Abstract
Background
Limited data exist on induction mortality of pediatric patients with acute promyelocytic leukemia in the United States, usage of all-trans retinoic acid (ATRA) during acute promyelocytic leukemia induction, and the resources needed to deliver induction therapy.
Procedure
Using the Pediatric Health Information System database we established a retrospective cohort of patients treated for newly diagnosed acute promyelocytic leukemia with ATRA between January 1999 and September 2009 in 32 of 43 PHIS contributing free-standing pediatric hospitals in the United States. Standard statistical methods were used to determine inhospital induction mortality, ATRA administration, and resource utilization during a 60-day observation period.
Results
A total of 163 children were identified who met eligibility criteria for cohort inclusion; 52% were female and 76% were white with an average age of 12.7 years. A total of 12 patients (7.4%) died, with 7 (58.3%) dying within the first 7 days of first admission. The mean time to first ATRA exposure increased with decreasing age (P=0.0016). Resource utilization for management of retinoic acid syndrome was higher than anticipated based on prior studies and differed significantly from patients with non-M3 acute myeloid leukemia.
Conclusions
The induction mortality for pediatric acute promyelocytic leukemia remains substantial with wide variation in ATRA administration and high rates of resource utilization.
Keywords: APL, ATRA, induction mortality, resource utilization, retinoic acid syndrome
INTRODUCTION
Acute promyelocytic leukemia (APL) represents about 10% of pediatric acute myeloid leukemia (AML) [1] and is characterized by the t(15;17) translocation that fuses the promyelocytic leukemia gene on chromosome 15 to the retinoic acid receptor alpha gene on chromosome 17 [2]. Current pediatric APL therapy is based on anthracyclines, cytarabine, and all-trans retinoic acid (ATRA). In the past decade several cooperative group trials from around the world have reported on the success of ATRA containing regimens for children with APL [3–6]. These studies have defined induction mortality rate and APL differentiation syndrome rates ranging from 3.2–8.2% and 7.5–20%, respectively.
These reports have focused primarily on outcomes within cooperative group clinical trials and have not evaluated compliance of ATRA administration or resource utilization in patients with APL. In addition, there have been no published studies that focus on the clinical experience of children receiving ATRA containing induction chemotherapy for APL in the United States. We sought to address this data gap by assembling a cohort of pediatric APL patients that received ATRA as part of their induction chemotherapy in the United States. This cohort was established from the Pediatric Health Information Systems (PHIS) database, a national administrative database of 43 freestanding pediatric hospitals in the United States. The PHIS database has been effectively utilized to study various pediatric medical conditions, including renal failure in pediatric patients with AML, the risk of thrombosis associated with central venous catheters, and the comparative effectiveness of oral versus intravenous antibiotic treatment of osteomyelitis [7–9].
We hypothesized that PHIS data would allow accurate estimation of the case fatality rate of children with APL within 60 days of their first ATRA exposure, the patterns of ATRA use during hospitalization for APL induction, and the clinical resource utilization in children treated for APL with ATRA. We also hypothesized that the patterns of resource utilization would differ significantly from other acute myeloid leukemia subtypes.
MATERIALS AND METHODS
Study Design
We performed a retrospective cohort study to describe the inpatient experience of patients receiving ATRA for newly diagnosed APL during their incident hospitalization. Children entered the cohort on the day of admission during which the APL diagnosis was first reported. Each patient was followed for up to 60 days from the first day of ATRA exposure. If patients were discharged and readmitted during this 60-day follow-up period then data for these readmissions were also captured. Patients who did not have a subsequent admission in the PHIS database were considered lost to follow-up at the completion of their final known hospital admission. Patients were censored if they suffered an inpatient death prior to 60 days from first ATRA exposure.
Data Source
Data contained in the PHIS database include the following: encrypted patient medical record number, demographics, dates of admission and discharge, up to 41 International Classification of Diseases-9-Clinical Modification (ICD-9-CM) discharge diagnosis and procedure codes per hospital admission, and specific billing/resource utilization data, including pharmaceuticals, blood products, laboratory tests, radiology imaging studies ordered, and clinical services utilized. All billing/resource utilization data include date of order. Additionally, pharmaceutical data includes medication type and route of administration. However, laboratory and radiology result data are not available.
PHIS member hospitals have access to the multi-center database via a virtual private network. Data are de-identified at the time that each hospital submits their data. Oversight of PHIS data quality methods is a joint effort between the Children's Hospital Association (data management center), Truven Health Analytics (data processing partner), and participating hospitals. After file submission to Truven Health Analytics, data quality audits are performed. These audits primarily check for valid entries (e.g., valid ICD-9-CM diagnosis codes) and reasonable patient information (e.g., birthweight). Reports are generated that identify errors needing correction by the respective hospitals. Error rates above threshold values require hospitals to review their data and resubmit until error rates fall below the threshold values. Known data quality issues are transparently communicated to all PHIS data users. These data quality reports allow the data users to exclude data for data quality reasons.
The PHIS database was queried to extract each patient's data for each hospital day identified during the study period of interest. Once data were extracted, SAS version 9.2 (Cary, NC) and STATA statistical software version 11.0 (College Station, TX) were used to convert the PHIS data into a database representing information for the daily inpatient experience of each child in the final cohort. For each inpatient day, information on medications ordered and hospital resources utilized (see below) was available. The specific disposition at the time of hospital discharge was recorded for those patients completing their hospitalization within the 60-day study period. The final database contained each APL patient's daily medication and resource utilization information from their index admission up to 60 days after their first ATRA exposure.
Defining the Study Cohort
The source population for our study was patients with a hospital discharge date in the PHIS database between January 1, 1999 and September 30, 2009. Since ICD-9-CM codes do not include a code specific for APL, children were considered for study cohort inclusion if their index hospitalization in the database was assigned an ICD-9-CM code (either as a primary or secondary diagnosis) for any type of myeloid or unspecified leukemia (205.xx: myeloid leukemia, 206.xx: monocytic leukemia, 207.xx: other specified leukemia, and 208.xx: leukemia of unspecified cell type). Additionally, patients were required to have received at least one dose of oral ATRA within the first 30 days of this index hospitalization. In order to further restrict the cohort to children with likely newly diagnosed APL, the final cohort was limited to patients that did not have any hospitalization in the 90 days preceding the admission where ATRA was first given. Once included in the final cohort, the patients were followed from the date of their admission until 60 days after the first ATRA dose, hospital discharge, or until death. The cohort of patients with non-APL acute myeloid leukemia (AML) was defined as previously reported [10].
Defining Chemotherapy Regimens
A manual review of the specific chemotherapy regimen administered to each patient in his or her index admission was performed (S.S.). Pharmaceutical data for each patient were manually reviewed and compared to currently accepted APL induction chemotherapy regimens as defined by two pediatric oncologists (R.A. and J.G.). A specific chemotherapy regimen was assigned to a patient based on the chemotherapy agents delivered and timing of chemotherapy administration. In certain instances, patients received ATRA plus other chemotherapy agents but not in an identifiable pattern, and thus, were classified as “ATRA with other regimen.” Lastly, some patients were found to have received only ATRA, and thus, they were defined as “ATRA only.” No differentiation was made between patients enrolled on a study protocol (i.e., CALGB9710 or AAML0631) and those treated according to the protocol but not enrolled on study.
Study Variables
Demographics
Demographic data (age, gender, and race) were extracted for each patient in the cohort at the time of their first APL hospitalization. Gender was coded as a dichotomous variable (male/female), age in years, and race was collected as a categorical variable (white, black, Asian, Native American, other, or missing). Since a substantial number of patients had missing ethnicity data, no analyses by ethnicity were conducted.
Hospital resources utilized
The need for vasopressor support (epinephrine, dopamine, norepinephrine, or dobutamine), steroids (prednisone, dexamethasone, methylprednisolone, or hydrocortisone), diuretics (listed in Appendix 1), blood product replacement (platelets, packed red blood cells (PRBC), fresh frozen plasma (FFP), and cryoprecipitate) and radiology studies (chest radiograph [CXR], computerized tomography [CT], and magnetic resonance imaging [MRI]) was documented based on daily billing data.
Hospital discharge status
The conclusion of each hospitalization is coded in the PHIS database as discharged to home, discharged to another care facility or inpatient death. Using this disposition data, the case fatality rate for new onset APL within the first 30 days from admission and within the first 30 days from ATRA exposure was calculated.
Statistical Analyses
Baseline and demographic characteristics were summarized by standard descriptive statistics. Each patient's day-by-day ATRA exposure from admission to 60 days post-admission was presented graphically. Time from admission to the first ATRA exposure and percent of hospital days with ATRA exposure were compared by demographics (age, race, and gender), using ANOVA or linear regression. Percent of patients using vasopressors, steroids, diuretics, and CXR was plotted week by week (starting from the first day of ATRA exposure). Percent of patients exposed to each blood product replacement and CNS imaging was plotted day by day (starting from the first day of admission), by two patient groups of APL and non-APL AML. Number of exposure days (to each blood product replacement and CNS imaging) in the first seven hospital days was compared by the two patient groups, using a two-sample t-test. SAS 9.2 (Cary, NC) was used for all statistical analyses. A two-sided P-value of <0.05 was considered statistically significant.
RESULTS
Between January 1, 1999 and September 30, 2009, 198 patients in the PHIS database were hospitalized, assigned an ICD-9-CM code consistent with myeloid or unspecified leukemia (205.xx–208.xx), and received at least one dose of oral ATRA in the first 30 days of the hospitalization. Of these 198 patients, 27 were excluded because they had been admitted at least once in the 90 days prior to this hospitalization. Manual review of the chemotherapy administered to the remaining 171 patients was performed with 8 patients having chemotherapy exposures not at all consistent with APL. This resulted in a final study population of 163 patients.
Table I displays patient characteristics as well as the length of hospital stay and frequency of inpatient death for the first APL admission. The average age of patients was 12.67 years (IQR: 4.99-16.07) with females representing 52.2% of the cohort and white patients represented more than 75% of the cohort. There were 12 patients (7.4%) that died during the study period. The in-hospital case fatality rate for patients within 30 days of admission was 6.14%, and was slightly higher at 6.75% within 30 days from first ATRA exposure. Of the patients that died, all but one patient died during their index hospitalization. Furthermore, the majority of these in-hospital fatalities (7/12 or 58.33%) occurred within the first 7 days of admission.
TABLE I.
Cohort Characteristics
| Total (N = 163) | ||
|---|---|---|
|
|
|
|
| Age | ||
| Median | 12.67 | 4.99–16.07 (IQR) |
| <3 | 26 | 15.9% |
| ≥3 and <11 | 44 | 27.0% |
| ≥11 and <18 | 81 | 49.7% |
| ≥18 | 12 | 7.4% |
| Female (%) | 85 (52.2%) | 52.2% |
| Race (%) | ||
| White | 124 | 76.07% |
| Black | 15 | 9.26% |
| Asian | 3 | 1.84% |
| Native American | 0 | 0% |
| Other | 10 | 5.99% |
| Missing | 11 | 6.13% |
| Length of first hospitalization (days) | ||
| Median in-Hospital case fatality rate | 23 | 13–37 (IQR) |
| 30 Days from hospital admit date | 10 | 6.14% |
| 30 Days from 1st ATRA exposure | 11 | 6.75% |
| 60 Days from hospital admit date | 12 | 7.4% |
Table II illustrates the variation in chemotherapy regimens administered as induction therapy for APL patients. The majority of patients (44%) received ATRA with other chemotherapy agents in an unidentifiable pattern. Of the identifiable patterns CALGB9710 was the most frequently administered chemotherapy regimen. The case fatality rate was found to be highest in patients who received “ATRA only” for both death within 30 days of admission (11.11%) and death within 30 days of initial ATRA use (16.67%).
TABLE II.
Initial Chemotherapy Regimens Administered
| Chemo regimen | Total (n=163) | 30 Day mortality from admission (n=10) | 30 Day mortality from 1st ATRA dose (n = 11) |
|---|---|---|---|
| AAML0631 | 6 | 1 | 1 |
| AIDA | 4 | 0 | 0 |
| CALGB9710 | 62 | 2 | 2 |
| EAPLG | 3 | 0 | 0 |
| ATRA with unidentified chemotherapy regimens | 73 | 6 | 6 |
| ATRA only | 15 | 1 | 2 |
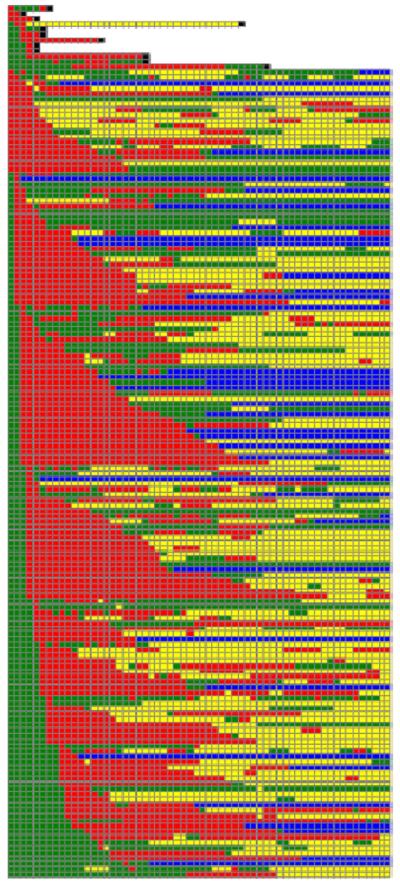
Figure 1 is a graphical representation of each patient's day-byday ATRA exposure from admission to 60 days post-admission. Variable timing of the first ATRA exposure is seen with some patients receiving the drug on the first day of their admission and others receiving it as late as the 28th day of admission. Time to ATRA initiation and percent of hospital days with ATRA exposure did not vary by gender or race. Time to ATRA initiation increased with decreasing age with a mean time to first ATRA exposure of 5.5, 3.6, 3.7, and 2.4 days, respectively, for age quartiles (0–5, 5–12.7, 12.7–16, and greater than 16 years; P = 0.0016 for trend across groups). The percentage of days in the first hospitalization with an ATRA exposure ranged from 55.4% in patients between 0 and 5 years of age to 67.1% in patients older than 16 years. However, this difference was not statistically significant.
Fig. 1.
Daily ATRA exposure, hospitalization and mortality status for 60 days after index admission: in the hospital but NOT exposed to ATRA (green); in the hospital AND exposed to ATRA (red); outpatient status but ultimately readmitted (n = 71; yellow); outpatient status and no future admissions (n = 38; blue); inpatient death on that day (black). Patients are sorted by mortality status and first ATRA exposure.
Figure 2 depicts the week-by-week healthcare resources that are commonly utilized in the setting of retinoic acid differentiation syndrome (vasopressors, steroids, diuretics, and chest radiographs). Within the first week of ATRA exposure, almost 11% of patients required vasopressors, nearly 40% received steroids, over 50% received diuretics, and 61% underwent chest radiographs. There was a substantial drop in the utilization of these resources between weeks one and two and by the seventh week the utilization of each of these resources was minimal.
Fig. 2.
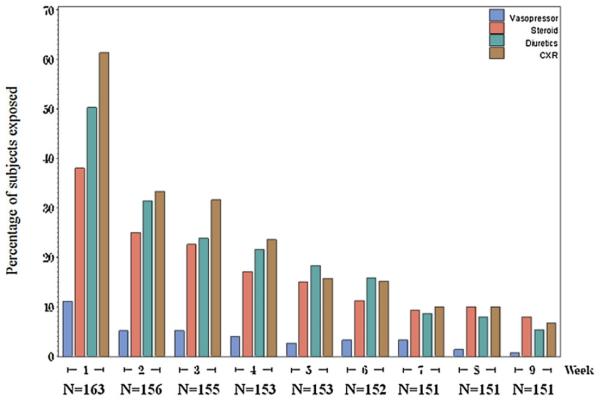
Resources typically used for the management of retonoic acid syndrome by week (starting from first ATRA exposure).
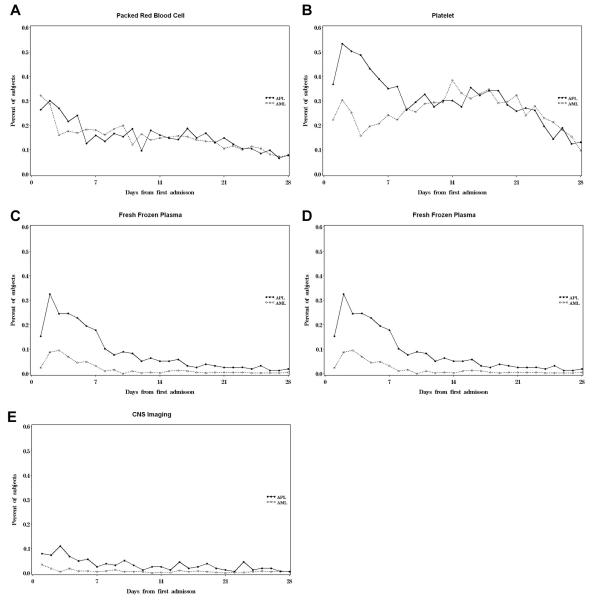
Figure 3 graphically compares the frequency of daily blood product replacement and CNS imaging in patients with APL and newly diagnosed non-APL AML. The most frequently administered blood product was platelets, followed by PRBC, FFP, and cryoprecipitate. Similar to other resource utilization, the necessity of blood product administration dropped precipitously after the first week from admission. The average number of exposure days in the first seven hospital days was significantly higher in patients with APL for platelet, FFP, cryoprecipitate, and CNS imaging than for patients with non-APL AML (Table III). There was no significant difference in red cell transfusion requirements.
Fig. 3.
Packed red blood cell (A), platelet (B), fresh frozen plasma (C), cryoprecipitate (D), and CNS imaging usage (E) in the first 28 days of hospitalization.
TABLE III.
Average Number of Days With at Least One Exposure to PRBC, Platelet, FFP, Cryoprecipitate, and CNS Imaging During the First Seven Hospital Days
| APL | AML | P-value | |
|---|---|---|---|
| Packed red blood cell | 1.56 | 1.47 | 0.45 |
| Platelet | 3.0 | 1.6 | <0.0001 |
| Fresh frozen plasma | 1.56 | 0.40 | <0.0001 |
| Cryoprecipitate | 0.40 | 0.10 | <0.0001 |
| CNS imaging | 0.46 | 0.10 | <0.0001 |
DISCUSSION
This study describes the only cohort of pediatric patients in the United States receiving chemotherapy and ATRA for presumed APL as well as the first cohort of patients with APL created in an administrative database. Although the diagnosis of APL was not confirmed by laboratory data, the established cohort has substantial face validity for several reasons. First, a substantial fraction of patients had an identifiable pattern of chemotherapy exposure, and these patterns of chemotherapy exposure varied over time in concert with open cooperative group clinical trials. Second, blood product, medication, and radiology resources used were consistent with expected patterns for patients with APL and clearly differed from patterns seen in patients with newly diagnosed non-APL AML.
The case fatality rate in the first 60 days from hospital admission is substantial at 7.4%. This mortality rate is similar to mortality rates described in pediatric APL clinical trials [3–6]. Furthermore, this early case fatality rate is similar to the mortality rate seen in older non-APL AML trials [11,12], but modestly greater than current UK Medical Research Council (MRC) based regimens [13]. Importantly, more than half of these fatal events happened in the first 7 days of hospitalization with a decrease in fatality rates thereafter. This drop suggests that children with newly diagnosed APL are quite vulnerable in the immediate presentation period but the risk of mortality decreases once a patient has survived the first week of admission.
As shown in Figure 1, substantial variation in ATRA administration was observed. This variation indicates that, despite current recommendations [14], rapid initiation of ATRA does not occur in all patients. The delay in ATRA initiation is more prolonged in younger patients and these patients have the lowest percentage of hospital days with an ATRA exposure. This delay may be due to the absence of an intravenous formulation of ATRA and a lower index of clinical suspicion for APL in younger children. This delay may be of particular clinical importance given data from the European APL 93 and APL 2000 trials demonstrating poorer outcomes in children less than 4 years of age despite standard dose chemotherapy [15]. Future work with administrative/billing data sets may enable the investigation of medication adherence as a potential source of disparate outcomes in these patients. A substantial fraction of patients in our study had ATRA initiated after hospital day seven. While the available data do not permit a conclusive analysis of the etiology of this delay, these patients may either be too ill to receive ATRA in the first week of hospitalization or have a delayed diagnosis.
While there was significant variation in the type of chemotherapy regimens administered, the cohort is too small to make definitive statements about the comparative effectiveness relative to 60-day mortality across each regimen. The patients who received “ATRA only” had the highest case fatality rate but this may be due to confounding by indication. That is, patients who did not receive chemotherapy agents other than ATRA may have been too ill at presentation to tolerate other chemotherapeutic agents.
Based on the available data, the frequent administration of blood product replacement within the first week of the index APL admission suggests an increased risk of coagulopathy for APL patients. This hypothesis is supported by an increased rate of platelets, FFP, cryoprecipitate, and CNS imagining in the APL cohort relative to the non-APL AML cohort. This is consistent with prior publications that showed that the cause of early death in children with APL is not necessarily from chemotherapy related toxicities, such as severe infections, cardiac failure, or differentiation syndrome, but rather complications from coagulopathy [3–6].
Although the introduction of ATRA for APL therapy has resulted in improved outcomes, APL differentiation syndrome continues to be a feared complication of such therapy and can contribute to significant morbidity and mortality in APL patients. Because of limitations in ICD-9-CM codes, patients with APL differentiation syndrome could not be explicitly identified. However, it was possible to demonstrate that the resources commonly administered in the setting of APL differentiation syndrome (vasoactive agents, steroids, diuretics, and chest radiographs) were prevalent during the first week after ATRA exposure and declined over time. These data suggest that the published rates of pediatric APL differentiation syndrome (7.5–20%) may substantially underestimate the resources administered to treat this complication [2,4,6,13,15]. In addition, these data suggest that, unlike adult patients, pediatric patients may not experience a bimodal distribution in the incidence of APL differentiation syndrome [16].
Despite the substantial strengths of PHIS data, this analysis has important limitations. Our case definition required that a patient be assigned an ICD-9-CM code consistent with AML and had received at least one dose of ATRA during their hospitalization. While this case definition is likely very specific, patients with APL but without an ATRA exposure during initial therapy are not included in the cohort. Therefore, our findings are limited to APL patients treated with ATRA. Additionally, the lack of an ICD-9-CM code for APL differentiation syndrome prevents estimation of the incidence APL differentiation syndrome. Furthermore, despite the large cohort size, it is still too small to allow definitive comparison of treatment regimens. Finally, PHIS data do not currently include laboratory results. Thus, it is not possible to confirm the diagnosis of APL with cytogenetic data or to determine presenting white blood cell count, an important prognostic factor.
Despite these limitations, this study describes the only cohort of children treated for APL from the United States and the first cohort established in an administrative database. In addition, the use of administrative data enabled an analysis of ATRA administration during induction therapy. These data demonstrate that, despite current recommendations for rapid initiation of continuous ATRA administration, a substantial fraction of patients may receive only intermittent ATRA dosing and that ATRA administration is significantly delayed in younger children compared to children over 5 years of age. Our data also define the treatment related induction mortality rate for children treated with current APL induction regimens. The induction mortality rate is substantial, higher than the induction mortality associated with current non-APL AML therapy, and appears related to hemorrhagic complications. Finally, the rate of resources necessary for the management of APL differentiation syndrome management is substantially higher than would be expected from the published incidence of APL differentiation syndrome.
These data support the current treatment recommendation of early ATRA initiation in all patients in whom APL is clinically suspected, particularly younger children [14,17]. Once laboratory results are available in a subset of PHIS centers [18], this APL cohort may be expanded and further studies performed to refine the other current treatment recommendations for adult patients with APL, specifically, transfusion support to maintain a fibrinogen concentration above 100–150 mg/dl, a platelet count above and 30–50 × 109/L, and steroid prophylaxis for patients at high risk of APL differentiation syndrome [17,19].
ACKNOWLEDGMENTS
This study was supported by NIH (grant R01CA165277 to R.A.) and by the Slovenian Ministry of Education, Science, Culture and Sport (grant J3-4220 to M.K.).
Footnotes
Conflict of interest: Nothing to declare.
REFERENCES
- 1.Stone RM, Mayer RJ. The unique aspects of acute promyelocytic leukemia. J Clin Oncol. 1990;8:1913–1921. doi: 10.1200/JCO.1990.8.11.1913. [DOI] [PubMed] [Google Scholar]
- 2.De Botton S, Dombret H, Sanz M, et al. Incidence, clinical features, and outcome of all trans-retinoic acid syndrome in 413 cases of newly diagnosed acute promyelocytic leukemia. The European APL Group. Blood. 1998;92:2712–2718. [PubMed] [Google Scholar]
- 3.Creutzig U, Zimmermann M, Dworzak M, et al. Favourable outcome of patients with childhood acute promyelocytic leukaemia after treatment with reduced cumulative anthracycline doses. Br J Haematol. 2010;149:399–409. doi: 10.1111/j.1365-2141.2010.08107.x. [DOI] [PubMed] [Google Scholar]
- 4.de Botton S, Coiteux V, Chevret S, et al. Outcome of childhood acute promyelocytic leukemia with all-trans-retinoic acid and chemotherapy. J Clin Oncol. 2004;22:1404–1412. doi: 10.1200/JCO.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 5.Mann G, Reinhardt D, Ritter J, et al. Treatment with all-trans retinoic acid in acute promyelocytic leukemia reduces early deaths in children. Ann Hematol. 2001;80:417–422. doi: 10.1007/s002770100304. [DOI] [PubMed] [Google Scholar]
- 6.Ortega JJ, Madero L, Martin G, et al. Treatment with all-trans retinoic acid and anthracycline monochemotherapy for children with acute promyelocytic leukemia: A multicenter study by the PETHEMA Group. J Clin Oncol. 2005;23:7632–7640. doi: 10.1200/JCO.2005.01.3359. [DOI] [PubMed] [Google Scholar]
- 7.Fisher BT, Zaoutis TE, Leckerman KH, et al. Risk factors for renal failure in pediatric patients with acute myeloid leukemia: A retrospective cohort study. Pediatr Blood Cancer. 2010;55:655–661. doi: 10.1002/pbc.22601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raffini L, Huang YS, Witmer C, et al. Dramatic increase in venous thromboembolism in children's hospitals in the United States from 2001 to 2007. Pediatrics. 2009;124:1001–1008. doi: 10.1542/peds.2009-0768. [DOI] [PubMed] [Google Scholar]
- 9.Zaoutis T, Localio AR, Leckerman K, et al. Prolonged intravenous therapy versus early transition to oral antimicrobial therapy for acute osteomyelitis in children. Pediatrics. 2009;123:636–642. doi: 10.1542/peds.2008-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aplenc R, Fisher BT, Huang YS, et al. Merging of the National Cancer Institute-funded cooperative oncology group data with an administrative data source to develop a more effective platform for clinical trial analysis and comparative effectiveness research: A report from the Children's Oncology Group. Pharmacoepidemiol Drug Saf. 2012;21(Suppl 2):37–43. doi: 10.1002/pds.3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lange BJ, Smith FO, Feusner J, et al. Outcomes in CCG-2961, a children's oncology group phase 3 trial for untreated pediatric acute myeloid leukemia: A report from the children's oncology group. Blood. 2008;111:1044–1053. doi: 10.1182/blood-2007-04-084293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woods WG, Kobrinsky N, Buckley JD. Timed-sequential induction therapy improves postremission outcome in acute myeloid leukemia: A report from the Children's Cancer Group. Blood. 1996;87:4979–4989. [PubMed] [Google Scholar]
- 13.Gibson BE, Webb DK, Howman AJ, et al. Results of a randomized trial in children with Acute Myeloid Leukaemia: Medical research council AML12 trial. Br J Haematol. 2011;155:366–376. doi: 10.1111/j.1365-2141.2011.08851.x. [DOI] [PubMed] [Google Scholar]
- 14.Sanz MA, Lo-Coco F. Modern approaches to treating acute promyelocytic leukemia. J Clin Oncol. 2011;29:495–503. doi: 10.1200/JCO.2010.32.1067. [DOI] [PubMed] [Google Scholar]
- 15.Bally C, Fadlallah J, Leverger G, et al. Outcome of acute promyelocytic leukemia (APL) in children and adolescents: An analysis in two consecutive trials of the European APL Group. J Clin Oncol. 2012;30:1641–1646. doi: 10.1200/JCO.2011.38.4560. [DOI] [PubMed] [Google Scholar]
- 16.Montesinos P, Bergua JM, Vellenga E, et al. Differentiation syndrome in patients with acute promyelocytic leukemia treated with all-trans retinoic acid and anthracycline chemotherapy: Characteristics, outcome, and prognostic factors. Blood. 2009;113:775–783. doi: 10.1182/blood-2008-07-168617. [DOI] [PubMed] [Google Scholar]
- 17.Sanz MA, Grimwade D, Tallman MS, et al. Management of acute promyelocytic leukemia: Recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2009;113:1875–1891. doi: 10.1182/blood-2008-04-150250. [DOI] [PubMed] [Google Scholar]
- 18.Gouripeddi R, Warner PB, Mo P, et al. Federating clinical data from six pediatric hospitals: Process and initial results for microbiology from the PHIS+ consortium. AMIA Annu Symp Proc. 2012;2012:281–290. [PMC free article] [PubMed] [Google Scholar]
- 19.Dohner H, Estey EH, Amadori S, et al. Diagnosis and management of acute myeloid leukemia in adults: Recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115:453–474. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]