Abstract
The risk from cumulative erythrocyte transfusions is poorly understood in oncology populations. This analysis among long-term survivors explored variation in transfusional burden over progressive eras of treatment identifying those at risk for iron overload. Transfusion records of 982 survivors of hematological malignancies treated at St. Jude were reviewed. After exclusions, 881 (90%) were assessed for cumulative volume, weight-adjusted volume, and transfusion number. Treatment intensity was assigned using the ITR-3 scale. Hematopoietic stem cell transplant and acute myeloid leukemia survivors had greater transfusional burden than conventional therapy recipients and acute lymphoblastic leukemia survivors respectively. Survivors of 5-10 years were more likely than survivors of >10 years to receive ≥10 transfusions (OR=2.0, 95% CI 1.5-2.8). Those with higher ITR-3 scores and more recent decades of treatment had a higher transfusional burden. Comprehensive transfusion histories are useful in identifying those at highest risk for iron overload.
Keywords: Transfusion medicine, Iron Overload, Cancer survivors, Pediatric leukemia
INTRODUCTION
Important progress has been made in the treatment of childhood hematologic malignancies over the past several decades.[1] The 5-year survival rate for childhood acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML) is now more than 85% and 60%, respectively.[2-6] Advancements in risk-directed therapeutics are largely responsible for these successes, but concurrent improvement in supportive care, such as availability of broad-spectrum antibiotics, transfusions, hematopoietic growth factors, and nutritional support have also contributed substantially.
Packed red blood cell (PRBC) transfusions are an essential component of leukemia and hematopoietic stem cell transplant (HSCT) treatment regimens since the majority of chemotherapeutic agents will stunt the innate production of red cells. Most pediatric leukemia patients receive combination chemotherapy over many months or years, and it is rare for a patient to complete therapy without PRBC transfusions. Information regarding the risk of cumulative erythrocyte transfusions and tissue iron accumulation is well understood in non-malignant populations; however, very little is known about transfusion-related iron burden in oncology populations. In patients with compromised red cell production, such as thalassemia or bone marrow failure syndromes, and in those chronically transfused to abate complications of the underlying disease (e.g., sickle cell disease and other hemolytic disorders), laboratory and histological signs of transfusion-induced iron overload can be seen after approximately 10 to 20 transfusions.[7,8] Excess iron accumulates rapidly due to the lack of a physiologic mechanism for its excretion and deposits in tissues throughout the body, particularly the liver, myocardium, and endocrine organs, resulting in organ dysfunction.[9-14] Extrapolating from these populations, it is plausible that iron overload may be an undiagnosed condition among cancer survivors and that the transfusional burden may be a potentially important risk determinant.[7]
Iron overload in childhood cancer survivors has been demonstrated in small studies, but how one’s transfusional burden correlates with accumulation of iron has not been sufficiently investigated. Although we did not evaluate for iron overload in this study, our retrospective review was conducted to evaluate transfusional burden among long-term survivors of hematologic malignancies treated with HSCT or conventional chemotherapy alone, with the aim of exploring variation in transfusion burden within progressive eras of treatment identifying those at highest risk for iron overload.
MATERIALS AND METHODS
Patient Population
Patients were included in the analysis if they were treated for a hematologic malignancy at St. Jude Children’s Research Hospital (St. Jude) between 1962 and 2004, and were long-term (≥5 years) survivors. Subjects treated with conventional chemotherapy, with or without HSCT, were included in the analysis. Survivors undergoing routine survivorship care were included (those between 5 and 10 years post diagnosis regardless of age), as were survivors who were no longer in active follow-up care but eligible to return to the institution for participation in clinical research (those age 18 years or older and ≥ 10 years post diagnosis). Patients were excluded from the analysis if they received transfusions at other institutions because transfusion records were incomplete. Longstanding practice at St. Jude has been to initiate erythrocyte transfusion therapy when hemoglobin concentration falls below 8 g/dl, or if symptoms of anemia develop or worsen.
Data Ascertainment
Blood bank records were reviewed (MC) and the number of PRBC transfusions and transfusion volumes were recorded. A 10% random sample was re-abstracted to verify accuracy. Uniform medical record abstraction was performed to obtain cancer diagnosis, year of treatment, presence or absence of relapse, and mean body weight during therapy. Transfusional burden was defined as (1) number of transfusions, (2) total volume of PRBCs (ml), and (3) volume of PRBCs/mean kilogram of body weight during therapy (ml/kg). To account for variability in treatment intensity among different diagnoses and different disease risk categories, a score of 1, 2, 3, or 4 was assigned using the Intensity of Treatment Rating Scale version 3.0 (ITR-3).[15] Decade at time of treatment was used to evaluate trends in treatment intensity and transfusional burden over time. This study was approved by the St. Jude Institutional Review Board with a waiver of consent from participants.
Statistical Analysis
Associations between categorical variables were evaluated with the Chi-Square test and differences in group medians were compared with the Wilcoxon Mann Whitney test. Transfusion data by decade and ITR-3 group are presented with boxplots. P-values less than 0.05 are considered statistically significant and all analyses were conducted in SAS Version 9.2 (Cary, NC).
RESULTS
Patients identified as eligible included 982 survivors treated from 1963 to 2004. Ninety-two survivors were excluded for incomplete blood bank records, resulting in 881 (90%) evaluable patients. Characteristics of the evaluable patients are shown in Table I. The median age at initiation of treatment was 5.64 years (range: 0.1-24.6) with slightly more males (55%) than females (45%) in the cohort, a 1.2:1 M:F ratio. HSCT survivors comprised 24% (n=215) of the patient population, with half (50%) of the study cohort being survivors of 5-10 years, and half >10 years. Of the survivors treated with conventional therapy alone, 14% (n=91) were survivors of 5 - 10 years and 86% (n=575) were > 10 years. AML was the most prevalent diagnosis among HSCT survivors (41%, n=89), and ALL was most prevalent among those treated with conventional therapy only (88%, n=584).
Table 1.
Clinical characteristics of 881 survivors of childhood hematological malignancy treated at St. Jude Children’s Research Hospital
| Characteristic | N=881 |
|---|---|
| Age at diagnosis (years) | |
| Median (range) | 5.6(0.1-24.6) |
| Follow-up time (years) | |
| Median (range) | 15.4 (5.3-48.1) |
| Gender | |
| Male | 487 (55%) |
| Female | 394 (45%) |
| Patient Type | |
| HSCT Survivors | 215 (24%) |
| 5-10 year duration | 107 (50%) |
| >10 year survivors | 108 (50%) |
| Non-HSCT Survivors | 666 (76%) |
| 5-10 year duration | 91 (14%) |
| >10 year survivors | 575 (86%) |
| Diagnosis | |
| HSCT Survivors | |
| Acute Lymphoblastic Leukemia | 43 (20%) |
| Acute Myeloid Leukemia | 89 (41%) |
| Chronic Myeloid Leukemia | 30 (14%) |
| Hodgkin Lymphoma | 21 (10%) |
| Non-Hodgkin Lymphoma | 11 (5%) |
| Other | 21 (10%) |
| Non-HSCT Survivors | |
| 5-10 year duration | |
| Acute Lymphoblastic Leukemia | 67 (74%) |
| Acute Myeloid Leukemia | 23 (25%) |
| Other | 1 (1%) |
| >10 year survivors | |
| Acute Lymphoblastic Leukemia | 517 (90%) |
| Acute Myeloid Leukemia | 49 (9%) |
| Other | 9 (2%) |
| ITR-3 Score | |
| 1 (lowest intensity) | 6 (1%) |
| 2 | 228 (26%) |
| 3 | 304 (35%) |
| 4 (highest intensity) | 343 (39%) |
HSCT survivors were transfused more heavily than conventional therapy recipients with regard to median number of transfusions (20 vs. 7), median cumulative volume (3890 ml vs. 1250 ml), and median volume per kilogram of body weight (140 ml/kg vs. 61 ml/kg), all p-values <0.001. (Table II) Conventional therapy only recipients with AML had a significantly higher transfusional burden compared to those with ALL – i.e. median 21 vs. 6 transfusions, 3,793 ml vs. 1,134 ml, and 182 ml/kg vs. 57 ml/kg (all p-values <0.001), respectively. Recent survivors (5-10 years) were twice as likely (OR=2.0, 95% CI 1.5-2.8, p-value < 0.001) to have received 10 or more transfusions than were survivors of >10 years duration. (Table III)
Table II.
Transfusion burden among survivors of hematological malignancies by primary treatment modality and diagnosis
| HSCT Recipients (N=215) |
Recipients of Conventional Therapy only (N=666) |
p-value | |
|---|---|---|---|
| Median transfusion number (range) | 20 (1-92) | 7 (1-85) | p < 0.001 |
| Median volume transfused (ml) | 3,890 ml | 1,250 ml | p < 0.001 |
| Median volume/kg (ml/kg) | 140 ml/kg | 61 ml/kg | p < 0.001 |
| Recipients of Conventional Therapy only | |||
|
ALL
(N=584) |
AML
(N=72) |
p-value | |
| Median transfusion number (range) | 6 (1-62) | 21 (1-85) | p < 0.001 |
| Median volume transfused (ml) | 1,134 ml | 3,793 ml | p < 0.001 |
| Median volume/kg (ml/kg) | 57 ml/kg | 182 ml/kg | p< 0.001 |
Table III.
Comparison of survivor time versus number of transfusions
| SURVIVOR TIME | Total | ||
|---|---|---|---|
| 5-10 years | >10 years | ||
|
10 OR MORE
TRANSFUSIONS |
116 | 280 | 396 (45%) |
|
l to 9
TRANSFUSIONS |
82 | 403 | 485 (55%) |
| Total | 198 (22.5%) | 683 (77.5%) | 881 (100%) |
• Chi-Square p-value < 0.001
• Survivors of 5-10 years are twice as likely to have received 10 or more transfusions than survivors of >10 years. Unadjusted odds ratio: 2.0 (95% CI 1.5-2.8)
An analysis of transfusion number and volume according to ITR-3 scores and treatment decade is shown in Figure I. Transfusion number and weight-adjusted volume (ml/kg) increased with increasing ITR-3 score and with more recent decade of treatment. Transfusional burden is highest among those treated in the 1990’s and 2000’s, particularly in those with an ITR-3 score of 4.
Figure 1. Transfusion burden as a function of decade of treatment and treatment intensity scores.
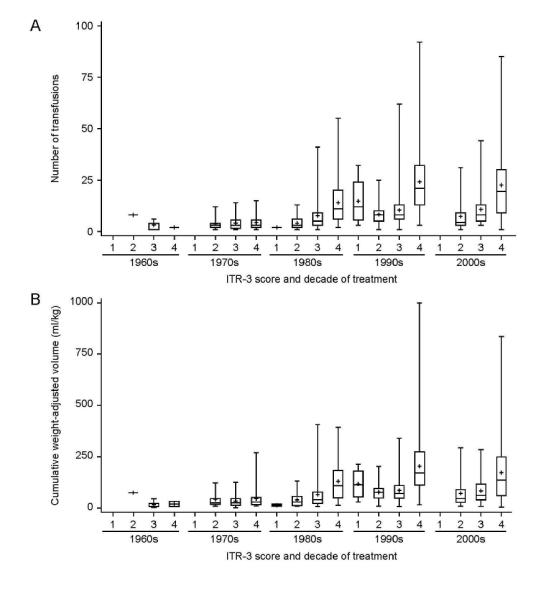
(A) Total number of transfusions and (B) weight-adjusted volume is compared among different treatment intensity scores and decade of treatment. Boxplots are presented with the lower and upper fences at the minimum and maximum values. Group means are represented by the + symbol.
DISCUSSION
Our data illustrate that hematological malignancy survivors of 5-10 years received a higher number of transfusions and a higher volume of PRBCs than similar survivors treated >10 years ago. Survivors received greater amounts of blood in the setting of higher treatment intensity scores and more recent treatment decades. More liberal transfusion practices among clinicians could explain this finding, however the standard institutional transfusion threshold guidelines at St. Jude did not change throughout the time period under study. The evolution of more intensive chemotherapy regimens is another explanation as these treatments induce more severe or prolonged anemia necessitating greater transfusion support. Though we show that there are more survivors with greater treatment intensity scores, this may be impacted by survivor bias since patients from remote decades who had more aggressive disease, more intensive treatment regimens, and potentially more PRBC transfusions, may have died in greater proportion than more recent patients before meeting the eligibility requirement of surviving at least 5 years from diagnosis to be part of this analysis.
The clinical implications of our data are that more modern survivors have received substantially more erythrocyte transfusions, placing them at risk for complications associated with repeated transfusions, namely iron overload. Our observation is that the risk for iron overload is higher for patients treated recently than that of survivors treated more than 10 years ago. Other important conclusions from our analysis are that, of the survivors treated with conventional chemotherapy alone, AML survivors have a significantly higher transfusional burden than survivors of ALL. In addition, the transfusional burden among survivors of HSCT is significantly greater than those treated with conventional chemotherapy alone.
Understanding transfusion patterns is an essential step toward characterizing those at risk for iron overload and any related organ dysfunction. Two previous studies have quantified PRBC transfusions in pediatric cancer populations. Eng and Fish reviewed 107 non-relapsed and non-transplanted pediatric ALL patients treated between 1995 and 2007 and concluded that those with high risk ALL received a greater volume of PRBC transfusions than did those with standard risk disease.[16] Ruccione et al. reported on 214 pediatric oncology patients that were treated between 2004 and 2009 for a variety of hematological and solid malignancies and found that higher treatment intensity scores, were associated with more transfusions.[17] Our analysis extends that beyond the previous studies cited, and is the first to delineate how survivor time, decade of treatment, primary diagnosis, and type of treatment (HSCT vs. conventional therapy only) may reflect transfusional burden and resultant risk of iron overload.
Importantly, as more pediatric patients become long-term cancer survivors, the relevance of comprehensive transfusion histories will be ever increasing. In 2009, there were an estimated 363,000 childhood cancer survivors living in the United States [18] and the prevalence of patients with a history of a large transfusional burden will continue to grow. It is well known that childhood leukemia survivors have a high risk of chronic health conditions as a result of their prior therapy.[19] In a study by investigators from the Childhood Cancer Survivor Study (CCSS), ALL survivors were nearly seven times more likely than their siblings to report the presence of a chronic cardiac condition.[20] The CCSS has also shown that survivors of leukemia are 1.5 times more likely to be hospitalized for cardiac causes and ALL survivors are 4.2 times more likely to die of cardiac-related illness compared to age-, calendar year–, and sex-specific rates in the United States population.[21,22] Endocrinopathies, in particular growth hormone deficiency and insulin resistance as a component of the metabolic syndrome, are also well established late effects in long-term ALL survivor populations.[23-25] These organ systems, while vulnerable to chemotherapy toxicity, are also the target of iron accumulation. Though it is not yet clear if iron overload is contributing to these chronic conditions, given that iron overload is a reversible condition with phlebotomy or chelating agents, further investigation is warranted.
Cancer survivors do not routinely undergo surveillance for iron overload, with the exception of HSCT survivors in whom an annual ferritin measurement is recommended by the Children’s Oncology Group Long-term Follow-up Guidelines. Since ferritin is an acute phase reactant, it may be falsely elevated due to any systemic ongoing inflammatory process and thus may not be the most reliable measurement of body iron burden. Detailed transfusion histories may be a superior assessment of one’s risk of iron overload; however, research is lacking to confirm this assumption and transfusion data have not traditionally been collected by survivorship programs.
Direct quantification of tissue iron burden can be performed using invasive methods, such as liver biopsy, and non-invasive methods [e.g., superconducting quantum interference device (SQUID) susceptometer,[26-28] and R2 and R2* magnetic resonance imaging (MRI) techniques].[29-31] Liver iron content (LIC) is considered the reference method for body iron burden assessment, and R2 and R2* MRI modalities are increasingly being used for LIC assessment due to the non-invasive nature and growing availability of the technique. The value of these scans in routine survivorship care is yet unknown since its use as a tool to quantify LIC has been sparsely utilized. The high usage of blood in pediatric malignancy survivors justifies the utilization of precise techniques to define the breadth and depth of iron overload in this population.
In summary, our data show that modern survivors of hematological malignancies, particularly those who underwent high intensity therapy, have received a large transfusion burden leaving them at risk for iron overload. Higher survivor rates of pediatric hematological malignancies have paralleled the increased usage of blood. We suggest that comprehensive transfusion histories should become standard among survivors treated since 1980 to identify those at highest risk for iron overload. Further studies are needed to better delineate surveillance guidelines, the use of MRI, and, ultimately, when intervention is warranted.
ACKNOWLEDGEMENTS
This work was supported by the Cancer Center Support (CORE) grant CA 21765 from the National Cancer Institute, and by the American Lebanese Syrian Associated Charities (ALSAC).
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Rubnitz JE, Inaba H, Dahl G, et al. Minimal residual disease-directed therapy for childhood acute myeloid leukaemia: results of the AML02 multicentre trial. Lancet Oncol. 2010;11:543–552. doi: 10.1016/S1470-2045(10)70090-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Creutzig U, Zimmermann M, Lehrnbecher T, et al. Less toxicity by optimizing chemotherapy, but not by addition of granulocyte colony-stimulating factor in children and adolescents with acute myeloid leukemia: results of AML-BFM 98. J Clin Oncol. 2006;24:4499–4506. doi: 10.1200/JCO.2006.06.5037. [DOI] [PubMed] [Google Scholar]
- 4.Lehrnbecher T, Zimmermann M, Reinhardt D, Dworzak M, Stary J, Creutzig U. Prophylactic human granulocyte colony-stimulating factor after induction therapy in pediatric acute myeloid leukemia. Blood. 2007;109:936–943. doi: 10.1182/blood-2006-07-035915. [DOI] [PubMed] [Google Scholar]
- 5.Pui CH, Campana D, Pei D, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med. 2009;360:2730–2741. doi: 10.1056/NEJMoa0900386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmiegelow K, Forestier E, Hellebostad M, et al. Long-term results of NOPHO ALL-92 and ALL-2000 studies of childhood acute lymphoblastic leukemia. Leukemia. 2010;24:345–354. doi: 10.1038/leu.2009.251. [DOI] [PubMed] [Google Scholar]
- 7.Olivieri NF. Progression of iron overload in sickle cell disease. Semin Hematol. 2001;38:57–62. doi: 10.1016/s0037-1963(01)90060-5. [DOI] [PubMed] [Google Scholar]
- 8.Inati A, Musallam KM, Wood JC, Taher AT. Iron overload indices rise linearly with transfusion rate in patients with sickle cell disease. Blood. 2010;115:2980–2981. doi: 10.1182/blood-2009-09-243568. author reply 2981-2982. [DOI] [PubMed] [Google Scholar]
- 9.Anderson L, Holden S, Davis B, et al. Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. Eur Heart J. 2001;22:2171–2179. doi: 10.1053/euhj.2001.2822. [DOI] [PubMed] [Google Scholar]
- 10.Thakerngpol K, Fucharoen S, Boonyaphipat P, et al. Liver injury due to iron overload in thalassemia: histopathologic and ultrastructural studies. Biometals. 1996;9:177–183. doi: 10.1007/BF00144623. [DOI] [PubMed] [Google Scholar]
- 11.Risdon R, Barry M, Flynn D. Transfusional iron overload: the relationship between tissue iron concentration and hepatic fibrosis in thalassaemia. J Pathol. 1975;116:83–95. doi: 10.1002/path.1711160204. [DOI] [PubMed] [Google Scholar]
- 12.Olivieri N, Nathan D, MacMillan J, et al. Survival in medically treated patients with homozygous beta-thalassemia. N Engl J Med. 1994;331:574–578. doi: 10.1056/NEJM199409013310903. [DOI] [PubMed] [Google Scholar]
- 13.Zurlo M, De Stefano P, Borgna-Pignatti C, et al. Survival and causes of death in thalassaemia major. Lancet. 1989;2:27–30. doi: 10.1016/s0140-6736(89)90264-x. [DOI] [PubMed] [Google Scholar]
- 14.Merkel PA, Simonson DC, Amiel SA, et al. Insulin resistance and hyperinsulinemia in patients with thalassemia major treated by hypertransfusion. N Engl J Med. 1988;318:809–814. doi: 10.1056/NEJM198803313181303. [DOI] [PubMed] [Google Scholar]
- 15.Kazak AE, Hocking MC, Ittenbach RF, et al. A revision of the intensity of treatment rating scale: Classifying the intensity of pediatric cancer treatment. Pediatr Blood Cancer. 2011 doi: 10.1002/pbc.23320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eng J, Fish JD. Insidious iron burden in pediatric patients with acute lymphoblastic leukemia. Pediatr Blood Cancer. 2011;56:368–371. doi: 10.1002/pbc.22851. [DOI] [PubMed] [Google Scholar]
- 17.Ruccione KS, Mudambi K, Sposto R, Fridey J, Ghazarossian S, Freyer DR. Association of projected transfusional iron burden with treatment intensity in childhood cancer survivors. Pediatr Blood Cancer. 2011 doi: 10.1002/pbc.24046. [DOI] [PubMed] [Google Scholar]
- 18.Howlader N, Noone A, Krapcho M, et al. SEER Cancer Statistics Review. National Cancer Institute; Bethesda, MD: 2012. pp. 1975–2009. [Google Scholar]
- 19.Ness KK, Armenian SH, Kadan-Lottick N, Gurney JG. Adverse effects of treatment in childhood acute lymphoblastic leukemia: general overview and implications for long-term cardiac health. Expert Rev Hematol. 2011;4:185–197. doi: 10.1586/ehm.11.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mody R, Li S, Dover DC, et al. Twenty-five-year follow-up among survivors of childhood acute lymphoblastic leukemia: a report from the Childhood Cancer Survivor Study. Blood. 2008;111:5515–5523. doi: 10.1182/blood-2007-10-117150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Armstrong GT, Liu Q, Yasui Y, et al. Late mortality among 5-year survivors of childhood cancer: a summary from the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2328–2338. doi: 10.1200/JCO.2008.21.1425. United States. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurt BA, Nolan VG, Ness KK, et al. Hospitalization rates among survivors of childhood cancer in the Childhood Cancer Survivor Study cohort. Pediatr Blood Cancer. 2012;59:126–132. doi: 10.1002/pbc.24017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gurney J, Ness K, Sibley S, et al. Metabolic syndrome and growth hormone deficiency in adult survivors of childhood acute lymphoblastic leukemia. Cancer. 2006;107:1303–1312. doi: 10.1002/cncr.22120. [DOI] [PubMed] [Google Scholar]
- 24.Link K, Moëll C, Garwicz S, et al. Growth hormone deficiency predicts cardiovascular risk in young adults treated for acute lymphoblastic leukemia in childhood. J Clin Endocrinol Metab. 2004;89:5003–5012. doi: 10.1210/jc.2004-0126. [DOI] [PubMed] [Google Scholar]
- 25.Taskinen M, Saarinen-Pihkala U, Hovi L, Lipsanen-Nyman M. Impaired glucose tolerance and dyslipidaemia as late effects after bone-marrow transplantation in childhood. Lancet. 2000;356:993–997. doi: 10.1016/S0140-6736(00)02717-3. [DOI] [PubMed] [Google Scholar]
- 26.Sheth S. SQUID biosusceptometry in the measurement of hepatic iron. Pediatr Radiol. 2003;33:373–377. doi: 10.1007/s00247-003-0877-x. [DOI] [PubMed] [Google Scholar]
- 27.Fischer R, Piga A, Harmatz P, Nielsen P. Monitoring long-term efficacy of iron chelation treatment with biomagnetic liver susceptometry. Ann N Y Acad Sci. 2005;1054:350–357. doi: 10.1196/annals.1345.043. [DOI] [PubMed] [Google Scholar]
- 28.Nielsen P, Engelhardt R, Düllmann J, Fischer R. Non-invasive liver iron quantification by SQUID-biosusceptometry and serum ferritin iron as new diagnostic parameters in hereditary hemochromatosis. Blood Cells Mol Dis. 2002;29:451–458. doi: 10.1006/bcmd.2002.0583. [DOI] [PubMed] [Google Scholar]
- 29.St Pierre TG, Clark PR, Chua-anusorn W, et al. Noninvasive measurement and imaging of liver iron concentrations using proton magnetic resonance. Blood. 2005;105:855–861. doi: 10.1182/blood-2004-01-0177. [DOI] [PubMed] [Google Scholar]
- 30.Wood JC, Enriquez C, Ghugre N, et al. MRI R2 and R2* mapping accurately estimates hepatic iron concentration in transfusion-dependent thalassemia and sickle cell disease patients. Blood. 2005;106:1460–1465. doi: 10.1182/blood-2004-10-3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hankins JS, McCarville MB, Loeffler RB, et al. R2* magnetic resonance imaging of the liver in patients with iron overload. Blood. 2009;113:4853–4855. doi: 10.1182/blood-2008-12-191643. [DOI] [PMC free article] [PubMed] [Google Scholar]


