Freeman and collaborators (1) report in the current issue of the Journal of Lipid Research important findings indicating that cholesterol-loaded macrophages secrete unesterified cholesterol (UC) complexes that are deposited as microdomains within the cell surface of the macrophages, as well as in their secreted extracellular matrix (ECM). Furthermore, the experiments indicate that these microdomains participate in the initial steps of the reverse-cholesterol pathways (1). The UC-microdomains are rich in cholesterol monohydrate aggregates that are recognized by MAb 58B1, an antibody generated against cholesterol crystals and that specifically recognizes ordered arrays with a minimum of 12 cholesterol monohydrate molecules (2). Deposition of the ECM and cell surface UC-cholesterol microdomains required overloading of macrophages with cholesterol as well as functional ABCA1 and ABCG1 transporters (3). Importantly, Freeman et al. show that the deposition of cholesterol-rich microdomains in the macrophage's surface and in the ECM as a consequence of acetylated (Ac)-LDL loading could be inhibited by the presence of 50 μg/ml apoAI in the incubation. However, when intact HDL was used at a similar concentration, it did not inhibit deposition of the UC-microdomains in the cell surface, although this did occur when HDL was used at a concentration of 200 μg/ml.
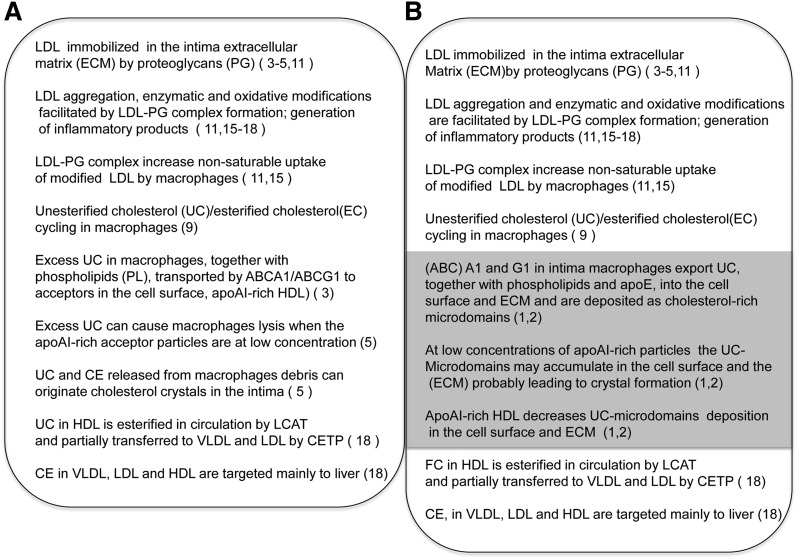
These findings are the most recent product of sustained research from this laboratory about phenomena taking place in the interface between macrophages and the extracellular environment and their potential significance in atherosclerosis. The implications of these experimental observations can be best appreciated by placing them within a framework of what we know about the interplay between cells, ECM, and lipoproteins in the arterial intima in atherogenesis. In order to guide the reader through our comments, we will use the diagrams in Fig. 1. In the 1A frame are summarized the processes that have led to formulation of the current response-to-retention hypothesis of atherosclerosis (4–6). In 1B, we inserted (shaded area) the most relevant findings from the current article by Freeman et al. (1) and from previous findings from their laboratory (1, 2).
Fig. 1.
Possible steps contributing to atherogenesis initiated by apoB-100 lipoprotein deposition in the arterial intima. A: Steps according to the response-to-retention hypothesis (4–6). B: Outline including the additional steps associated with deposition of cholesterol microdomains in the extracellular matrix (shaded area) (1, 2). The numbers in parentheses are those of pertinent references.
One important tool used in the experiments reported by Freeman et al. is the MAb 58B1 that was developed and characterized in 1996 by Perl-Treves et al. (7) at the Weizmann Institute in Israel. Ong and collaborators (2) have previously used this MAb for similar experiments with macrophage cultures as well as for showing that in human aortic early lesions, rich in foam cells, UC-microdomains exist in association with Oil Red O-positive foam cells. The authors concluded that the immuno-histochemical results are compatible with the hypothesis that UC-rich microdomains are generated by foam cells (macrophages) in human lesions. However, because these observations with MAb 58B1 were made in lesions from only one subject, we do not yet know how prevalent such structures are in human lesions. Likewise, there are not yet publications about the detailed biochemical composition of the UC-microdomains, neither from human lesions nor from macrophage cultures. Such characterizations, once available, would put on a more solid base their hypothesis about similarities of the structures detected in cell cultures and those found in human lesions. However, we speculate that because the MAb recognizes a small structure containing few UC molecules, the microdomains in the cell culture model and those present in lesion may differ in their associated phospholipids and proteins. A previous publication from Ong et al. (2) indicates that the cholesterol aggregates may require phospholipids and apoE in order to be secreted from differentiated cholesterol-loaded macrophages. The possible contribution of apoE to generation and secretion of the UC-microdomains is supported by findings described in the article by Freeman et al. (1) in this issue of the JLR. Freeman et al. show that the liver X receptor (LXR) agonist TO901317 increases the deposition of cholesterol microdomains in the ECM produced by macrophages. It is known that, besides upregulation of ABCA1 and ABCG1, LXR agonists both in vivo and in vitro upregulate apoE expression (8). Future colocalization experiments with MAb(s) against the microdomains and against apoE in cell cultures and in human lesions could be informative in this respect.
As mentioned, secretion and deposition of the UC-microdomains at the cell surface and within the ECM secreted by macrophages requires overloading the cells with LDL cholesterol. However, this can only be achieved in macrophages with LDL that has been modified (9). The modification used in the current experiments is acetylation of amine groups of amino acids in the LDL apoB-100 (1, 2). This modification reduces markedly the net positive charge of LDL and blocks recognition of LDL by the LDL receptor. In addition, this Ac-LDL no longer binds to heparan sulfate proteoglycans that are on the macrophage's cell surface (glycocalix) or to the chondroitin/dermatan sulfate proteoglycans present in the ECM secreted by cultured macrophages (10). Thus, the modified LDL used by Freeman et al. probably remains in solution during the incubations, as indicated by the fact that it can be washed out from the cell cultures, as described (1). Although Ac-LDL is much used in experiments aimed to overload macrophages with LDL cholesterol, this covalent modification has not been observed in humans. The better documented modifications of LDL in arterial tissue are: aggregation, oxidative changes of unsaturated fatty acids and formation of covalent adducts with apoB, proteolysis of apoB-100, and hydrolysis of LDL phospholipids (11, 12). These modifications appear to follow retention of LDL particles caused by rapid binding of arginine and lysine-rich specific surface segments of the apoB-100 to the ECM proteoglycans (13). It should be stressed that in animal models of atherogenesis (14) and in human developing lesions, apoB-100 lipoprotein accumulation in the ECM of intima-media seems to precede macrophage accretion in the same regions (15, 16). Several of the modifications that appear to take place in the intima have been shown in vitro to induce uncontrolled uptake by macrophages. They include complex formation with arterial proteoglycans (17, 18), oxidative alterations (11, 19), and enzymatic hydrolysis of phospholipids (20). LDL oxidation and hydrolysis of its phospholipids induce increase in negative charge of the lipoproteins. It is then possible that in vivo, in a situation where nonmodified and modified apoB-100 lipoproteins exist, the deposition of UC-microdomains may be different to that observed in experiments in which only negatively charged LDL is available, as those discussed by Freeman et al. (1). This could be the case if apoE-containing microdomains and native LDL compete for proteoglycan binding sites in the macrophage-secreted ECM. This possibility could be explored in experiments where native LDL is coincubated with LDL that has been modified by aggregation, oxidation, or enzyme treatment.
Another aspect that needs further investigation is how the UC-microdomains are deposited and retained in the ECM and cell surface. In a previous article, Ong et al. (2) suggest that apoE may be part of the complex secreted by the cholesterol loaded macrophages. If this is the case, apoE may be one of the ligands anchoring the UC-microdomains to heparin sulfate proteoglycans in the cell surface and to chondroitin/dermatan sulfate proteoglycans in the secreted ECM. It should be noted that apoB-100 and apoE share consensus positive sequences that bind to sulfated proteoglycans from the cell surface and the ECM (13). One important observation in the article of Freeman et al. (1) is that when apoA-I at 50 μg/ml is present during the cholesterol-overloading step, it completely blocks the deposition of UC-microdomains in the cell surface and in the ECM of macrophages. This is particularly interesting because this occurs without altering the uptake of Ac-LDL measured as 125I-Ac-LDL internalization (Fig. 5 in the article). However, the increase in cell cholesterol content caused by incubation with Ac-LDL was reduced when HDL or apoA-I was present in the incubation media at similar concentrations, but apoA-I was more efficient than HDL (supplementary Fig. I for the article). In addition, HDL was a less effective inhibitor of the deposition of UC-microdomains on the cell surface and ECM than apoA-I at similar concentrations, as shown by the signal intensity observed with the MAb 58B1 (Fig. 3 in the article). The authors suggest that this difference was related to the capacity of apoA-I to associate with phospholipids from the cells to form discoidal particles (there was no serum present in the cell culture media during the incubations). This could generate more efficient UC acceptors than HDL, which is already loaded with CE and UC. As indicated by Freeman et al. (1), when cells are present, the apoA-I acceptors could compete more effectively than HDL for the UC-microdomains deposition within the cell surface and ECM and could also deplete better the components of those aggregates already deposited. Additionally, the results suggest that the differential inhibitory effects of apo-A-I and HDL may result from their dissimilar capacity to reduce cell-cholesterol content (supplementary Fig. I in the article). Without kinetic data, it is difficult to assign the relative contribution of each of these possibilities.
The capacity of apoA-I acceptors to block the accumulation of UC-microdomains in vitro and the ability that they may have in vivo seems to be different. Freeman et al. (1) found that 12.5 μg/ml of apoA-I was capable of blocking the appearance of UC-microdomains induced by incubation with 50 μg/ml of Ac-LDL and that 50 μg/ml of HDL blocked their deposition within the ECM. Then we need to explain how in the arterial intima, with concentrations of apoA-I lipoproteins higher than 50 μg/g of tissue (19), UC-microdomains can be generated and remain as stable immuno-detectable structures (2). Although, extrapolation from data obtained with cell cultures to the far more complex situation in the arterial intima deserves caution, the above apparent paradox needs to be addressed. A good starting point could be to evaluate the prevalence of the UC-microdomains at different stages of lesion development and from arterial lesions from several subjects.
In conclusion, the data in the article by Freeman and collaborators (1) supports the main hypothesis proposing that macrophages may use several secretion pathways to maintain nontoxic cellular levels of UC (21). Furthermore, the results presented clearly document the possibility that macrophages can use ABCA1- and ABCG1-mediated secretion and deposition of UC-microdomains as extracellular reservoirs, which can then donate UC to apoA-I containing discoidal particles. As usual, such novel results generate further questions, some of which we have tried to describe. The authors addressed other issues as well, and this made the article's Discussion section valuable reading. Experimental exploration of all of the issues raised will be needed to ascertain the biological significance of the novel observations presented in this article.
REFERENCES
- 1.Freeman S. R., Jin X., Anzinger J. J., Xu Q., Purushothaman S., Fessler M. B., Addadi L., Kruth H. S. 2013. ABCG1-mediated generation of extracellular cholesterol microdomains. J. Lipid Res. 55: 115–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ong D. S., Anzinger J. J., Leyva F. J., Rubin N., Addadi L., Kruth H. S. 2010. Extracellular cholesterol-rich microdomains generated by human macrophages and their potential function in reverse cholesterol transport. J. Lipid Res. 51: 2303–2313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang X., Collins H. L., Ranalletta M., Fuki I. V., Billheimer J. T., Rothblat G. H., Tall A. R., Rader D. J. 2007. Macrophage ABCA1 and ABCG1, but not SR-BI, promote macrophage reverse cholesterol transport in vivo. J. Clin. Invest. 117: 2216–2224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams K. J., Tabas I. 1995. The response-to-retention hypothesis of early atherogenesis. Arterioscler. Thromb. Vasc. Biol. 15: 551–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tabas I., Williams K. J., Boren J. 2007. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation. 116: 1832–1844 [DOI] [PubMed] [Google Scholar]
- 6.Fogelstrand P., Boren J. 2012. Retention of atherogenic lipoproteins in the artery wall and its role in atherogenesis. Nutr. Metab. Cardiovasc. Dis. 22: 1–7 [DOI] [PubMed] [Google Scholar]
- 7.Perl-Treves D., Kessler N., Izhaky D., Addadi L. 1996. Monoclonal antibody recognition of cholesterol monohydrate crystal faces. Chem. Biol. 3: 567–577 [DOI] [PubMed] [Google Scholar]
- 8.Verschuren L., de Vries-van der Weij J., Zadelaar S., Kleemann R., Kooistra T. 2009. LXR agonist suppresses atherosclerotic lesion growth and promotes lesion regression in apoE*3Leiden mice: time course and mechanisms. J. Lipid Res. 50: 301–311 [DOI] [PubMed] [Google Scholar]
- 9.Goldstein J. L., Ho Y. K., Brown M. S., Innerarity T. L., Mahley R. W. 1980. Cholesteryl ester accumulation in macrophages resulting from receptor-mediated uptake and degradation of hypercholesterolemic canine beta-very low density lipoproteins. J. Biol. Chem. 255: 1839–1848 [PubMed] [Google Scholar]
- 10.Asplund A., Stillemark-Billton P., Larsson E., Rydberg E. K., Moses J., Hulten L. M., Fagerberg B., Camejo G., Bondjers G. 2010. Hypoxic regulation of secreted proteoglycans in macrophages. Glycobiology. 20: 33–40 [DOI] [PubMed] [Google Scholar]
- 11.Steinberg D., Parthasarathy S., Carew T. E., Khoo J. C., Witztum J. L. 1989. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N. Engl. J. Med. 320: 915–924 [DOI] [PubMed] [Google Scholar]
- 12.Witztum J. L., Steinberg D. 1991. Role of oxidized low density lipoprotein in atherogenesis. J. Clin. Invest. 88: 1785–1792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Camejo G., Hurt-Camejo E., Wiklund O., Bondjers G. 1998. Association of apo B lipoproteins with arterial proteoglycans: pathological significance and molecular basis. Atherosclerosis. 139: 205–222 [DOI] [PubMed] [Google Scholar]
- 14.Tamminen M., Mottino G., Qiao J. H., Breslow J. L., Frank J. S. 1999. Ultrastructure of early lipid accumulation in apoE-deficient mice. Arterioscler. Thromb. Vasc. Biol. 19: 847–853 [DOI] [PubMed] [Google Scholar]
- 15.Nakashima Y., Fujii H., Sumiyoshi S., Wight T. N., Sueishi K. 2007. Early human atherosclerosis: accumulation of lipid and proteoglycans in intimal thickenings followed by macrophage infiltration. Arterioscler. Thromb. Vasc. Biol. 27: 1159–1165 [DOI] [PubMed] [Google Scholar]
- 16.Nakashima Y., Wight T. N., Sueishi K. 2008. Early atherosclerosis in humans: role of diffuse intimal thickening and extracellular matrix proteoglycans. Cardiovasc. Res. 79: 14–23 [DOI] [PubMed] [Google Scholar]
- 17.Hurt-Camejo E., Camejo G., Rosengren B., Lopez F., Wiklund O., Bondjers G. 1990. Differential uptake of proteoglycan-selected subfractions of low density lipoprotein by human macrophages. J. Lipid Res. 31: 1387–1398 [PubMed] [Google Scholar]
- 18.Plihtari R., Kovanen P. T., Öörni K. 2011. Acidity increases the uptake of native LDL by human monocyte-derived macrophages. Atherosclerosis. 217: 401–406 [DOI] [PubMed] [Google Scholar]
- 19.Camejo G., Halberg C., Manschik-Lundin A., Hurt-Camejo E., Rosengren B., Olsson H., Hansson G. I., Forsberg G. B., Ylhen B. 1998. Hemin binding and oxidation of lipoproteins in serum: mechanisms and effect on the interaction of LDL with human macrophages. J. Lipid Res. 39: 755–766 [PubMed] [Google Scholar]
- 20.Hurt-Camejo E., Camejo G., Peilot H., Oorni K., Kovanen P. 2001. Phospholipase A(2) in vascular disease. Circ. Res. 89: 298–304 [DOI] [PubMed] [Google Scholar]
- 21.Rosenson R. S., Brewer H. B., Jr, Davidson W. S., Fayad Z. A., Fuster V., Goldstein J., Hellerstein M., Jiang X. C., Phillips M. C., Rader D. J., et al. 2012. Cholesterol efflux and atheroprotection: advancing the concept of reverse cholesterol transport. Circulation. 125: 1905–1919 [DOI] [PMC free article] [PubMed] [Google Scholar]