Abstract
Alterations in social behavior are a hallmark of many neurodevelopmental disorders in humans. In rodents, social behavior is affected by prenatal insults. The outcomes are dependent on the timing of the insult as well as the sex and age of the animal tested. The limbic system is particularly important for social behavior, and a peak of neurogenesis within this system occurs on gestational day (G)15. Neurons appear particularly vulnerable to ethanol insult around the time they become post-mitotic. We tested the hypothesis that acute exposure to ethanol on G15 would result in significant social behavior deficits. Accordingly, Long Evans pregnant females were injected with ethanol (2.9 g/kg) or an equivalent volume of saline on G15. Offspring were assessed in a modified social interaction test on postnatal day (P) 28, P42, or P75, i.e., during early adolescence, late adolescence, or young adulthood. Prenatal ethanol exposure decreased social investigation in P28 females and transformed social preference into social avoidance in 75-day-old females. Contact behavior, play fighting, and locomotor activity differed as a function of age, but were not significantly affected by ethanol exposure. Males demonstrated significantly more contact behavior and play fighting at P42 than at P28 or P70, whereas there were no age-related changes in females. Adult females showed more locomotor activity than adult males. Overall, prenatal ethanol exposure on G15 enhanced social anxiety in females, with these effects seen in adulthood only.
Keywords: adolescence, autism, fetal alcohol syndrome, sex differences
Alterations in social behavior are among symptoms of many neurodevelopmental disorders, including fetal alcohol spectrum disorder (see [1] for references and review). Preclinical research has shown that acute or chronic prenatal exposure to ethanol alters social behavior of offspring [2–6]. These alterations are dependent on the timing of the exposure as well as the sex and age of the animal tested.
Many neural systems are implicated in the regulation of social behavior, with the limbic system playing a substantial role. The limbic system includes the hippocampus, septal nuclei, nucleus accumbens, amygdala, and cortical regions often including orbito-frontal cortex (OFC), occipital and temporal cortices, and the anterior cingulate cortex. Together, the OFC, amygdala, and anterior cingulate cortex have been termed the “social brain”, with these structures playing an important role in processing of social information and making decisions regarding social behavior [7].
During development of the central nervous system, neurons pass through critical periods of vulnerability to ethanol, with the time during which neurons undergo their final mitoses being one of such periods (e.g., [8]). In the rat, neurons of the OFC are born between gestational day (G) 13 and G20, with the majority born between G15 and G17 [9]. Neurons of the amygdala are born between G12 and G19, with the most neurons produced between G14 and G16 [10]. Many neurons of the ACC are also generated around G15 [11]. Therefore, the present study investigated possible alterations in social behavior following exposure to a high dose of ethanol on G15 during the peak neuronal generation for brain structures implicated in regulation of social behavior.
Pregnant Long Evans rats (Harlan, Indianapolis, IN) were received on G4. G1 was defined as the first day on which a sperm-positive plug was seen. Animals were maintained on a 12/12-hr light/dark cycle (lights off at 0700) in a temperature-controlled (22ºC) facility that was accredited by Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). All procedures were approved by the Committee for Humane Use of Animals (SUNY Upstate Medical University) and the Institutional Animal Care and Use Committee (Syracuse Veteran’s Affairs Medical Center).
On G15, pregnant rats were injected with 2.9 g/kg ethanol intraperitoneally (20% v/v solution in physiological saline, 0.9%, w/v, pH 7.4) at 9.00 AM (EtOH-exposed). Two hours later, they received a second intraperitoneal injection of 1.45 g/kg ethanol [5, 6, 8]. Control females received isovolumetric saline injections at the same times (Sal-exposed). Previous work with this model shows that the mean (± standard error) blood ethanol concentration is 287 ± 3.5 mg/dl two hr after the first injection [5].
On postnatal day (P) 3, litters were weighed and culled to 8–10 animals. Pups remained with their biological dam until weaning on P21 and were then housed in same sex groups of three to four littermates. Animals underwent social interaction (SI) testing on P28, P42, or P75 as previously described [5, 6]. Briefly, experimental rats (Sal- or EtOH-exposed prenatally) were placed alone in a partitioned testing apparatus for 30 min the day prior to SI testing. On the test day, experimental subjects (Sal- and EtOH-exposed) were marked with Sharpie™, put alone in a standard housing cage for 30 min, and then placed in the testing chamber for five min. Test chambers were made of Plexiglas and were 30 x 20 x 20 cm for adolescents (P28 and P42) and 45 x 30 x 20 cm for adults (P75). A semi-circular aperture (7 x 5 cm for adolescents and 9 x 7 cm for adults) in a central clear Plexiglas partition allowed animals to move between compartments. A prenatally non-exposed partner (not marked with Sharpie™), matched for age, sex, and weight, was also placed into the apparatus, and social interactions were recorded for 10 min. The test was performed under dim light conditions.
The frequencies of social investigation, play fighting and contact behavior were assessed from video recordings by an observer without knowledge of exposure condition of any given animal. Social investigation was defined as sniffing of any part of the partner’s body. Contact behavior consisted of social grooming and crawling over or under the partner. Play fighting was scored as a sum of following, chasing, playful nape attacks, and pinning. Social motivation was assessed using a coefficient of social preference/avoidance by scoring the number of crossovers (movements between compartments) an experimental subject made towards and away from the test partner. Coefficient (%) = (crossovers to – crossovers from) / (crossovers to + crossovers from) x 100. Social preference was defined by positive values of the coefficient, whereas social avoidance was indicated by negative values. In addition, the total number of crossovers was used as an index of general locomotor activity under social test circumstances.
Data were analyzed using separate for each behavioral measure 3 (age: P28, P42, P75) x 2 (prenatal exposure: Sal, EtOH) x 2 (sex) analyses of variance (ANOVAs). In order to avoid inflating the possibility of type II errors on tests with at least 3 factors [12], Fisher’s planned pairwise comparison test was used to explore significant effects and interactions. Significance was set at p<0.05, and all data are expressed as mean ± standard error (M ± SEM).
Maternal and litter data
The average daily weight gain of dams did not differ between Sal- and EtOH-injected pregnant females, nor was dam weight on G21 different between these groups (371.6g ± 6.7 in Sal-exposed dams vs. 355.6 g ± 10 in EtOH-exposed dams). There were no significant differences in the size of the litter or the proportion of males in the litters (Table 1). There was no difference in average pup weight between Sal- or EtOH-exposed groups.
Table 1.
Maternal and litter data.
| Prenatal Exposure | Dam weight on G21 (g) | Average daily weight gain by dams (g) | # pups | % male | Average pup weight on P3 (g) |
|---|---|---|---|---|---|
| Saline | 371.56 ± 6.66 | 7.70 ± 0.34 | 12.45 ± 0.43 | 57.73 ± 5.73 | 7.86 ± 0.17 |
| Ethanol | 355.57 ± 10.02 | 6.55 ± 0.48 | 11.73 ± 0.86 | 45.68 ± 4.49 | 8.04 ± 0.31 |
Data shown as mean ± standard error of the mean.
Social Behavior
Social investigation and social motivation showed a significant age x sex x prenatal exposure interaction (F2, 115 = 3.23, p = 0.041 for social investigation; F2, 115 = 3.69, p = 0.028 for social motivation; Fig. 1). Prenatal ethanol exposure significantly decreased social investigation in 28-day-old females relative to their Sal-exposed counterparts, but no effect was seen in 42- or 75-day-old animals. Social motivation was not affected by prenatal ethanol exposure in 28- or 42-day-old females, whereas it substantially decreased the coefficient in 75-day-old females, transforming social preference into social avoidance. The coefficient of social preference was not affected by prenatal exposure to ethanol in male rats at any age.
Figure 1.
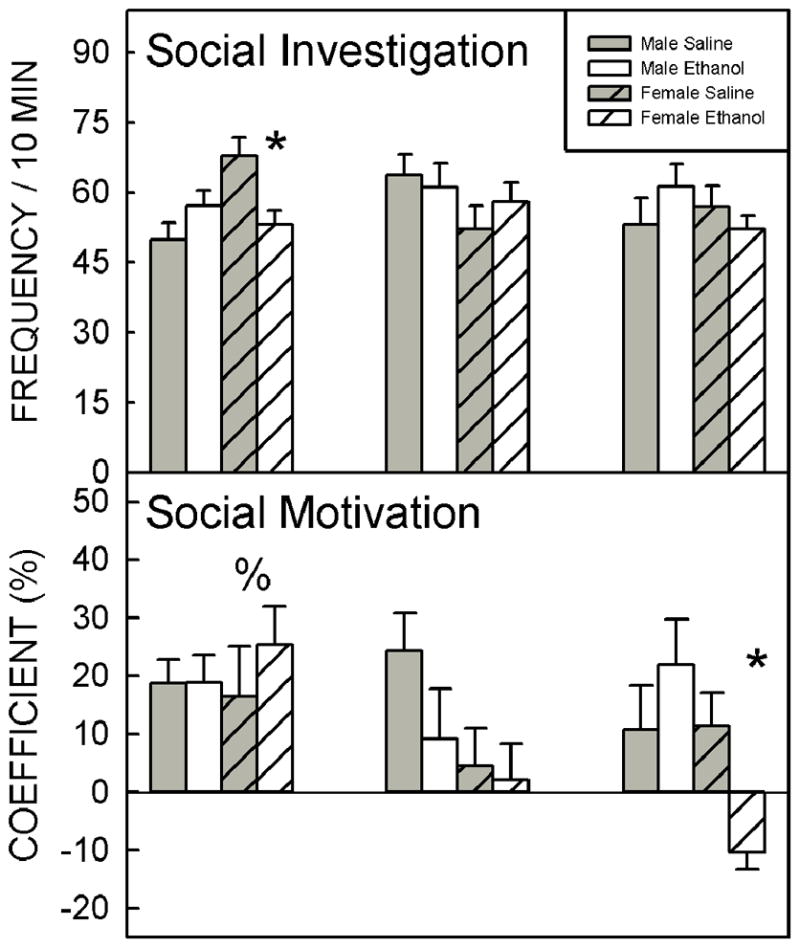
Social investigation and social motivation showed significant sex x prenatal exposure x age interactions. Social investigation was significantly lower in EtOH-exposed 28-day-old females relative to Sal-exposed counterparts. Within females, social motivation was highest at P28, and by P75 EtOH-exposed females showed social avoidance. Bars show the mean values, t-bars are the standard error of the mean. * EtOH-exposed animals are significantly (p<0.05) different from Sal-exposed age- and sex-matched controls; % significantly (p<0.05) different to females at other ages.
Contact behavior, play fighting, and locomotor activity were not significantly altered by prenatal ethanol exposure, however significant sex x age interactions were identified for all three measures (F2, 115 = 6.31, p = 0.004 for contact behavior; F2, 115 = 3.79, p = 0.026 for play fighting, and F2, 115 = 3.20, p = 0.044 for locomotor activity; Table 2).
Table 2.
Contact behavior, play fighting, and locomotor activity indexed as total number of crossovers in P28, P42, and P75 males and females, with data collapsed across prenatal exposure.
| Age | Sex | Contact Behavior Frequency | Play Fighting Frequency | Number of Crossovers |
|---|---|---|---|---|
| P28 | male | 15.48 ± 1.06 | 11.24 ± 1.03 | 21.09 ± 1.37 |
| female | 22.45 ± 1.95 a | 17.60 ± 2.20 a | 23.10 ± 1.61 b | |
| P42 | male | 27.86 ± 2.20 | 27.36 ± 4.02 c | 30.18 ± 1.94 c |
| female | 21.68 ± 1.57 a | 19.54 ± 2.58 | 29.00 ± 1.68 | |
| P75 | male | 16.29 ± 2.60 | 16.48 ± 2.76 | 21.43 ± 1.61 |
| female | 17.52 ± 1.89 | 15.29 ± 1.94 | 28.33 ± 1.42 a |
Data shown as mean ± standard error of the mean. Significant differences (p < 0.05) from males within each age are indicated by (a); (b) denotes significant differences from two other age groups in females; (c) denotes significant differences from other two age groups in males.
Contact behavior was significantly higher in females than in males at P28 and significantly higher in males than in females at P42. Contact behavior did not differ as a function of age in females, whereas 42-day-old males demonstrated significantly more contact behavior than younger and older males.
Play fighting was also significantly higher in females than in males at P28 and significantly higher in males than in females at P42. Play did not differ as a function of age in females, whereas 42-day-old males demonstrated significantly higher levels of play fighting than younger and older males.
No significant sex differences in overall locomotor activity were observed at P28 or P42, whereas adult females demonstrated more crossovers than adult males (Table 2). Males showed more locomotor activity at P42 than P28 and P70, whereas locomotor activity in females increased from P28 to P42, then remained stable.
The social consequences of acute exposure to ethanol on G15 during the peak of neuronal generation for parts of the limbic system were examined in the present study. This exposure caused alterations of social behavior that were largely confined to female offspring. Prenatal ethanol exposure decreased social investigation in 28-day-old females and transformed social preference into social avoidance in 75-day-old females.
Social investigation was decreased in ethanol-exposed females at P28, however, the values of the coefficient were positive at this age. Thus, when animals moved between compartments, they were more likely to move towards the play partner than away from it. Non-significant decreases in contact behavior and play fighting were also apparent at this age, thus ethanol-exposed young adolescent females, although moving towards the test partner, interacted substantially less with this partner relative to their saline-exposed counterparts.
It was surprising that behavioral alterations associated with prenatal ethanol exposure were seen in 28-day-old and 75-day-old females, with no effects of prenatal ethanol apparent in their counterparts tested at P42. Whereas 28-day-old females showed less social investigation than Sal-exposed controls, 75-day-old females demonstrated substantial alterations in social motivation, indexed via social avoidance. Both measures of social interactions, namely social investigation and social preference/avoidance are extremely sensitive to anxiogenic manipulations [13] and anxiolytic effects of ethanol (e.g., [14]). Therefore, this pattern of results shows that females exposed to ethanol prenatally and tested during early adolescence or adulthood demonstrated anxiety-like behavioral alterations under social test circumstances, with the indices of this anxiety differing among ages. In contrast, late adolescent females showed no anxiety-like changes in social interactions. Interestingly, social anxiety-like alterations were evident in pre-pubertal and adult females, whereas females that just underwent puberty (see [15] were not socially anxious. Although we did not assess the effects of prenatal ethanol exposure on puberty onset in the present study, there is some evidence suggesting a delayed onset of puberty in female rats prenatally exposed to ethanol (e.g., [16]). Thus, it is possible that the 42-day-old females in the present study could still have been peripubertal.
One of the possible mechanisms of the observed anxiety-like behavior in EtOH-exposed females tested at P28 and P75, but not at P42 may be related, at least in part, to alterations in neurosteroids. Neurosteroids, such as the progesterone metabolite allopregnanolone, produce positive modulatory actions on the GABAa receptor, which play a substantial role in mood regulation and anxiety relief [17]. Increases in anxiety are correlated with decreases in circulating levels of endogenous allopregnanolone [18]. Paradoxically, allopregnanolone increases anxiety-like behavior in pubertal mice [19], in contrast to its anxiolytic action before puberty and in adulthood [20]. The results of the present study revealed increases in anxiety-like behavior in pre-pubertal and adult females, indexed via decreases in social investigation before puberty, and emergence of social avoidance in adulthood. Given the reported age-related differences in sensitivity to anxiolytic/anxiolgenic effects of allopregnanolone, it is possible that prenatal ethanol exposure alters the circulating levels of neurosteroids, substantially decreasing them in EtOH-exposed females. These decreases, in turn, produce anxiety-like behavior in females tested at P28 (i.e., before puberty) or P75 (adulthood), but not in peripubertal females.
In addition to behavioral alterations associated with prenatal ethanol exposure, age-related changes in behavior were also seen. Males showed significantly more locomotor activity and play fighting in late adolescence than either early adolescence or young adulthood. The same trend was seen for contact behavior in males, but failed to reach statistical significance. This “inverted U-shape” pattern of ontogenetic changes in play fighting behavior is in agreement with outcomes reported previously [21, 22]. In contrast, females show increased locomotor activity at P42 and P75 relative to their 28-day-old counterparts, but did not demonstrate any age-related changes in play fighting or contact behavior.
Intriguingly, social behavior of male offspring was not affected by exposure to ethanol on G15, whereas our earlier findings demonstrated pronounced social deficits in male offspring following exposure to ethanol on G12 [5, 6]. In those studies that used the same mode of prenatal exposure to ethanol and social testing at the same ages, males exposed to ethanol prenatally exhibited substantial reductions of social investigation, contact behavior, and play fighting at all ages examined. Alterations in social motivation were age-dependent, with transformation of social preference into social avoidance evident in older adolescent and adult males and females. Other recent studies also report sex differences in outcome following an acute prenatal exposure to toxins (e.g., [23, 24]). Thus, it is important to include sex as a factor when performing such studies.
In summary, prenatal exposure on G15 enhanced social anxiety in females, with these effects seen in early adolescence and adulthood, but not in late adolescence, whereas males were previously shown to be vulnerable to an earlier exposure [5, 6]. At present the mechanism(s) underlying these effects are unknown.
Highlights.
Prenatal ethanol exposure decreased social investigation in young adolescent females
Prenatal ethanol exposure transformed social preference into social avoidance in adult females
Males in late adolescence demonstrated significantly more contact behavior and play fighting than older or younger animals
Females did not show age-related changes in social interactions.
Acknowledgments
The authors thank Renee Mezza, Wendi Burnette, Terri Novak, and Bill Bondi for technical assistance. This research was supported by the National Institute of Alcohol Abuse and Alcoholism (AA018693 and AA0178231 to SMM; AA012453 to EIV) and Autism Speaks (SMM). None of the funding sources had any role in study design, data collection, analysis or interpretation, in the writing of the report; or in the decision to submit the article for publication
Footnotes
The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kelly SJ, Day N, Streissguth AP. Effects of prenatal alcohol exposure on social behavior in humans and other species. Neurotoxicol Teratol. 2000;22:143–149. doi: 10.1016/s0892-0362(99)00073-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meyer LS, Riley EP. Social play in juvenile rats prenatally exposed to alcohol. Teratology. 1986;34:1–7. doi: 10.1002/tera.1420340102. [DOI] [PubMed] [Google Scholar]
- 3.Lugo JN, Jr, Marino MD, Cronise K, Kelly SJ. Effects of alcohol exposure during development on social behavior in rats. Physiol Behav. 2003:78185–194. doi: 10.1016/s0031-9384(02)00971-x. [DOI] [PubMed] [Google Scholar]
- 4.Hamilton DA, Akers KG, Rice JP, Johnson TE, Candelaria-Cook FT, Maes LI, Rosenberg M, Valenzuela CF, Savage DD. Prenatal exposure to moderate levels of ethanol alters social behavior in adult rats: relationship to structural plasticity and immediate early gene expression in frontal cortex. Behav Brain Res. 2010;207:290–304. doi: 10.1016/j.bbr.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mooney SM, Varlinskaya EI. Acute prenatal exposure to ethanol and social behavior: effects of age, sex, and timing of exposure. Behav Brain Res. 2011;216:358–364. doi: 10.1016/j.bbr.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Middleton FA, Varlinskaya EI, Mooney SM. Molecular substrates of social avoidance seen following prenatal ethanol exposure and its reversal by social enrichment. Dev Neurosci. 2012;34:115–128. doi: 10.1159/000337858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kennedy DP, Adolphs R. The social brain in psychiatric and neurological disorders. Trends Cogn Sci. 2012;16:559–572. doi: 10.1016/j.tics.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mooney SM, Miller MW. Time-specific effects of ethanol exposure on cranial nerve nuclei: gastrulation and neuronogenesis. Exp Neurol. 2007;205:56–63. doi: 10.1016/j.expneurol.2007.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bayer SA, Altman J. Neocortical development. New York, NY: Raven Press; 1991. [Google Scholar]
- 10.Bayer SA. Quantitative 3H-thymidine radiographic analyses of neurogenesis in the rat amygdala. J Comp Neurol. 1980;194:845–875. doi: 10.1002/cne.901940409. [DOI] [PubMed] [Google Scholar]
- 11.Bayer SA. Neurogenetic patterns in the medial limbic cortex of the rat related to anatomical connections with the thalamus and striatum. Exp Neurol. 1990;107:132–142. doi: 10.1016/0014-4886(90)90151-h. [DOI] [PubMed] [Google Scholar]
- 12.Carmer SG, Swanson MR. An evaluation of ten pairwise multiple comparison procedures by Monte Carlo methods. J Am Stat Assoc. 1973;68:66–74. [Google Scholar]
- 13.Doremus-Fitzwater TL, Varlinskaya EI, Spear LP. Social and non-social anxiety in adolescent and adult rats after repeated restraint. Physiol Behav. 2009;97:484–494. doi: 10.1016/j.physbeh.2009.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Varlinskaya EI, Doremus-Fitzwater TL, Spear LP. Repeated restraint stress alters sensitivity to the social consequences of ethanol in adolescent and adult rats. Pharmacol Biochem Behav. 2010;96:228–235. doi: 10.1016/j.pbb.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schneider M. Puberty as a highly vulnerable developmental period for the consequences of cannabis exposure. Addict Biol. 2008;13:253–263. doi: 10.1111/j.1369-1600.2008.00110.x. [DOI] [PubMed] [Google Scholar]
- 16.McGivern RF, Yellon SM. Delayed onset of puberty and subtle alterations in GnRH neuronal morphology in female rats exposed prenatally to ethanol. Alcohol. 1992;9:335–340. doi: 10.1016/0741-8329(92)90077-n. [DOI] [PubMed] [Google Scholar]
- 17.Smith SS, Shen H, Gong QH, Zhou X. Neurosteroid regulation of GABA(A) receptors: Focus on the alpha4 and delta subunits. Pharmacol Ther. 2007;116:58–76. doi: 10.1016/j.pharmthera.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frye CA, Petralia SM, Rhodes ME. Estrous cycle and sex differences in performance on anxiety tasks coincide with increases in hippocampal progesterone and 3alpha,5alpha-THP. Pharmacol Biochem Behav. 2000;67:587–596. doi: 10.1016/s0091-3057(00)00392-0. [DOI] [PubMed] [Google Scholar]
- 19.Shen H, Gong QH, Aoki C, Yuan M, Ruderman Y, Dattilo M, Williams K, Smith SS. Reversal of neurosteroid effects at alpha4beta2delta GABAA receptors triggers anxiety at puberty. Nat Neurosci. 2007;10:469–477. doi: 10.1038/nn1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bitran D, Dugan M, Renda P, Ellis R, Foley M. Anxiolytic effects of the neuroactive steroid pregnanolone (3 alpha-OH-5 beta-pregnan-20-one) after microinjection in the dorsal hippocampus and lateral septum. Brain Res. 1999;850:217–224. doi: 10.1016/s0006-8993(99)02150-2. [DOI] [PubMed] [Google Scholar]
- 21.Vanderschuren LJ, Niesink RJ, Van Ree JM. The neurobiology of social play behavior in rats. Neurosci Biobehav Rev. 1997;21:309–326. doi: 10.1016/s0149-7634(96)00020-6. [DOI] [PubMed] [Google Scholar]
- 22.Varlinskaya EI, Spear LP, Spear NE. Social behavior and social motivation in adolescent rats: role of housing conditions and partner's activity. Physiol Behav. 1999;67:475–482. doi: 10.1016/s0031-9384(98)00285-6. [DOI] [PubMed] [Google Scholar]
- 23.Olczak M, Duszczyk M, Mierzejewski P, Meyza K, Majewska MD. Persistent behavioral impairments and alterations of brain dopamine system after early postnatal administration of thimerosal in rats. Behav Brain Res. 2011;223:107–118. doi: 10.1016/j.bbr.2011.04.026. [DOI] [PubMed] [Google Scholar]
- 24.Kim KC, Kim P, Go HS, Choi CS, Park JH, Kim HJ, Jeon SJ, Dela Pena IC, Han SH, Cheong JH, Ryu JH, Shin CY. Male-specific alteration in excitatory post-synaptic development and social interaction in pre-natal valproic acid exposure model of autism spectrum disorder. J Neurochem. 2013;124:832–843. doi: 10.1111/jnc.12147. [DOI] [PubMed] [Google Scholar]


