increase and decrease of side-on attached and end-on attached kinetochores, respectively. Cells were treated with 10 μM monastrol for 4 h before fixation. One 3D-SIM section from the 3D-SIM stack is shown for each condition. The boxed regions are magnified 4× to show typical kinetochores. ~150 kinetochores from at least 5 cells were counted for each experiment and condition.
Scale bars, 5 μm. Error bars, SEM. Student’s t-test: *p<0.05, **p<0.01, ***p<0.001 from triplicates.
To understand whether the chromosome misalignment upon the depletion of BuGZ was due to defective interactions between MTs and kinetochores, we assessed the stability of kinetochore MTs using cold treatment to depolymerize unstable MTs. We found that kinetochore MTs were depolymerized in the cold in the majority of mitotic HeLa cells depleted of BuGZ, whereas in control cells they remained stable (Figure 2E). Consistent with the defects in kinetochore MTs, we found a significant reduction of inter-kinetochore distances in cells treated with BuGZ RNAi compared to controls (Figure 2F).
To further analyze the effect of BuGZ depletion on kinetochore-MT interactions in detail, we used three dimensional (3D) Structured Illumination Microscopy (3D-SIM) to image mitotic cells. Recent studies show that monastrol treatment allows easy assessment of unattached, side-on attached (kinetochore binding along MT walls), or end-on (kinetochore binding to the plus ends of MTs) attached kinetochores, due to the formation of monopolar spindle (Shrestha and Draviam, 2013). Thus, we treated BuGZ-depleted and control cells with monastrol. By quantifying the three types of kinetochores, we found that BuGZ depletion resulted in an increase of kinetochores that assumed side-on attachment and a corresponding decrease of end-on attachments compared to controls (Figure 2G). Thus BuGZ regulates mitosis by ensuring proper kinetochore-MT interactions.
BuGZ binds directly to Bub3 to form a complex of equal stoichiometry
To understand how BuGZ regulates the MT-kinetochore interaction, we performed mass spectrometry to identify BuGZ-interacting proteins in either the cytostatic factor (CSF)-arrested Xenopus egg extracts (Desai et al., 1999) or mESCs. We found Bub3 as one of the top hits among the candidate interacting proteins identified in both CSF-egg extracts and mESCs (Table S1). The interaction between the endogenous and tagged BuGZ and Bub3 was confirmed by immunoprecipitation in HeLa cells (Figure 3A and S3A) and CSF-egg extracts (Figure 3B and S3B). Purified BuGZ and Bub3 showed a direct interaction with one another (Figure 3C). Importantly, co-expression of untagged mouse Bub3 and mouse 6His-BuGZ in Sf9 cells produced a well-behaved complex of Bub3-BuGZ as revealed by gel filtration chromatography, and the two proteins were present at 1:1 stoichiometry in the complex (Figure 3D).
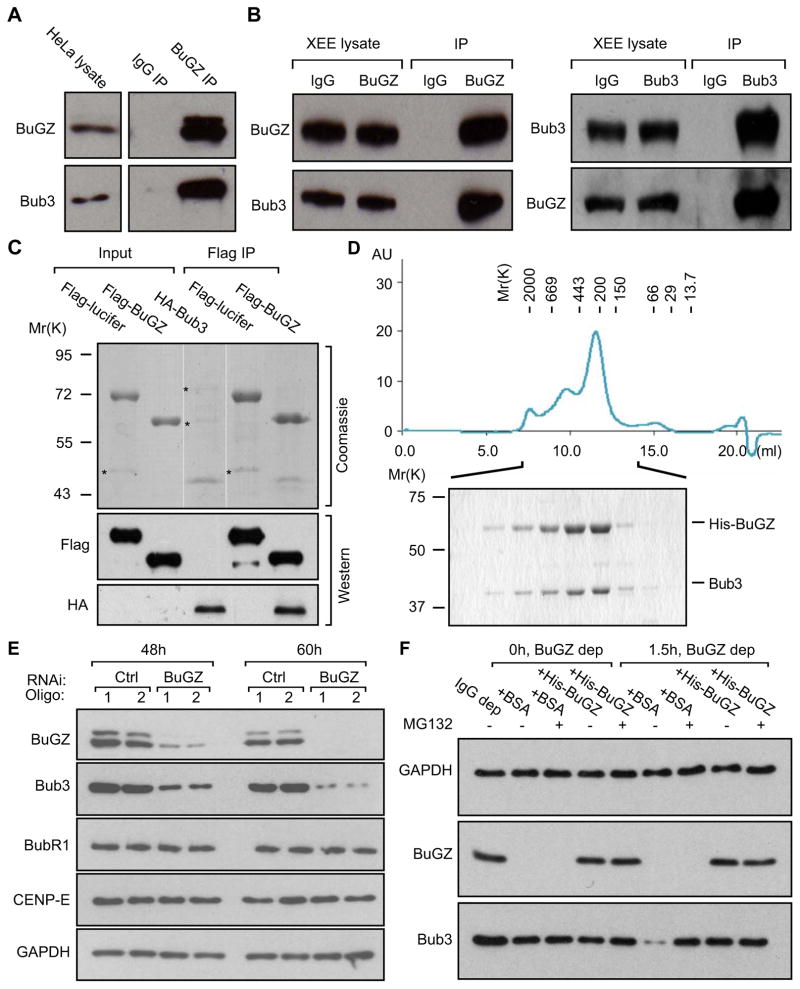
Figure 3. BuGZ directly binds and stabilizes Bub3.
A and B. Endogenous BuGZ and Bub3 interact with one another in HeLa cells (A) and Xenopus CSF-egg extracts (XEE) (B) as revealed by immunoprecipitation (IP).
C. BuGZ and Bub3 interact directly. Purified Flag-BuGZ, but not Flag-luciferase, pulled down purified HA-tagged Bub3 as judged by Coomassie blue staining (top) and Western blotting analyses (bottom). For Coomassie staining: 10% of Flag-BuGZ or Flag-luciferase and 25% of HA-Bub3 present in the reaction were loaded for input and 27% of total Flag-BuGZ or Flag-luciferase pull-downs were loaded. For Western blotting: 3% of Flag-BuGZ or Flag-luciferase and 2.5% of HA-Bub3 present in the reaction were loaded for input and 3% of total Flag-BuGZ or Flag-luciferase pull-downs were loaded. Asterisks, co-purified contaminating proteins.
D. BuGZ and Bub3 form a complex of 1:1 stoichiometry when co-expressed in Sf9 cells using baculovirus. The complex was first purified via the 6His-tag on BuGZ and then analyzed by gel filtration chromatography with the indicated size standards. The complex in the indicated fractions were further analyzed by SDS-PAGE and Coomassie blue staining.
E. RNAi depletion of BuGZ reduced the overall Bub3 protein levels without affecting BubR1 and CENP-E in HeLa cells. Loading controls, GAPDH.
F. Immunodepletion of BuGZ in CSF-egg extracts destabilized Bub3, which was rescued by MG132 or purified His-BuGZ but not BSA. Depletion of BuGZ caused a reduction of Bub3 due to co-immunoprecipitation. The reduced Bub3 level was further diminished in the absence of either MG132 or His-BuGZ upon incubation for 1.5 h at room temperature. Loading controls, GAPDH.
BuGZ protects Bub3 from degradation
The binding of BuGZ to Bub3 suggests that BuGZ could regulate chromosome alignment via Bub3. Since BuGZ is a nuclear protein, it might regulate Bub3 transcription. However, quantitative reverse transcriptase PCR analyses showed that the knockdown of BuGZ did not affect mRNA levels of Bub3, Bub1, or BubR1 (Figure S3C), but it resulted in a reduction of overall Bub3 protein levels in several cell lines examined (Figure 3E and S3D). By contrast, depletion of Bub3 by RNAi did not affect the level of BuGZ (Figure S3E). Although Bub3 protein level is reduced upon BuGZ knockdown, other kinetochore proteins such as BubR1 and CENP-E remained unchanged (Figure 3E). Using CSF-egg extracts, we found that depletion of BuGZ also resulted in Bub3 degradation, which could be rescued by either adding the purified His-BuGZ or the proteasome inhibitor MG132 (Figure 3F). Thus BuGZ protects Bub3 from degradation by the proteasome.
BuGZ promotes chromosome alignment by directly binding to and stabilizing Bub3 via the GLEBS motif
The above findings suggest that BuGZ could regulate chromosome alignment by stabilizing Bub3, which would in turn promote proper kinetochore loading of Bub3. Consistent with this, depletion of BuGZ in HeLa cells by RNAi resulted in reduced kinetochore Bub3 (Figure S4A). We showed that mitotic cells with BuGZ depletion and Bub3 reduction underwent mitotic arrest followed by death (see Figure S1D and E), but Bub3 depletion is known to cause SAC override. The mitotic death observed in BuGZ depleted cells might be due to some unknown interphase function(s) of BuGZ. On the other hand, the mitotic arrest in BuGZ RNAi versus mitotic exit in Bub3 RNAi could be due in part to the different degree of Bub3 depletion because we found that there was much less reduction of Bub3 by BuGZ RNAi than Bub3 RNAi (see Figure S3E). The remaining Bub3 protein in the BuGZ siRNA-treated cells might be sufficient to trigger SAC (see Figure 2D) but insufficient to support proper MT-kinetochore interactions. Consistently, whereas nocodazole-treated cells depleted of BuGZ arrested in mitosis similar to controls, cells depleted of Bub3 by RNAi were unable to arrest (Figure S4B).
To determine whether the binding of BuGZ to Bub3 is indeed required for Bub3 stability, Bub3 kinetochore localization, and chromosome alignment, we mapped the Bub3-binding site on BuGZ by deletion studies. Although no clear BuGZ homologs are present in lower eukaryotes such as the yeasts, the metazoan BuGZ proteins exhibit high sequence conservation (Figure S4C). We found that the conserved 50 amino acids within the C-terminal half of BuGZ (BuGZC50) (Figure S4C, red outline) mediated the interaction with Bub3 (Figure S4D and E).
To further narrow down the sequence involved in Bub3 interaction, we inspected the BuGZC50 sequences and found a GLEBS motif known to be present in several other proteins (Pritchard et al., 1999), including Bub1 and BubR1 (Harris et al., 2005; Larsen et al., 2007; Wang et al., 2001) (Figure 4A). In the accompany study, BuGZ was also identified to contain Bub3 and regulate Bub3 stability (XX). Since the GLEBS in Bub1 and BubR1 mediates their direct binding to Bub3, we mutated a number of conserved amino acids in the BuGZ GLEBS either individually or in combinations to A. We found that mutating the highly conserved EE to AA (BuGZAA, see the outlined EE in Figure 4A) disrupted the binding of BuGZ to Bub3 (Figure 4B).
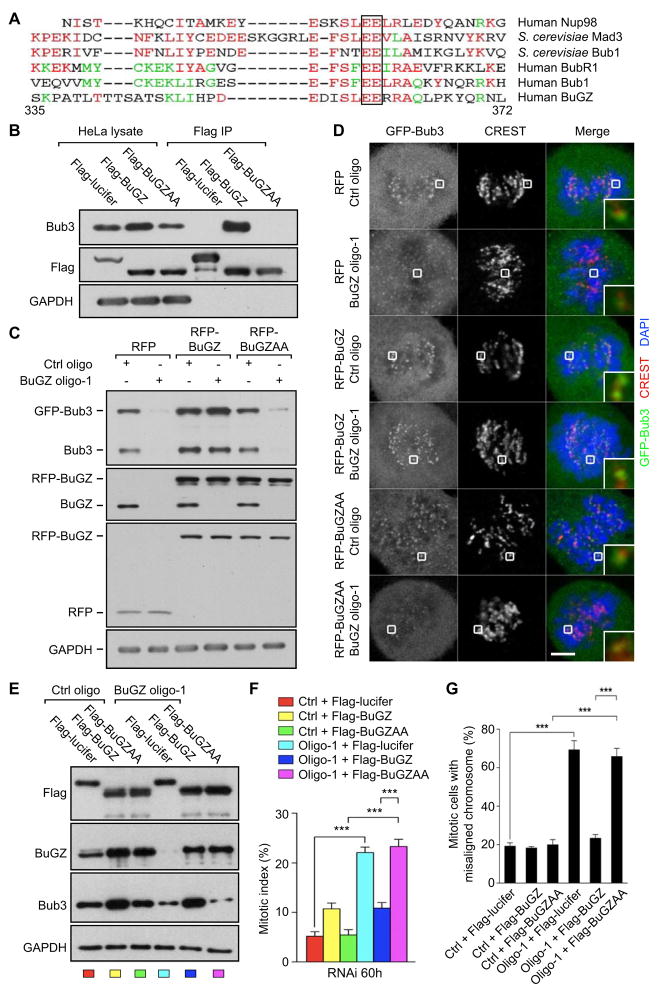
Figure 4. The conserved GLEBS motif in BuGZ binds and stabilizes Bub3 and promotes Bub3 kinetochore loading and function.
A. Sequence alignment of human BuGZ amino acids 335–372 with the known GLEBS motifs from the indicated proteins. The completely conserved amino acids, EE, are boxed.
B. Flag-BuGZ, but not Flag-luciferase (Flag-Lucifer) or Flag-BuGZAA (with the conserved EE boxed in A mutated to AA), pulled down endogenous Bub3 in HeLa cells. Loading control, GAPDH.
C. Expression of the RNAi-insensitive versions of RFP-BuGZ, but not RFP or RFP-BuGZAA, stabilized both endogenous Bub3 and stably expressed GFP-Bub3 in HeLa cells treated with control or BuGZ siRNA. Note that the levels of rescue RFP-BuGZ and RFP-BuGZAA exceeded those of the endogenous BuGZ by ~2-fold, but only RFP-BuGZ caused a modest increase of Bub3.
D. Expression of the RNAi-insensitive RFP-BuGZ, but not RFP-BuGZAA or RFP, rescued the kinetochore localization of Bub3 in HeLa cells treated with BuGZ siRNA. The appreciably stronger kinetochore Bub3 signal in cells treated with control or BuGZ siRNA and expressing RFP-BuGZ is consistent with the increased Bub3 protein levels in these cells compared to other controls (see C). CREST was used to mark centromeres. The boxed kinetochore was enlarged to the bottom right of each merged images. Scale bar, 5 μm.
E. Expression of RNAi-insensitive Flag-BuGZ, but not Flag-BuGZAA or Flag-luciferase, stabilized Bub3 upon BuGZ RNAi. Note the levels of rescue Flag-BuGZ and Flag-BuGZAA exceeded that of endogenous BuGZ by ~2-fold, but only Flag-BuGZ caused a modest increase of Bub3. The color code for each lane corresponds to the bars in the graph in (F).
F. Stabilization of Bub3 by Flag-BuGZ significantly reduced the mitotic index in HeLa cells treated with BuGZ siRNA. The elevated Bub3 levels in cells expressing the rescue Flag-BuGZ (see E) correspond to the moderate increase in the mitotic index (compare yellow and dark blue bars to the red bar). ~300 cells were counted for each experiment and condition.
G. Flag-BuGZ, but not Flag-BuGZAA, rescued the chromosome misalignment in cells treated with BuGZ siRNA. Flag-luciferase served as a negative control. ~40 mitotic cells were counted for each experiment and condition.
Error bars, SEM. Student’s t-test: ***p<0.001 from triplicates.
Next, we created RNAi-insensitive RPF-BuGZ and RFP-BuGZAA. To test whether these proteins could stabilize Bub3, we introduced each into HeLa cells that stably expressed GFP-Bub3 at levels similar to the endogenous Bub3 (Figure 4C), which would allow easy assessment of Bub3 localization. Only the expression of the RNAi-insensitive wild-type RFP-BuGZ, but not RFP-BuGZAA or RFP, prevented the reduction of the endogenous Bub3 and GFP-Bub3 in HeLa cells treated by BuGZ RNAi (Figure 4C). This stabilization of Bub3 resulted in the corresponding rescue of kinetochore GFP-Bub3 to levels similar to the control siRNA-treated cells expressing RFP (Figure 4D). Compared to RFP and RFP-BuGZAA, RFP-BuGZ also augmented the endogenous Bub3 and GFP-Bub3 expression in cells treated by control RNAi (Figure 4C). The modest difference in GFP-Bub3 levels positively correlated with the kinetochore Bub3 signals in these cells (Figure 4D). The RNAi-insensitive Flag-BuGZ, but not Flag-BuGZAA, also stabilized Bub3 (Figure 4E) and significantly rescued the mitotic defects caused by BuGZ RNAi as judged by the marked attenuation of mitotic arrest and chromosome misalignment (Figure 4F and G). The higher Bub3 protein level due to the modestly elevated Flag-BuGZ expression compared to the endogenous BuGZ (Figure 4E) correlated with a mild increase of the mitotic index of cells treated with control RNAi (Figure 4F). Thus the direct binding of BuGZ to Bub3 through GLEBS is required for BuGZ to regulate Bub3 stability, Bub3 kinetochore localization, and chromosome alignment.
BuGZ directly binds to microtubules via its conserved N-terminus
Since BuGZ binds along spindle MTs (see Figure 1E and F), we wish to further study whether BuGZ directly binds to MTs and whether such binding regulates Bub3. We stimulated MT polymerization by adding taxol in CSF-egg extracts and found that BuGZ co-pelleted with taxol-stabilized MTs along with Bub3, whereas neither BuGZ nor Bub3 was found in the pellet when egg extracts were treated with nocodazole to prevent MT assembly (Figure 5A). We repeated these experiments in either mock-depleted (IgG dep) or BuGZ-depleted (BuGZ dep) CSF-egg extracts (Figure 5B left panels) and found that in the absence of BuGZ, the remaining Bub3 failed to co-pellet with taxol-stabilized MTs (Figure 5B right panels). This suggests that Bub3 requires BuGZ for MT association. Next we purified Flag-BuGZ or BuGZ (obtained by removing the 6His tag from 6His-BuGZ) (Figure S5A–C). By mixing the purified BuGZ with taxol-stabilized MTs assembled from pure tubulin in vitro, we found BuGZ directly bound to MTs (Figure 5C and S5A–C). We found that purified 6His-Bub3 failed to bind to taxol-stabilized MTs in vitro (Figure S5D). However, the purified BuGZ-Bub3 complex (from Sf9 cells) exhibited MT binding (Figure 5D). This strongly suggests that BuGZ recruits Bub3 to MTs. Only a fraction of BuGZ co-pelleted with MTs in the above assays, which might suggest a low affinity interaction, but further analyses would be required to determine the binding affinity.
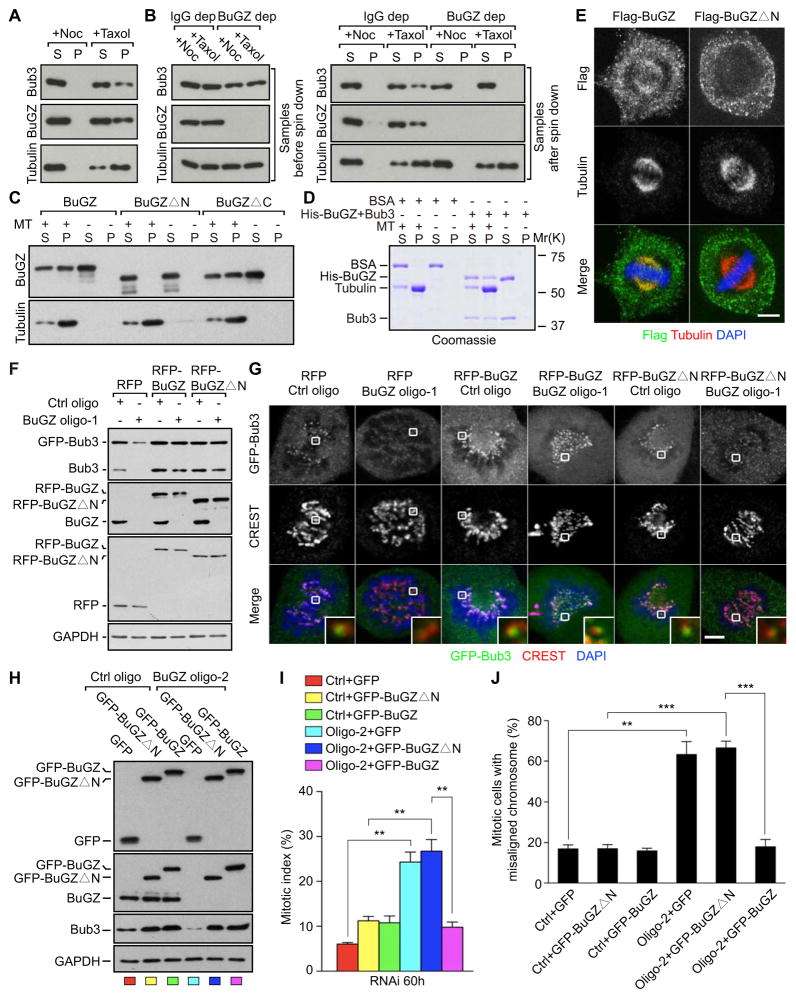
Figure 5. The MT-binding domain of BuGZ is required for efficient loading of Bub3 to kinetochores and chromosome alignment.
A. BuGZ and Bub3 co-pelleted with MTs in the CSF egg extracts.
B. Depletion of BuGZ in CSF egg extracts resulted in ~50% reduction of Bub3 due to co-depletion (left panel, samples before spin down), but the remaining Bub3 failed to bind to MTs (right panel, samples after spin down).
C. Only the full-length BuGZ and BuGZΔC, but not BuGZΔN, bound directly to taxol-stabilized MTs assembled from purified tubulin.
D. The purified BuGZ-Bub3 complex co-pelleted with taxol-stabilized MTs assembled from pure tubulin.
E. Flag-BuGZ, but not Flag-BuGZΔN, localized to spindle MTs as revealed by confocal microscopy. Cells were extracted with detergent followed by immunostaining. Scale bar, 5μm.
F. Expression of the RNAi-insensitive RFP-BuGZ or RFP-Zin1ΔN similarly stabilized Bub3 and GFP-Bub3.
G. RFP-BuGZΔN failed to promote the kinetochore loading of GFP-Bub3 compared to controls. The strong kinetochore GFP-Bub3 signal correlated with the overall protein levels in RFP-BuGZ-expressing cells treated with control or BuGZ siRNA (see F above). CREST was used to mark centromeres. The boxed kinetochore was enlarged to the bottom right of each merged images. Scale bar, 5 μm.
H. Expression of the RNAi-insensitive GFP-BuGZ and GFP-BuGZΔN in HeLa cells treated with BuGZ siRNA oligo-2 also stabilized the endogenous Bub3. The color codes at the bottom of each lane correspond to the color bars in I below.
I. Expression of GFP-BuGZ, but not GFP-BuGZΔN or GFP, significantly suppressed the mitotic arrest caused by BuGZ RNAi. The ~2-fold higher Bub3 levels in cells expressing GFP-BuGZ or GFP-BuGZΔN and treated by control or BuGZ siRNA compared to cells expressing GFP and treated by similar siRNAs (see H) explain the respective moderate increase in the mitotic index (compare yellow, green, and pink bars to the red bar). For quantifications, ~300 cells were counted for each experiment and condition.
J. Expression of GFP-BuGZ, but not GFP-BuGZΔN or GFP, significantly rescued chromosome misalignment caused by BuGZ RNAi. For quantifications, ~100 mitotic cells were counted for each experiment and condition.
Error bars, SEM. Student’s t-test: **p<0.01, ***p<0.001 from triplicates.
Next we mapped the MT-binding domain on BuGZ. Sequence analyses of BuGZ showed that the conserved N-terminal 92 or C-terminal 86 amino acids (see Figure S4C) exhibit an overall positive charge, which could mediate the binding to the negatively charged MTs. By creating mutants lacking either region (BuGZΔN and BuGZΔC), we found that the purified BuGZΔN failed to bind to MTs (Figure 5C, S5B and C). Thus the MT binding domain of BuGZ is present within the N-terminal 92 amino acids.
The MT binding of BuGZ is required for efficient loading of Bub3 onto kinetochores during prometaphase
To verify the MT-binding domain mapped in vitro in cells, we expressed Flag-BuGZΔN in HeLa cells. Compared to HeLa cells expressing Flag-BuGZ, which showed both spindle and cytoplasmic localization, Flag-BuGZΔN was only found in the cytoplasm (Figure 5E). Next, we created RNAi-insensitive RFP-BuGZΔN to examine whether the MT binding of BuGZ is required for BuGZ to stimulate Bub3 loading onto kinetochores. Consistent with the presence of GLEBS in BuGZΔN, RFP-BuGZΔN was able to protect the endogenous Bub3 and GFP-Bub3 from degradation upon BuGZ RNAi to a similar degree as that of RFP-BuGZ in HeLa cells (Figure 5F). However, compared to RFP-BuGZ, RFP-BuGZΔN failed to increase the kinetochore localization of GFP-Bub3 in BuGZ siRNA-treated cells (Figure 5G). Similarly, although the expression of either RNAi-insensitive GFP-BuGZ or GFP-ZinsΔN stabilized the endogenous Bub3 to a similar degree in cells treated by BuGZ RNAi (Figure 5H), only GFP-BuGZ suppressed the mitotic arrest and chromosome misalignment caused by BuGZ RNAi (Figure 5I and J). The increase in kinetochore GFP-Bub3 (Figure 5G) and a moderate increase of mitotic index (Figure 5I) in cells expressing the rescue BuGZ or BuGZΔN (Figure 5F and H) correlated with the modest increase of the overall Bub3 protein levels in these cells. Together, these show that BuGZ uses its MT-binding domain to facilitate the loading of Bub3 to kinetochores, which in turn is required for chromosome alignment and mitotic progression.
Microtubules and BuGZ promote the loading of Bub3, BubR1, and CENP-E onto kinetochores and end-on kinetochore-MT binding during prometaphase
The above findings suggest that BuGZ could use MTs to facilitate kinetochore loading of Bub3, but this would contradict the previous observation that MT depolymerization in metaphase cells increases the amount of SAC proteins including Bub3 and BubR1 at kinetochores (Hoffman et al., 2001; Howell et al., 2004). However, in the previous analyses the kinetochore signals of Bub3 and BubR1 measured upon nocodazole-induced MT depolymerization were compared to the signals measured on metaphase kinetochores, but not to the signals on prometaphase kinetochores (Hoffman et al., 2001; Howell et al., 2004). Additionally MT-kinetochore interactions are regulated by cyclin-A at different stages of mitosis (Kabeche and Compton, 2013). It would be important to analyze whether mitotic stage and MTs affect the loading of kinetochore proteins.
To assess whether MTs could promote Bub3 and BubR1 binding to kinetochores upon NEBD in prometaphase, we first compared their intensities on kinetochores of different mitotic stages. By quantifying the immunofluorescence signals at kinetochores based on a previously used method (Hoffman et al., 2001), we found that the overall kinetochore Bub3, BubR1, and CENP-E signals in prometaphase cells were stronger than those of the nocodazole-treated cells, and the lowest signals were observed on the metaphase kinetochores (Figure 6A–F), supporting the involvement of MTs in the enhanced kinetochore loading of Bub3, BubR1, and CENP-E in prometaphase.
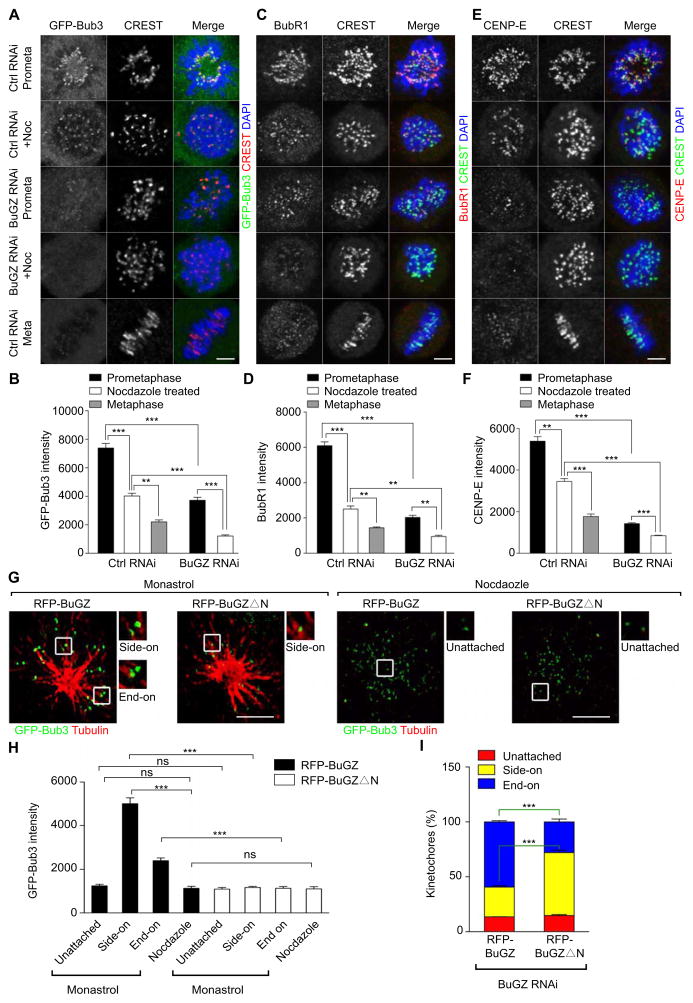
Figure 6. MTs and BuGZ promote the kinetochore loading of Bub3, BubR1, and CENP-E, and end-on MT-kinetochore interaction.
A–F. The kinetochore signals for Bub3 (as revealed by GFP-Bub3 localization, A and B), BubR1 (C and D), or CENP-E (E and F) are highest in prometaphase cells followed by that of nocodazole-treated (3.3 μM nocodazole for 4 h before fixation) mitotic cells and then metaphase cells (all cells were treated by control siRNA). BuGZ depletion by siRNA caused a reduction of kinetochore signals of all three proteins. Scale bars, 5 μm. ~30 kinetochores from 5–10 cells were measured for each experiment and condition.
G. 3D-SIM imaging of GFP-Bub3 signals in BuGZ-depleted mitotic cells expressing RFP-BuGZ or RFP-BuGZΔN and treated by monastrol (10 μM for 4 h before fixation) or nocodazole (3.3 μM for 4 h before fixation). One SIM section from the 3D-SIM stack is shown for each condition. The boxed regions are magnified 2× to show representative kinetochores. Scale bars, 5 μm.
H. Expression of RFP-BuGZ in BuGZ-depleted and monastrol- or nocodazole-treated cells significantly enhanced the loading of Bub3 onto kinetochores that have either side-on or end-on MT-kinetochore interactions compared to that of RFP-BuGZΔN, whereas neither proteins affected Bub3 levels on kinetochores with no MT attachment. Cells as shown in G were used for quantifications. GFP-Bub3 intensities on ~30 kinetochores from 5 cells were measured for each experiment and condition.
I. Expression of RFP-BuGZ in BuGZ-depleted cells significantly rescued the end-on MT-kinetochore interactions compared to that of RFP-BuGZΔN. Monastrol-treated cells as shown in G were used for quantifications. ~150 kinetochores from at least 5 cells were measured for each experiment and condition.
Error bars, SEM. Student’s t-test: **p<0.01, ***p<0.001 from triplicates.
Next, we studied the effect of BuGZ depletion on the loading of Bub3 onto kinetochores in prometaphase cells and nocodazole-treated cells. Compared to control RNAi-treatment, depleting BuGZ resulted in a significant reduction of Bub3 levels at kinetochores in both prometaphase cells and nocodazole-treated cells (Figure 6A and B). As expected from Bub3 reduction (Logarinho et al., 2008; Taylor et al., 1998), the depletion of BuGZ also resulted in a reduction of kinetochore BubR1 and CENP-E in prometaphase and nocodazole treated cells compared to the control siRNA-treated cells (Figure 6C–F) (Kim et al., 2008; Schaar et al., 1997; Shrestha and Draviam, 2013; Wood et al., 1997).
We then compared the ability of wild-type BuGZ and the BuGZΔN mutant to promote the loading of Bub3 onto prometaphase kinetochores that were unattached, side-on, or end-on attached to MTs. To facilitate the analyses, we treated BuGZ-depleted and rescued (by the RNAi-insensitive RFP-BuGZ or RFP-BuGZΔN) cells with monastrol to induce monopolar spindle formation (Shrestha and Draviam, 2013) or nocodozole to disassemble MTs. Using 3D-SIM, we analyzed kinetochore-MT interactions and Bub3 signal at the kinetochores (Figure 6G). We found that BuGZ-depleted cells rescued by the wild-type RFP-BuGZ exhibited higher Bub3 signals at the kinetochores that assumed either side-on or end-on MT attachment as compared to unattached kinetochores (Figure 6H). By contrast, in the cells rescued by RFP-BuGZΔN, Bub3 signals were similarly low on kinetochores that were unattached (also see the nocodazole treatment), side-on attached, or end-on attached to MTs (Figure 6H). These low kinetochore Bub3 signals were comparable to those seen on the unattached kinetochores in cells rescued by the wild-type RFP-BuGZ (Figure 6H). These analyses further support the idea that BuGZ uses MTs to promote the loading of Bub3 onto kinetochores during prometaphase.
Since Bub3 is required for end-on kinetochore-MT interactions (Elowe, 2011; Elowe et al., 2007; Guo et al., 2012; Huang et al., 2008; Lampson and Kapoor, 2005; Logarinho et al., 2008; Meraldi and Sorger, 2005; Windecker et al., 2009), the increased loading of Bub3 observed in prometaphase promoted by BuGZ could facilitate proper chromosome alignment and mitotic progression. To test this, we asked whether the MT-binding domain of BuGZ is required for promoting the end-on kinetochore-MT interactions. By analyzing monastrol-treated cells as shown in Figure 6G, we found that although both RFP-BuGZ- and RFP-BuGZΔN-transfected cells contained similar low percentages of unattached kinetochores, the RFP-BuGZ-rescued cells had significantly more end-on attached kinetochores than the RFP-BuGZΔN-rescued cells (Figure 6I). This suggests that BuGZ uses MTs to stimulate Bub3 loading onto the kinetochores that have achieved the initial MT attachment, which in turn promotes end-on kinetochore-MT interactions.
Discussion
Our findings is consistent with the idea that BuGZ functions as a Bub3-binding partner and chaperone that promote the end-on kinetochore-MT interaction by both stabilizing Bub3 and facilitating the loading of Bub3 onto kinetochores. Specifically, we find that BuGZ uses its GLEBS motif to bind and stabilize Bub3, which in turn is critical for BuGZ to promote kinetochore loading of Bub3, chromosome alignment, and mitotic progression. Since BuGZ, BubR1, and Bub1 all use their GLEBS motif to bind to the same surface of Bub3, the binding of Bub3 to BubR1/Bub1 or BuGZ could be mutually exclusive. As the binding of Bub3 to the GLEBS motif of BubR1 and Bub1 is required for the kinetochore loading of these proteins (Krenn et al., 2012; London et al., 2012; Primorac et al., 2013; Shepperd et al., 2012; Taylor et al., 1998; Yamagishi et al., 2012), it will be important to further understand whether and how BuGZ promotes Bub3 loading onto kinetochores in the context of BubR1 and Bub1. Despite great progress toward understanding the kinetochores-MT interactions in mitosis, what fraction of Bub3 is bound to BubR1 or Bub1 or whether all three proteins form a complex in the cytoplasm remains not well understood. By binding and stabilizing Bub3, BuGZ could chaperone the formation of functional complexes consisting of Bub3-Bub1, Bub3-BubR1, or BubR1-Bub3-Bub1 in the mitotic cytosol for either kinetochore targeting or cytoplasmic functions involving these proteins. Our ability to purify the well-behaved Bub3-BuGZ complex in milligram quantities should aid the effort of studying how the binding of BuGZ to Bub3 affects the interaction of Bub3 with BubR1 and Bub1.
Previous studies show that upon proper kinetochore-MT interaction in metaphase, substantial amounts of kinetochore proteins, such as Bub3, dissociate from the kinetochore (Howell et al., 2004; Shah et al., 2004) and MT depolymerization reverses the dissociation. This phenomenon has led to a general belief that MTs negatively regulate the binding of proteins to kinetochores. However, our analyses show that the effect of MTs on the binding of Bub3, BubR1, and CENP-E to kinetochores depends on the stage of mitosis. These proteins exhibit significantly higher kinetochore signals during prometaphase than that of metaphase or nocodazole-treated cells (Figure 6A–F). Consistent with previous findings (Howell et al., 2004; Shah et al., 2004), we show that the kinetochore signals for Bub3, BubR1, and CENP-E in nocodazole-treated cells are higher than that of metaphase cells. Since the kinetochore Bub3, BubR1, and CENP-E are required for the conversion from side-on to end-on kinetochore-MT interactions, it is tempting to speculate that MTs could play a role in promoting their own end-on interactions with kinetochores.
Our analyses show that the enhanced kinetochore loading of Bub3 during prometaphase requires MTs and the MT-binding domain of BuGZ. Importantly, the MT-binding domain of BuGZ is required for end-on kinetochore-MT interactions. It is possible that BuGZ uses MTs to promote the handing off of Bub3 to BubR1 and Bub1, which could in turn stimulate the kinetochore loading of these proteins during prometaphase and end-on interactions. Since Bub3 binding to Bub1 and BubR1 is required for their kinetochore targeting, one way to test whether BuGZ uses MTs to promote kinetochore loading of these proteins would be to analyze whether MT depolymerization and/or depletion of BuGZ decrease the amount of Bub3 associated with BubR1 and Bub1. Although our data is consistent with the idea that BuGZ uses MTs to promote Bub3 loading, we note that further studies are required to test this idea. For example, since the MT-binding domain of BuGZ covers 92 amino acids, it remains possible that other functional sequences exist within this region that could promote Bub3 loading onto kinetochores independent of MTs. Additional mutagenesis studies to define critical amino acids required for MT binding should help to establish whether BuGZ uses MTs to load Bub3 onto kinetochores during prometaphase.
Our findings show that the MT-binding domain is required for BuGZ to promote a larger increase of Bub3 loading onto side-on attached kinetochores than the end-on kinetochores, with no effect on the unattached kinetochores. Currently, we do not understand how and why BuGZ exhibits such selectivity. One possibility is that MT attachment may modify kinetochores to facilitate further Bub3 loading. Alternatively both kinetochores and MTs may be required to facilitate BuGZ-mediated Bub3 loading onto kinetochores. Finally, if the binding of BuGZ to MTs promotes the formation of the kinetochore-loading competent Bub3, MT-attached kinetochores may encounter a higher concentration of these Bub3 molecules than those unattached kinetochores that are further away from MTs.
Interestingly, recent studies show that upon NEBD the orientation of kinetochores favor their side-on interactions with MTs (Magidson et al., 2011). Our findings suggest that upon NEBD, the MT-independent loading of the MT-capture machinery to kinetochores might facilitate the initial side-on MT capture, whereas the MT-dependent further loading of the machinery onto the kinetochore that has already established its MT interaction could facilitate end-on conversion. Since during mitosis the wall of MTs can nucleate new MTs at a shallow angle (Petry et al., 2013; Zheng and Iglesias, 2013), an increased loading of MT-capture complexes onto MT-tethered kinetochores might allow the kinetochore to interact with the plus ends of incoming MT to assume end-on interactions. As many of these kinetochores would still have side-on attached MTs, their stabilized MT associations through CENP-E and depolymerization of the side-on MTs by MCAK as described in a recent report (Shrestha and Draviam, 2013) may further aid in the conversion of kinetochores into all end-on interaction with MTs.
Experimental procedures
BuGZ and Bub3 were expressed as 6His-tagged, GST-tagged, or untagged proteins in bacteria, HEK293T cells, or Sf9 cells, according to the manufacturer’s instructions. The purified proteins were used for antibodies production or functional studies. For live imaging of HeLa cells or E14 ESCs expressing H2B-GFP (Vong et al., 2005; Vong et al., 2010), cells were transfected with siRNA for 48 h or 12 h, respectively, followed by imaging at 20× magnification for at least 20 h in a stage incubator at 37°C with 5% CO2, and 70% humidity (Pathology Devices, Inc.). Images were captured with a Nikon ECLIPSE TE2000-U Microscope at 5 min intervals controlled by MetaMorph (Molecular Devices).
To visualize BuGZ, Bub3, BubR1 and CENP-E, cells were fixed in 4% paraformaldehyde followed by immunostaining (see below). Cells were imaged by epifluorescence microscopy (Nikon ECLIPSE E800), confocal microscopy (Leica SP5), or 3D-SIM (Zeiss ELYRA SIM) as indicated. To better visualize the MT-associated BuGZ, cells were first permeabilized with PEM buffer (100 mM PIPES pH6.8, 5 mM EGTA, 2 mM MgCl2, 5 nM taxol, 0.05% Triton X100) for 20–30 sec at room temperature to extract the soluble proteins before fixation in 4% paraformaldehyde for 7 min. The extracted or non-extracted cells were incubated with 0.3% Triton in PBS for 5 min and then blocked in 3% BSA in PBS for at least 1 h followed by incubation with primary and secondary antibodies. The primary antibody incubation was for overnight at 4°C. For details of antibodies and dilutions used see Table S2. For 3D-SIM, we used the ELYRA SIM microscopy system (Zeiss) with Andor EMCCD Camera IXON DU855, 63×/1.4 oil immersion objective (C-Apochromat), and ZEN2011 image capturing software to visualize MTs and kinetochores.
Mitotic index was determined by dividing the number of cells in prophase through anaphase (judged by DAPI staining) with the total number of cells counted. For the following measurements, cells were first treated with siRNA for the indicated time followed by incubation with 10 μM of MG132 for 1.5 h and then processed for immunofluorescence microscopy. The percentage of cells with misaligned chromosomes was defined as previously described (Meraldi and Sorger, 2005). Cells that formed the metaphase plate were included in the analyses. Mitotic chromosomes found outside of the rectangular area encompassing the central 30% of the spindle were considered as misaligned chromosomes. To determine the inter-kinetochore distance, only chromosomes congressed on the metaphase plate were measured. To determine the stability of kinetochore fibers, HeLa cells were treated with 10 μM of MG132 for 1.5 h and then washed with cold medium. After placing the cells on ice for 5 min, they were processed for immunofluorescence microscopy. The number of cells with stable kinetochore-MTs was counted. At least 100 mitotic cells were counted for each condition and at least three independent experiments were performed.
To obtain 3D-SIM images, each channel was set at a fixed laser power, exposure time, and gain. These parameters were set depending on the signal intensity with the goal of obtaining the best signal below the pixel saturation level. The maximum saturation value of the setup is 65000 and the maximum pixel values in all our pictures are under 20000. To capture the image of kinetochores with MTs, we set the first and last stage positions based on MTs (for those with MT attachment). To quantify the type of MT-kinetochore interactions, clearly separated individual kinetochores in each cell were analyzed section by section to judge their relationship with MTs. Kinetochores whose relationship with MTs were difficult to determine were excluded and the remaining ones were classified as unattached, side-on attached, or end-on attached kinetochores. For each cell, six clearly separated individual kinetochores from each experimental group were picked randomly for the quantification of Bub3 intensity. At least five cells were analyzed for each experiment and condition.
To quantify the kinetochore Bub3 intensity and correlate it with the nature of kinetochore-MT interaction, we merged the slices of Bub3 kinetochore images using the Maximum Intensity Mode similar to those we used in confocal microscopy and then used the Adobe PS software to quantify Bub3 intensity based on the method published previously (Hoffman et al., 2001). Briefly, computer-generated 9×9 and 13×13 pixel regions were centered over each kinetochore and the total integrated fluorescence counts were obtained for the 9×9 and 13×13 pixel regions. Since the 9×9 pixel region was typically large enough to contain 90% of kinetochore fluorescence, the intensity difference between 9×9 and 13×13 pixel regions was used as the background intensity. The same method was also used to quantify kinetochore signals from images obtained by confocal microscopy.
For the reciprocal immunoprecipitation of Bub3 and BuGZ in HeLa cells, 3×106 cells were plated one day before transfection. Cells were transfected with plasmids expressing tagged mouse Bub3 and BuGZ using FuGENE®6 (Roche). After 48 h post transfection, cells were collected and lysed in 650 μl of the lysis buffer. The cell lysate was incubated on ice for 10 min followed by centrifugation at 12,000 rpm for 30 min at 4°C. 50 μl of supernatant was taken as input samples. The remaining supernatant was incubated with 30 μl of the Flag or 25 μl GFP bead slurry for 2.5 h at 4°C. After the incubation, the beads were washed with 1 ml of the lysis buffer 3 times and then with 1 ml of the wash buffer 3 times. Proteins associated with the Flag beads were eluted with 30 μl of the Flag peptide (1 mg/ml). The GFP beads were eluted with 50 μl of 0.1 M glycine (pH 2.5) and then neutralized by adding 5 μl of 1 M Tris-base. The eluted samples were used for Western blotting analyses.
To study whether Bub3 and BuGZ directly bind to each other, 3×106 HEK293T cells were plated one day before transfection. 10 μg of the expression plasmid for Flag-tagged luciferase, BuGZ, or BuGZΔC50 along with 15 μg of the expression plasmid for HA-Bub3 were transfected into the cells. At 48 h post transfection, the cells were lysed with 650 μl of the lysis buffer and incubated on ice for 10 min. The lysates were centrifuged at 12,000 rpm for 30 min at 4°C. The supernatant was incubated with 30 μl Flag or HA bead slurry for 2.5 h at 4°C. After incubation, the beads were washed with 1 ml of the lysis buffer for 4 times and 1 ml of the wash buffer for 4 times. HA-Bub3 was eluted using 40 μl HA peptide at 1 mg/ml. 10 μl of the HA-eluted proteins were used as the input control. For Flag-tagged proteins, 1/4 of the Flag beads were eluted with 10 μl of the Flag peptide (1 mg/ml) as the input control. The rest of the Flag beads were then incubated with 30 μl HA peptide-eluted HA-Bub3 protein in a final volume of 500 μl of the lysis buffer for 3 h at 4°C. The beads were then washed with the lysis buffer followed by the wash buffer for 3 times each. The Flag beads were then eluted with 30 μl Flag peptide (1 mg/ml).
To study protein-protein interactions between Bub3 and BuGZ in CSF Xenopus egg extracts, 30 μg of rabbit antibodies to Xenopus BuGZ or Bub3 were coupled to 50 μl of protein A bead slurry. The protein A beads were added to 100 μl of CSF-egg extracts for immunoprecipitation. The immunoprecipitates were analyzed by Western blotting using antibodies to BuGZ and Bub3. One-Hour Western Complete Kit (GenScript) was used to avoid detecting the IgG band. Protein samples were run on 3–15% gradient gels for either Coomassie staining or Western blotting analyses. Antibodies for Western and immunofluorescence staining can be found in Supplementary Table S2.
To study whether BuGZ binds directly to MTs, we used in vitro MT spin-down assays. Since the 6His tag is slightly positively charged, to ensure proper assessment of MT binding of 6His-BuGZ, AcTEV protease (Invitrogen, cat# 12575-015) was used to cleavage the 6His on HisBuGZ, HisBuGZΔN, and HisBuGZΔC. ~20 μg of each of HisBuGZ (33 μl at 0.61 μg/μl), HisBuGZ (33 μl at 0.61 μg/μl), and HisBuGZΔC (8.3 μl at 2.4 μg/μl), all purified from Sf9 cells, were mixed with 7.5 μl of the 20X TEV Buffer, 1.7 μl 0.1 M DTT, 1.0 μl AcTEV protease (10 units), and distilled water to bring the final volume to 150 μl. The mixtures were incubated at 30°C for 1 h. 10 μl of each sample was removed at 0 and 1 h for Western blotting and PAGE analyses to examine the cleavage efficiency and protein integrity. The remaining 130 μl protein mix has a final concentration of 0.13 mg/ml. The Microtubule Binding Protein Spin-down Assay Kit (Cytoskeleton Inc. cat# BK029) was used for all the experiments. Briefly, 15 μl of each of the protein mix was added to 20 μl of tubulin solution (0.5 mg/ml stock) or tubulin buffer (supplied in the kits) and 15 μl of taxol at 20 μM. 2 μg of purified Flag-BuGZ, Flag-luciferase (both from HEK293T cells), His-Bub3 (from baculovirus), or BSA was used in the MT spin down assay as described above. The reactions were incubated at room temperature for 30 min. MTs spin down and the supernatants and pellets were analyzed following the instruction manual provided by the manufacturer. By comparing His-tagged proteins with the His-cleaved proteins, we found that the 6His tag on BuGZ or Bub3 did not have appreciable effect on MT binding of the proteins.
For additional detailed description of methods used please refer to the supplementary materials.
Supplementary Material
Highlights.
BuGZ regulates kinetochore-microtubule interaction via Bub3.
BuGZ and Bub3 form a complex of equal stoichiometry.
BuGZ uses its GLE-2-Binding Sequence (GLEBS) motif to bind and stabilize Bub3.
The microtubule-binding domain of BuGZ facilitates Bub3 loading onto kinetochores.
Acknowledgments
We thank Ona Martin for technical support, Ding Xiaoyan for mESCs, Li Juan for pcDNA3 plasmids, and the members of the Zheng lab for critical comments. Supported by the Chinese Academy of Sciences (XDA01010107) (X.Z.), Ministry of Science and Technology of China (2014CB964803) (X.Z.), National Science Foundation of China (31010103910) (X.Z. & Y.Z.), and R01 GM056312 and R01 GM06023 (Y.Z.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akiyoshi B, Biggins S. Reconstituting the kinetochore-microtubule interface: what, why, and how. Chromosoma. 2012;121:235–250. doi: 10.1007/s00412-012-0362-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai A, Murray A, Mitchison TJ, Walczak CE. The use of Xenopus egg extracts to study mitotic spindle assembly and function in vitro. Methods Cell Biol. 1999;61:385–412. doi: 10.1016/s0091-679x(08)61991-3. [DOI] [PubMed] [Google Scholar]
- Elowe S. Bub1 and BubR1: at the interface between chromosome attachment and the spindle checkpoint. Molecular and cellular biology. 2011;31:3085–3093. doi: 10.1128/MCB.05326-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elowe S, Hummer S, Uldschmid A, Li X, Nigg EA. Tension-sensitive Plk1 phosphorylation on BubR1 regulates the stability of kinetochore microtubule interactions. Genes & development. 2007;21:2205–2219. doi: 10.1101/gad.436007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X, Zhang P. Aneuploidy and tumorigenesis. Semin Cell Dev Biol. 2011;22:595–601. doi: 10.1016/j.semcdb.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley EA, Kapoor TM. Microtubule attachment and spindle assembly checkpoint signalling at the kinetochore. Nat Rev Mol Cell Biol. 2013;14:25–37. doi: 10.1038/nrm3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gache V, Waridel P, Winter C, Juhem A, Schroeder M, Shevchenko A, Popov AV. Xenopus meiotic microtubule-associated interactome. PloS one. 2010;5:e9248. doi: 10.1371/journal.pone.0009248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman B, Channels W, Qiu M, Iglesias P, Yang G, Zheng Y. Lamin B counteracts the kinesin Eg5 to restrain spindle pole separation during spindle assembly. The Journal of biological chemistry. 2010;285:35238–35244. doi: 10.1074/jbc.M110.140749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Kim C, Ahmad S, Zhang J, Mao Y. CENP-E--dependent BubR1 autophosphorylation enhances chromosome alignment and the mitotic checkpoint. The Journal of cell biology. 2012;198:205–217. doi: 10.1083/jcb.201202152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris L, Davenport J, Neale G, Goorha R. The mitotic checkpoint gene BubR1 has two distinct functions in mitosis. Experimental cell research. 2005;308:85–100. doi: 10.1016/j.yexcr.2005.03.036. [DOI] [PubMed] [Google Scholar]
- Hoffman DB, Pearson CG, Yen TJ, Howell BJ, Salmon ED. Microtubule-dependent changes in assembly of microtubule motor proteins and mitotic spindle checkpoint proteins at PtK1 kinetochores. Molecular biology of the cell. 2001;12:1995–2009. doi: 10.1091/mbc.12.7.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell BJ, Moree B, Farrar EM, Stewart S, Fang G, Salmon ED. Spindle checkpoint protein dynamics at kinetochores in living cells. Current biology: CB. 2004;14:953–964. doi: 10.1016/j.cub.2004.05.053. [DOI] [PubMed] [Google Scholar]
- Huang H, Hittle J, Zappacosta F, Annan RS, Hershko A, Yen TJ. Phosphorylation sites in BubR1 that regulate kinetochore attachment, tension, and mitotic exit. The Journal of cell biology. 2008;183:667–680. doi: 10.1083/jcb.200805163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson VL, Scott MI, Holt SV, Hussein D, Taylor SS. Bub1 is required for kinetochore localization of BubR1, Cenp-E, Cenp-F and Mad2, and chromosome congression. Journal of cell science. 2004;117:1577–1589. doi: 10.1242/jcs.01006. [DOI] [PubMed] [Google Scholar]
- Kebeche L, Compton DA. Cyclin A regulates kinetochore microtubules to promote faithful chromosome segregation. Nature. 2013;502:110–113. doi: 10.1038/nature12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Yu H. Mutual regulation between the spindle checkpoint and APC/C. Semin Cell Dev Biol. 2011;22:551–558. doi: 10.1016/j.semcdb.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Heuser JE, Waterman CM, Cleveland DW. CENP-E combines a slow, processive motor and a flexible coiled coil to produce an essential motile kinetochore tether. The Journal of cell biology. 2008;181:411–419. doi: 10.1083/jcb.200802189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura E, Tanaka K, Komoto S, Kitamura Y, Antony C, Tanaka TU. Kinetochores generate microtubules with distal plus ends: their roles and limited lifetime in mitosis. Developmental cell. 2010;18:248–259. doi: 10.1016/j.devcel.2009.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krenn V, Wehenkel A, Li X, Santaguida S, Musacchio A. Structural analysis reveals features of the spindle checkpoint kinase Bub1-kinetochore subunit Knl1 interaction. The Journal of cell biology. 2012;196:451–467. doi: 10.1083/jcb.201110013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampson MA, Kapoor TM. The human mitotic checkpoint protein BubR1 regulates chromosome-spindle attachments. Nature cell biology. 2005;7:93–98. doi: 10.1038/ncb1208. [DOI] [PubMed] [Google Scholar]
- Larsen NA, Al-Bassam J, Wei RR, Harrison SC. Structural analysis of Bub3 interactions in the mitotic spindle checkpoint. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:1201–1206. doi: 10.1073/pnas.0610358104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo KW, Kogoy JM, Pfister KK. The DYNLT3 light chain directly links cytoplasmic dynein to a spindle checkpoint protein, Bub3. The Journal of biological chemistry. 2007;282:11205–11212. doi: 10.1074/jbc.M611279200. [DOI] [PubMed] [Google Scholar]
- Logarinho E, Resende T, Torres C, Bousbaa H. The human spindle assembly checkpoint protein Bub3 is required for the establishment of efficient kinetochore-microtubule attachments. Molecular biology of the cell. 2008;19:1798–1813. doi: 10.1091/mbc.E07-07-0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London N, Ceto S, Ranish JA, Biggins S. Phosphoregulation of Spc105 by Mps1 and PP1 regulates Bub1 localization to kinetochores. Current biology: CB. 2012;22:900–906. doi: 10.1016/j.cub.2012.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Tsai MY, Wang S, Lu B, Chen R, Iii JR, Zhu X, Zheng Y. Requirement for Nudel and dynein for assembly of the lamin B spindle matrix. Nature cell biology. 2009;11:247–256. doi: 10.1038/ncb1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magidson V, O’Connell CB, Loncarek J, Paul R, Mogilner A, Khodjakov A. The spatial arrangement of chromosomes during prometaphase facilitates spindle assembly. Cell. 2011;146:555–567. doi: 10.1016/j.cell.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meraldi P, Sorger PK. A dual role for Bub1 in the spindle checkpoint and chromosome congression. The EMBO journal. 2005;24:1621–1633. doi: 10.1038/sj.emboj.7600641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra RK, Chakraborty P, Arnaoutov A, Fontoura BM, Dasso M. The Nup107–160 complex and gamma-TuRC regulate microtubule polymerization at kinetochores. Nature cell biology. 2010;12:164–169. doi: 10.1038/ncb2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musacchio A. Spindle assembly checkpoint: the third decade. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2011;366:3595–3604. doi: 10.1098/rstb.2011.0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musacchio A, Salmon ED. The spindle-assembly checkpoint in space and time. Nature reviews Molecular cell biology. 2007;8:379–393. doi: 10.1038/nrm2163. [DOI] [PubMed] [Google Scholar]
- Petry S, Groen AC, Ishihara K, Mitchison TJ, Vale RD. Branching microtubule nucleation in Xenopus egg extracts mediated by augmin and TPX2. Cell. 2013;152:768–777. doi: 10.1016/j.cell.2012.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primorac I, Weir JR, Chiroli E, Gross F, Hoffmann I, van Gerwen S, Ciliberto A, Musacchio A. Bub3 reads phosphorylated MELT repeats to promote spindle assembly checkpoint signaling. eLife. 2013;2:e01030. doi: 10.7554/eLife.01030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard CE, Fornerod M, Kasper LH, van Deursen JM. RAE1 is a shuttling mRNA export factor that binds to a GLEBS-like NUP98 motif at the nuclear pore complex through multiple domains. The Journal of cell biology. 1999;145:237–254. doi: 10.1083/jcb.145.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder CL, Cole RW, Khodjakov A, Sluder G. The checkpoint delaying anaphase in response to chromosome monoorientation is mediated by an inhibitory signal produced by unattached kinetochores. The Journal of cell biology. 1995;130:941–948. doi: 10.1083/jcb.130.4.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer G, Korner R, Hanisch A, Ries A, Nigg EA, Sillje HH. Proteome analysis of the human mitotic spindle. Molecular & cellular proteomics: MCP. 2005;4:35–43. doi: 10.1074/mcp.M400158-MCP200. [DOI] [PubMed] [Google Scholar]
- Schaar BT, Chan GKT, Maddox P, Salmon ED, Yen TJ. CENP-E function at kinetochores is essential for chromosome alignment. J Cell Biol. 1997;139:1373–1382. doi: 10.1083/jcb.139.6.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah JV, Botvinick E, Bonday Z, Furnari F, Berns M, Cleveland DW. Dynamics of centromere and kinetochore proteins; implications for checkpoint signaling and silencing. Current biology: CB. 2004;14:942–952. doi: 10.1016/j.cub.2004.05.046. [DOI] [PubMed] [Google Scholar]
- Shepperd LA, Meadows JC, Sochaj AM, Lancaster TC, Zou J, Buttrick GJ, Rappsilber J, Hardwick KG, Millar JB. Phosphodependent recruitment of Bub1 and Bub3 to Spc7/KNL1 by Mph1 kinase maintains the spindle checkpoint. Current biology: CB. 2012;22:891–899. doi: 10.1016/j.cub.2012.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha RL, Draviam VM. Lateral to end-on conversion of chromosome-microtubule attachment requires kinesins CENP-E and MCAK. Current biology: CB. 2013;23:1514–1526. doi: 10.1016/j.cub.2013.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K. Regulatory mechanisms of kinetochore-microtubule interaction in mitosis. Cellular and molecular life sciences: CMLS. 2013;70:559–579. doi: 10.1007/s00018-012-1057-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SS, Ha E, McKeon F. The human homologue of Bub3 is required for kinetochore localization of Bub1 and a Mad3/Bub1-related protein kinase. The Journal of cell biology. 1998;142:1–11. doi: 10.1083/jcb.142.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres JZ, Summers MK, Peterson D, Brauer MJ, Lee J, Senese S, Gholkar AA, Lo YC, Lei X, Jung K, et al. The STARD9/Kif16a kinesin associates with mitotic microtubules and regulates spindle pole assembly. Cell. 2011;147:1309–1323. doi: 10.1016/j.cell.2011.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai MY, Wang S, Heidinger JM, Shumaker DK, Adam SA, Goldman RD, Zheng Y. A mitotic lamin B matrix induced by RanGTP required for spindle assembly. Science. 2006;311:1887–1893. doi: 10.1126/science.1122771. [DOI] [PubMed] [Google Scholar]
- Tsai MY, Wiese C, Cao K, Martin O, Donovan P, Ruderman J, Prigent C, Zheng Y. A Ran signalling pathway mediated by the mitotic kinase Aurora A in spindle assembly. Nature cell biology. 2003;5:242–248. doi: 10.1038/ncb936. [DOI] [PubMed] [Google Scholar]
- Tsai MY, Zheng Y. Aurora A kinase-coated beads function as microtubule-organizing centers and enhance RanGTP-induced spindle assembly. Current biology: CB. 2005;15:2156–2163. doi: 10.1016/j.cub.2005.10.054. [DOI] [PubMed] [Google Scholar]
- Vong QP, Cao K, Li HY, Iglesias PA, Zheng Y. Chromosome alignment and segregation regulated by ubiquitination of survivin. Science. 2005;310:1499–1504. doi: 10.1126/science.1120160. [DOI] [PubMed] [Google Scholar]
- Vong QP, Liu Z, Yoo JG, Chen R, Xie W, Sharov AA, Fan CM, Liu C, Ko MS, Zheng Y. A role for Borg5 during trophectoderm differentiation. Stem cells. 2010;28:1030–1038. doi: 10.1002/stem.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Babu JR, Harden JM, Jablonski SA, Gazi MH, Lingle WL, de Groen PC, Yen TJ, van Deursen JM. The mitotic checkpoint protein hBUB3 and the mRNA export factor hRAE1 interact with GLE2p-binding sequence (GLEBS)-containing proteins. The Journal of biological chemistry. 2001;276:26559–26567. doi: 10.1074/jbc.M101083200. [DOI] [PubMed] [Google Scholar]
- Windecker H, Langegger M, Heinrich S, Hauf S. Bub1 and Bub3 promote the conversion from monopolar to bipolar chromosome attachment independently of shugoshin. EMBO reports. 2009;10:1022–1028. doi: 10.1038/embor.2009.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood KW, Sakowicz R, Goldstein LS, Cleveland DW. CENP-E is a plus end-directed kinetochore motor required for metaphase chromosome alignment. Cell. 1997;91:357–366. doi: 10.1016/s0092-8674(00)80419-5. [DOI] [PubMed] [Google Scholar]
- Yamagishi Y, Yang CH, Tanno Y, Watanabe Y. MPS1/Mph1 phosphorylates the kinetochore protein KNL1/Spc7 to recruit SAC components. Nature cell biology. 2012;14:746–752. doi: 10.1038/ncb2515. [DOI] [PubMed] [Google Scholar]
- Zheng Y. A membranous spindle matrix orchestrates cell division. Nature reviews Molecular cell biology. 2010;11:529–535. doi: 10.1038/nrm2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Iglesias PA. Nucleating new branches from old. Cell. 2013;152:669–670. doi: 10.1016/j.cell.2013.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.