Abstract
One mechanism of the lipid-lowering effects of the fish oil n-3 fatty acids [e.g., docosahexaenoic acid (DHA)] in cell and animal models is induced hepatic apolipoprotein B100 (apoB) presecretory degradation. This degradation occurs post-endoplasmic reticulum, but whether DHA induces it before or after intracellular VLDL formation remains unanswered. We found in McA-RH7777 rat hepatic cells that DHA and oleic acid (OA) treatments allowed formation of pre-VLDL particles and their transport to the Golgi, but, in contrast to OA, with DHA pre-VLDL particles failed to quantitatively assemble into fully lipidated (mature) VLDL. This failure required lipid peroxidation and was accompanied by the formation of apoB aggregates (known to be degraded by autophagy). Preventing the exit of proteins from the Golgi blocked the aggregation of apoB but did not restore VLDL maturation, indicating that failure to fully lipidate apoB preceded its aggregation. ApoB autophagic degradation did not appear to require an intermediate step of cytosolic aggresome formation. Taken with other examples in the literature, the results of this study suggest that pre-VLDL particles that are competent to escape endoplasmic reticulum quality control mechanisms but fail to mature in the Golgi remain subject to quality control surveillance late in the secretory pathway.
Keywords: endoplasmic reticulum, Golgi, autophagy, lipid peroxidation, apoB
Apolipoprotein B100 (apoB) is the major protein present on VLDLs and is required for the assembly and secretion of VLDL from the liver (1). VLDL is the precursor of LDL, and its overproduction is characteristic of insulin-resistant states (1). The number of VLDL particles secreted by the liver is dependent on the intracellular level of apoB, which is regulated primarily by post-transcriptional mechanisms controlled by several metabolic and nutritional factors (for a recent review, see Ref. 2).
There are three main processes responsible for regulating intracellular apoB levels by degradation (3): endoplasmic reticulum (ER)-associated degradation, post-ER presecretory proteolysis, and reuptake. N-3 fatty acids, such as docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), which are enriched in fish oils, possess lipid-lowering properties in part owing to their ability to increase intracellular apoB degradation (4). One mechanism of the hypolipidemic effect of fish oil-enriched diets is thought to be the induction by DHA or EPA of apoB degradation at the post-ER level (3). This process has been shown to involve the generation of lipid-peroxides, oxidative modification, and aggregation of monomeric apoB, followed by its autophagic degradation (5–7).
Post-ER presecretory proteolysis of apoB by DHA has been shown to exert a preferential effect on the secretion of apoB associated with the most mature (i.e., buoyant) VLDL particles (3). It remains to be determined at which stage of VLDL biogenesis the shunting of apoB to autophagic degradation occurs. For example, Wilkinson et al. (8) suggested that fish oils prevented apoB transfer from the ER to the Golgi lumen in rabbit hepatocytes. Kendrick and Higgins (9) reported ER degradation of apoB by fish oils in hamster hepatocytes, although previously we found no evidence of this in rodent cells (3). In the present studies, to address the issue of where and how in the secretory pathway the major fish oil fatty acid DHA exerts its effects on VLDL assembly and secretion, we have combined subcellular fractionation techniques with pulse-chase analysis of apoB turnover. The data are consistent with the transit of apoB from the ER to the Golgi as a pre-VLDL particle, which fails to fully mature and is shunted to autophagy. These results support a more general model in which preVLDL particles that do not mature properly are removed from the secretory pathway by a late-stage quality control program.
EXPERIMENTAL PROCEDURES
Materials
Rat hepatoma cell line McA-RH7777 (McA) was obtained from ATCC (Manassas, VA). Cell culture media and reagents were from Gibco/Invitrogen (Carlsbad, CA) and Cellgro (Herndon, VA). Rabbit polyclonal antiserum to rat apoB was developed in the author's laboratory. Rabbit anti-LC3 polyclonal antibody (free and cross-linked to Dynabead-Protein A) was provided by the laboratory of Dr. Zemin Yao (University of Ottawa). Rabbit anti-calnexin antibody was purchased from Stressgen (Ann Arbor, MI). Protein-A Sepharose was from Amersham Pharmacia Biotech (Piscataway, NJ). [35S]Methionine/Cysteine ([35S]Met/Cys), Solvable, and scintillation fluid were from Perkin Elmer (Boston, MA). Protease Inhibitor Cocktail tablets were from Roche (Indianapolis, IN). Autofluor was from National Diagnostics (Atlanta, GA). A cell disruption bomb was purchased from Parr Instrument Co. (Moline, IL). All other reagents were from Sigma (St. Louis, MO).
Cell culture
McA cells were regularly maintained at 37°C/5% CO2 in DMEM supplemented with 10% FBS, 10% horse serum (HS), 1% L-glutamine, and 1% penicillin/streptomycin on P100 dishes coated with type I collagen. Medium was changed every 3 days, and cells were used for experiments at 90–95% confluence. For some experiments, cells were also grown on 6-well plates.
Cell treatments and metabolic labeling
Cells were pretreated for 1 h with 0.6 mM DHA-BSA or 0.6 mM DHA-BSA with or without 100 µM desferrioxamine (DFX) to inhibit lipid peroxidation in Met/Cys-free DMEM supplemented with 0.5% FBS, 0.5% HS, 1% L-glutamine, and 1% penicillin/streptomycin. In all experiments, treatment with 0.6 mM OA-BSA was used as the reference. After preincubation, cells were pulse-labeled with 240 µCi [35S]Met/Cys for 10 min in the same medium. The pulse medium was then removed, and cells were washed quickly with PBS (2×) and chased in medium containing excess unlabeled Met (10 mM)/Cys (3 mM), 0.5% FBS, 0.5% HS, 1% L-glutamine, 1% penicillin/streptomycin, and the corresponding fatty acid treatments. The end of pulse was characterized as 0 min of chase (T0). Other chase time points were 15 min (T15), 30 min (T30), 45 min (T45), 60 min (T60), and 90 min (T90). At the end of each chase time point, the conditioned media and cells were collected. In some experiments, cells were labeled to steady-state in which fatty acid treatments were administered in the presence of 100 µCi [35S]Met/Cys for 3 h after the 1-h preincubation. In one series of experiments, pulse-chase studies as described above were carried out at 20°C to block protein trafficking out of the Golgi.
Lipoprotein isolation and fractionation
At the end of the chase period, cells were washed with PBS (2×) before being harvested by scraping in 2.5 ml of homogenization buffer [10 mM Hepes (pH 7.4), 250 mM sucrose, 0.5 mM DTT, 1× EDTA-free protease-inhibitor cocktail, 20 U/ml RNase inhibitor, 200 µM BHT, 2.5 mg/ml trypsin inhibitor, 2 mM MgCl2]. Cells were then homogenized by nitrogen decompression in a cell disruption bomb (500 psi for 15 min). Typically, cells from two P100 dishes were used for homogenization per experimental treatment. The homogenates were centrifuged at 10,000 g for 10 min to obtain the postnuclear supernatant, also referred to as postmitochondrial supernatant. The postnuclear supernatant was centrifuged at 100,000 g for 1 h at 4°C to obtain total microsomes. Purified Golgi microsomes were used in some experiments instead of total microsomes and were isolated as described (10, 11). Luminal contents of total and Golgi microsomes were released by treatment with 0.1 M sodium carbonate (pH 11) and deoxycholic acid (0.025%) (10–12). The supernatants containing the released lipoproteins were recovered by centrifugation at 60,000 rpm for 60 min at 4°C in a Beckman TLA 100.4 rotor. Luminal lipoproteins and those from conditioned media were separated according to density by sucrose-gradient ultracentrifugation (10, 11).
Immunoprecipitation and analysis
[35S]labeled apoB from each density fraction was immunoprecipitated using rabbit anti-rat apoB antiserum and separated in duplicate on two 4% gels: one set was subjected to fluorography, and the other was subjected to apoB quantification by scintillation counting. For scintillation counting of the [35S] label associated with apoB in each density fraction, the corresponding bands were excised from the gel and solubilized by SOLVABLE, and the associated radioactivity was measured using a Beckman LS 6000 scintillation counter after the addition of scintillation fluid. ApoB data from fluorograms were quantified by densitometry and adjusted for differences in exposure times.
Analysis of apoB-aggregates was performed as described in the figure legends after immunoprecipitation from whole cell lysates prepared in buffer containing 6 mM Na2HPO4, 4.5 mM NaH2PO4, 125 mM NaCl, 36 mM lithium dodecyl sulfate, 24 mM deoxycholate, and 1% Triton X-100 (pH 7.4). All apoB measurements obtained by scintillation counting were normalized to trichloroacetic acid (TCA)-precipitable radioactivity as a measure of total protein synthesis. Cellular TCA counts were also adjusted for total cell protein (TCA counts/mg) to account for variations in cell numbers among experimental treatments.
Confocal microscopy
McA cells were treated as described, fixed with 4% paraformaldehyde, and permeabilized with 0.1% saponin before the addition of antisera or antibodies. ApoB immunostaining was performed using the same antibody used for the immunoprecipitation experiment (rabbit anti-rat apoB at 1:250). The cells were stained for several markers: LAMP1 (lysosome marker) using the purified mouse antibody from Stressgen (Victoria, Canada) at 1:1,000, γ-tubulin using the mouse anti-γ-tubulin antibody (ref#T5326) at 1:5,000, and goat anti-vimentin antibody (ref#V4630) 1:100 from Sigma Aldrich (Munich, Germany). The staining was revealed after incubation with alexa-fluor (488 and 594) conjugated antibodies (Invitrogen) directed against the specific species. The stained cells were examined with a Leica TCS SP5 confocal laser scanning microscope.
Statistical analysis
Data reported are displayed as mean ± SEM, with the number of independent experiments provided in the figure legends. At least three replicates were performed for each independent experiment. Significance was calculated by two-tailed t-tests or ANOVA with Dunnett or Bonferroni post-testing for multiple comparisons. A P value of <0.05 was considered significant.
RESULTS
Reduced secretion of mature (fully lipidated) VLDL from DHA-treated hepatocytes is associated with reduced recovery of VLDL-associated apoB from the microsomal lumen
Although McA cells secrete apoB100 and apoB48, herein the abbreviation “apoB” refers only to apoB100. We did not analyze apoB48 data because they are not relevant to hepatic VLDL in the human liver (which only produces apoB100) and apoB48 also does not undergo marked degradation in rodent hepatic cells in response to fish oil fatty acids (3).
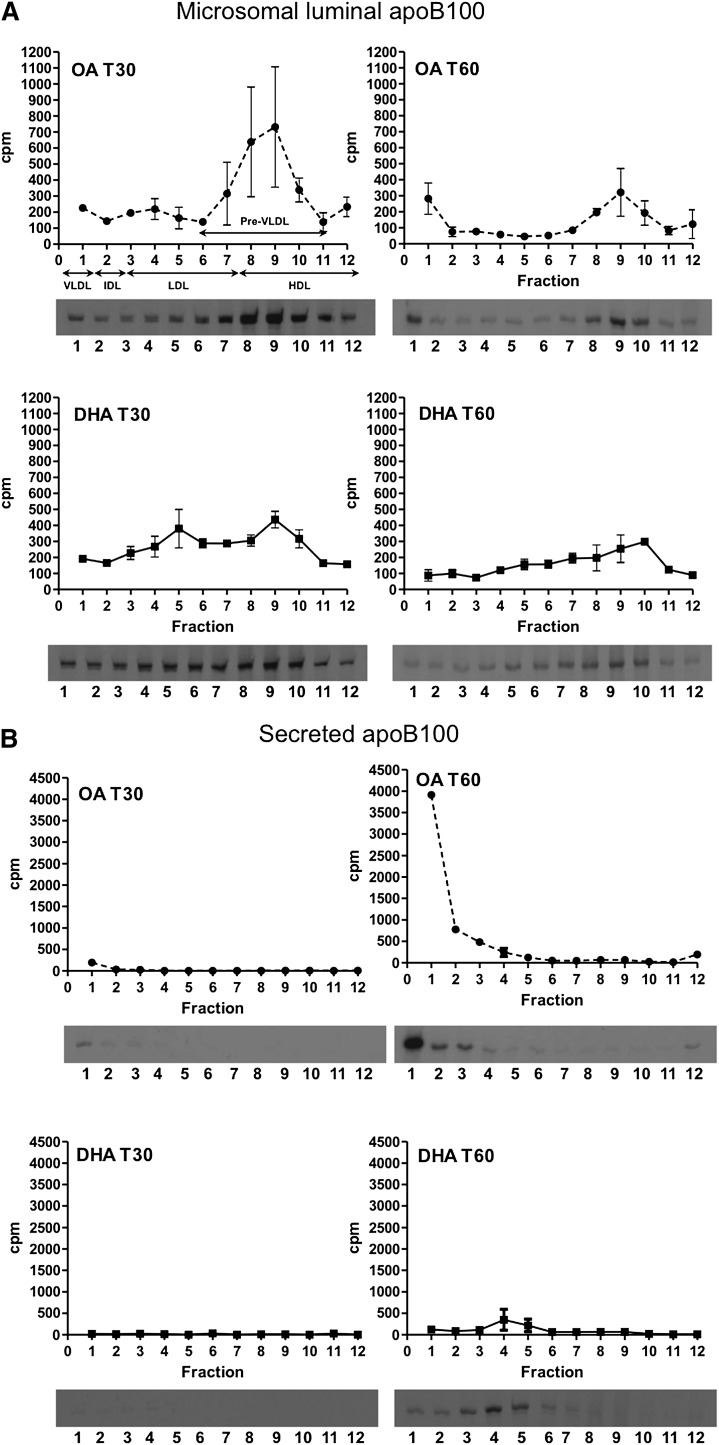
A time course of VLDL formation and secretion by DHA-treated cells was performed by a pulse-chase protocol in conjunction with density fractionation from microsomes isolated at the time points indicated in Fig. 1. Metabolically labeled apoB from total microsomes (Fig. 1A) and the conditioned media (Fig. 1B) was quantified at each time point. OA-treated cells served as the control. The density of the fractions from the gradient are: fraction 1: d = 1.006 g/ml (VLDL); fraction 2: d = 1.010 g/ml (IDL); fractions 3 through 7: d = 1.021–1.057 g/ml (LDL); and fractions 8 through 12: d = 1.069–1.200 g/ml (HDL). Of these, apoB-containing particles in fractions 6 through 11, in which are found the precursor particles to VLDL, were classified as “pre-VLDL” (1, 13).
Fig. 1.
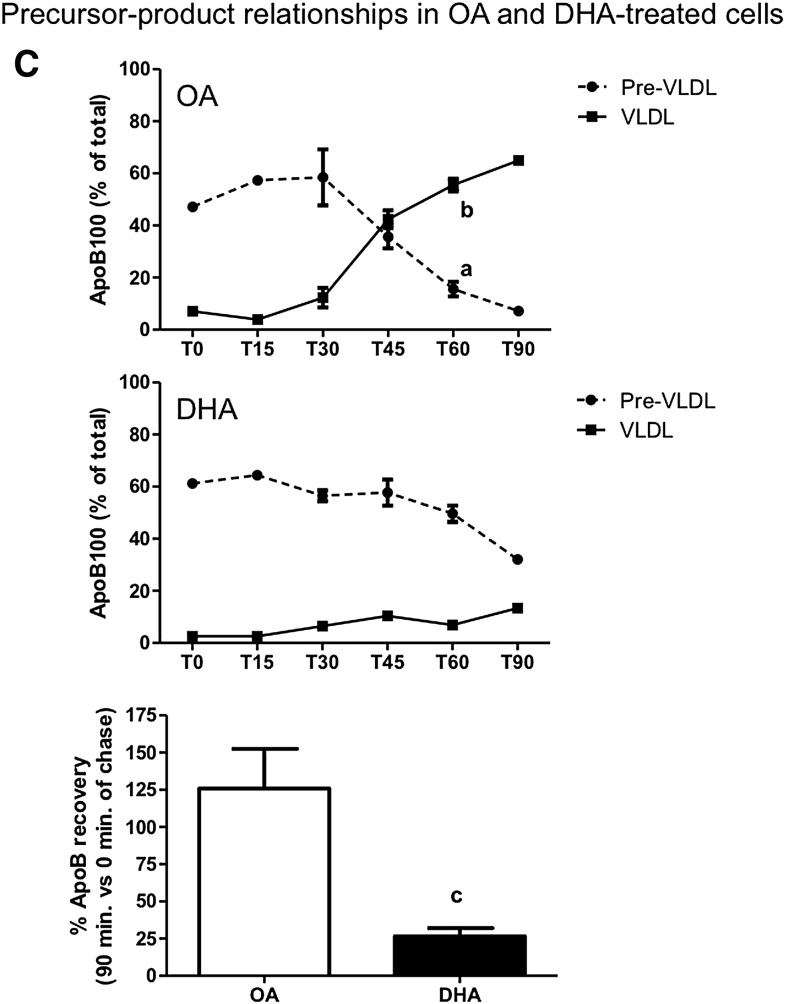
Effects of DHA on hepatic VLDL assembly and secretion. McA cells were pretreated with 0.6 mM OA-BSA or DHA-BSA complexes for 1 h, pulse labeled with [35S]Met/Cys for 10 min, and chased for up to 90 min in the continued presence of OA or DHA. At the end of chase, cells and conditioned media were collected. After cells were homogenized, total microsomes were isolated, and their luminal content was released by sodium carbonate/deoxycholate extraction. Lipoproteins in the indicated fractions were isolated by sucrose-gradient ultracentrifugation. [35S]labeled apoB in each density fraction was immunoprecipitated, separated by SDS-PAGE, and quantified by scintillation counts obtained from the excised apoB bands. Total apoB recovery (i.e., the sum of apoB in all intracellular and secreted density fractions) was determined by fluorography and densitometry. A: Density distribution profiles at T30 and T60 of apoB-lipoproteins isolated from total microsomes of cells treated with DHA or OA. B: Secreted apoB at T30 and T60. Fraction 1 corresponds to mature (fully lipidated) VLDL density particles, and fractions 6 through 11 correspond to preVLDL particles. Below each graph are the corresponding representative fluorograms. Results shown are representative of three separate experiments. C: Top two graphs: Percentage of apoB associated with the pre-VLDL and VLDL fractions at 0, 15, 30, 45, 60, and 90 min of chase (T0, T15, T30, T45, T60, and T90, respectively) from cells treated with OA or DHA. Bottom graph: Total apoB recovery at the end of chase. a and b indicate a significant decrease in pre-VLDL-apoB or increase in VLDL-apoB from T30 to T60, respectively (P < 0.05); c indicates a significant reduction in apoB recovery from T0 to T90 with DHA (P < 0.05). The results in C are based on three independent experiments for T30, T45, and T60; two independent experiments for T15; and replicate measurements from one experiment for T0 and T90.
For the apoB-containing lipoproteins isolated from the microsomal lumens, by T30 (Fig. 1A), although similar levels of luminal VLDL-apoB were recovered from both treatments, differences emerged in the other density subfractions. OA-treated cells demonstrated a distinct peak of HDL-apoB, whereas DHA treatment resulted in two peaks, one of HDL density and the other of LDL density. By T60 (Fig. 1A), the differences in lipoprotein density profiles were even more striking. There was a decrease in the HDL-density (pre-VLDL precursor) particles in the case of OA, with evidence that some were being converted to VLDL (note the increase in apoB recovery in fraction 1). DHA-treated cells also showed a decrease in microsomal apoB associated with HDL-density particles, but this was part of a broad decrease across all of the density fractions.
With both treatments, there was overall loss of apoB from the microsomes over time, and by examining the lipoproteins in the conditioned media samples, we were able to determine whether this was attributable to secretion. OA-treated cells secreted apoB almost entirely as mature VLDL (Fig. 1B). Consistent with previous studies (e.g., Ref. 4), apoB secretion from DHA-treated cells was significantly less than from OA-treated cells and was mainly as LDL-density particles (Fig. 1B). The first appearance of metabolically labeled apoB in the medium was VLDL associated from OA-treated cells at T30 (Fig. 1B). By considering the data in Fig. 1A and B together, the majority of microsomal apoB appeared to be secreted associated with VLDL by 60 min from OA-treated cells.
To better clarify the effects of OA and DHA on preVLDL conversion to VLDL and on apoB degradation, the data from Fig. 1A and B were expressed as the sum of apoB recovered from the corresponding density fractions of the microsomal and media samples. After T30 there was a decrease in pre-VLDL apoB in OA-treated cells and a corresponding increase in VLDL-apoB, strongly suggesting a precursor-product relationship (Fig. 1C). A similar precursor-product relationship was not observed in DHA-treated cells over the same time period (Fig. 1C). Furthermore, in pulse-chase experiments, there was a significant reduction of total apoB recovery to 26.4 ± 3.93% at T90 compared with T0 [comparable to what we have previously reported (3, 6)], whereas no significant degradation was seen in OA-treated cells. Thus, in OA-treated cells, pre-VLDL appears to be converted to and secreted as mature VLDL. In contrast, in DHA-treated cells, luminal loss of pre-VLDL did not seem to be associated with conversion to VLDL, nor was apoB quantitatively secreted into the medium, suggesting either a failure of VLDL maturation or the degradation of pre-VLDL or mature VLDL particles in DHA-treated cells. These possibilities were further explored in the following series of experiments.
Blocking intracellular lipid peroxidation by DFX enables conversion of pre-VLDL to VLDL and its secretion in DHA-treated cells
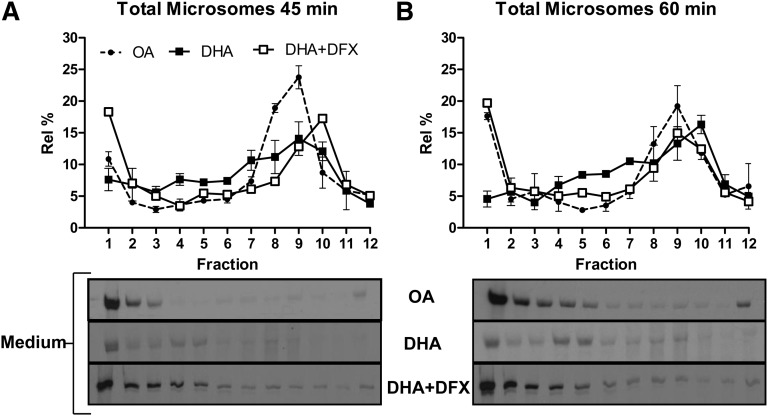
The mechanism of DHA-induced apoB degradation involves the formation of lipid peroxides and reactive oxygen species (6, 7). DFX has been shown to be a potent inhibitor of DHA-induced apoB degradation by preventing lipid peroxidation (5, 6). In the presence of DFX, the analysis of microsomal luminal lipoproteins revealed that DHA-treated cells were able to form pre-VLDL particles and convert them into mature VLDL (Fig. 2). Indeed, similar quantities of luminal VLDL were recovered from cells treated with OA and DHA+DFX at T45 and T60 (see graphs in Fig. 2A, B). The luminal VLDL from DHA+DFX-treated cells was subsequently recovered from the medium, and the density distribution of apoB-containing lipoproteins was similar to that in OA-treated cells at these time points (see the representative fluorograms in Fig. 2A, B). These results indicate that the decreased appearance of mature VLDL in DHA-treated hepatocytes was related to lipid peroxidation.
Fig. 2.
Blocking intracellular lipid peroxidation by cotreatment with DFX and DHA results in recovery of luminal VLDL formation and its secretion. Experiments identical in design to those described in Fig. 1 for OA and DHA were conducted with McA cells treated with DHA in the presence of 100 µM DFX. The density distribution profiles of luminal apoB from cells treated with OA, DHA, or DHA+DFX at 45 min (A) and at 60 min (B) are shown. Below each graph is a representative fluorogram for the density gradient analysis of apoB distribution in the corresponding conditioned media samples at that time point. Graphical results shown are representative of three separate experiments for OA and DHA and for two separate experiments with replicate measurements for DHA+DFX.
Blocking apoB-aggregation by inhibiting Golgi exit prevents loss of pre-VLDL but does not restore VLDL formation in DHA-treated cells
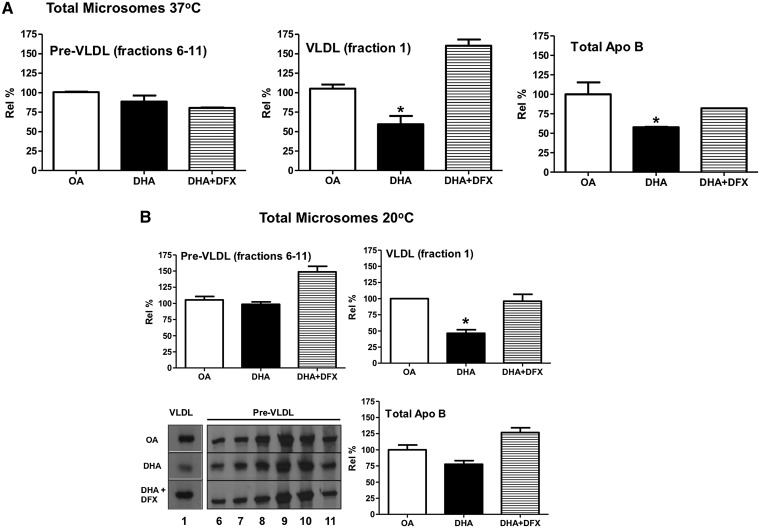
We have shown (5) that lipid peroxidation leads to the formation of apoB aggregates (confirmed by mass spectrometry) that appear near the top of the SDS-PAGE gels coincident upon or after exiting from the Golgi. These aggregates are not observed in significant levels in cells with relatively low levels of lipid peroxidation (i.e., cells incubated with BSA or OA or cotreated with DHA+DFX) (5). In addition, blocking Golgi exit using a 20°C incubation (14) prevents the formation of large apoB aggregates (5). Using the same protocol, after 45 min of chase at the permissive temperature of 37°C, the loss of apoB from the microsomal lumen was mainly accounted for by a deficiency of apoB associated with mature VLDL (Fig. 3A). Because the loss of pre-VLDL particles as a result of apoB aggregation could be the basis for reduced VLDL maturation with DHA treatment, we tested whether a 20°C temperature block would restore VLDL formation. To investigate this, the 45 min chase was also conducted at 20°C (Fig. 3B). Similar to 37°C, equivalent amounts of pre-VLDL particles were isolated from microsomes isolated from cells treated with DHA and OA at 20°C (compare Figs. 3A and 3B). The 20°C temperature block also restored total apoB levels in DHA-treated cells to levels comparable to OA-treated controls (77.67 ± 4.47% of OA at 20°C compared with 57.62 ± 0.43% of OA at 37°C). However, VLDL formation in DHA-treated cells was not restored, with relative percentages of mature VLDL remaining significantly lower than those in OA control cell microsome lumens independent of temperature (46.12 ± 3.74% of OA at 20°C and 59.42 ± 5.04% of OA at 37°C). DFX cotreatment with DHA was able to restore VLDL formation to control (OA) levels.
Fig. 3.
DHA-treated hepatocytes are able to form pre-VLDL particles but convert fewer to mature VLDL than OA-treated cells. Cells were treated as in Fig. 2, except that the experiment was concluded after 45 min of chase. A: The sum of apoB recovered from fractions 6 through 11 (pre-VLDL) or fraction 1 (fully lipidated VLDL) from total microsomes of cells treated with OA, DHA, or DHA+DFX at 37°C, as well as the total apoB recovered, are shown. *P < 0.05 compared with OA. B: The corresponding data were obtained from an experiment carried out at 20°C, with representative fluorograms also shown. *P < 0.01 compared with OA. Graphical results shown are from three separate experiments at 37°C and two separate experiments at 20°C, with replicate measurements for each treatment.
Although VLDL maturation was impaired in the DHA-treated cells, we wondered whether the fate of the smaller number of pre-VLDL particles that did successfully convert resembled that of the mature VLDL in OA-treated cells. Therefore, we examined the total VLDL-apoB recovery data at each chase time point from 0 to 90 min. The total VLDL-apoB recovered at 90 min compared with that recovered at earlier time points for the OA and DHA treatments did not indicate significant degradation of apoB associated with mature VLDL (data not shown). These data imply that once pre-VLDL particles were converted to mature VLDL in OA or DHA-treated cells, they were quantitatively secreted.
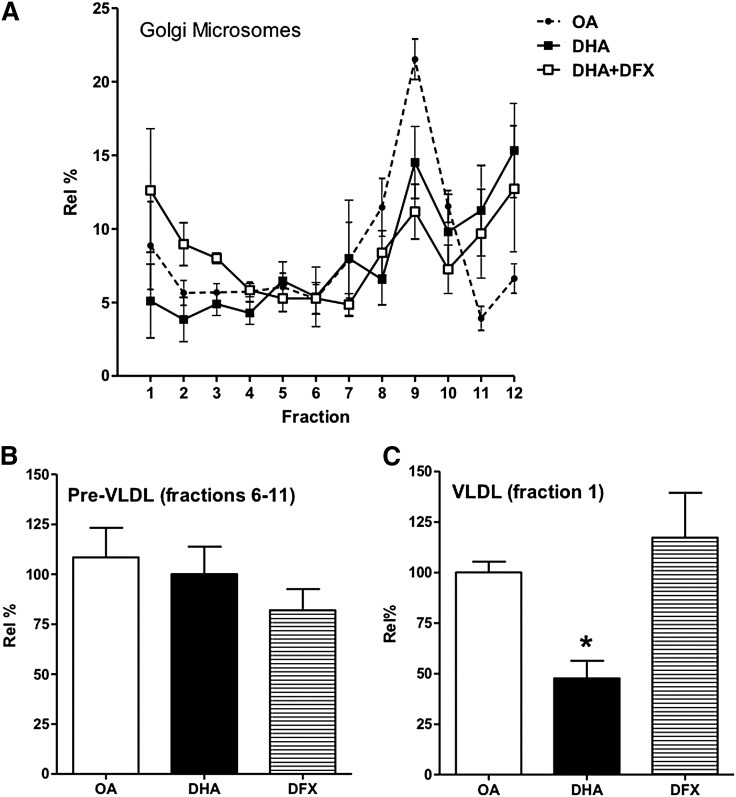
We (10, 11) and others (e.g., Ref. 1, 15) have shown that much of the maturation of VLDL particles occurs in the Golgi. We have assumed this to be the case in our experiments using total microsomes, which are a mixture of ER and Golgi-derived structures. To directly examine events in the Golgi, we repeated the 45 min time point with isolated Golgi microsomes (Fig. 4). The pattern of results, including the effects of DFX, was similar to that observed with total microsomes (i.e., less VLDL maturation in DHA-treated cells and its restoration upon DFX cotreatment).
Fig. 4.
Effect of DHA on the density distribution of apoB-lipoproteins in Golgi microsomes. Cells were treated as in Fig. 3A, but rather than the luminal contents of total microsomes being analyzed, only those of Golgi microsomes were. A: Density distributions of the apoB recovered under the different treatment conditions (OA, DHA, or DHA+DFX). The relative amounts of apoB associated with pre-VLDL (fractions 6–11) and mature VLDL (fraction1) are shown in B and C, respectively. *P < 0.01 compared with OA. Results shown are from five separate experiments.
Loss of VLDL formation and secretion coincides with the formation of intracellular apoB aggregates in DHA-treated cells, which colocalize with lysosomes in a microtubule-dependent, aggresome-independent process
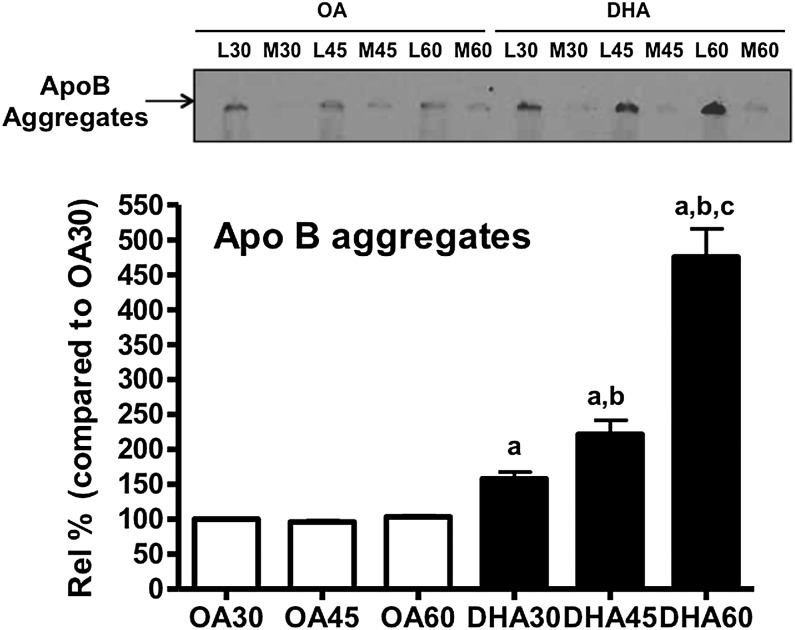
To further investigate the nature of the intracellular loss of monomeric (i.e., nonaggregated) apoB associated with pre-VLDL in DHA-treated cells, we carried out another series of pulse-chase studies. ApoB aggregates started to appear by T30 (Fig. 5) and accumulated over time, with greater quantities observed at 45 min (Fig. 5; T45 = 1.5× the levels at T30) and increasing further at T60 (Fig. 5; T60 = 3× T30). Although there were some aggregates in OA-treated cells, they did not accumulate over time (Fig. 5). The time course of apoB aggregate accumulation in DHA-treated cells parallels the decrease in the recovery of mature VLDL from the microsomes and conditioned media. The aggregates are recovered from whole cell lysate and never from microsomes, consistent with the post-Golgi formation we previously reported (5).
Fig. 5.
Loss of monomeric apoB from the microsomal lumen coincides with the formation of intracellular apoB aggregates. Cells were treated as in Fig. 1, except that immunoprecipitation/SDS-PAGE analysis for apoB was performed using aliquots of the cell lysates at the chase time points 30, 45, and 60 min. The aggregated apoB (which appears toward the top of the gels) was quantified by densitometry. a = significant increase compared with OA30 (P < 0.05 for DHA30; P < 0.01 for DHA45 and DHA60); b = significant increase compared with DHA30 (P < 0.01); c = significant increase compared with DHA45 (P < 0.001). The fluorogram shows the area of the gel expected to contain aggregated apoB and the source of the original sample [lysate (L) or conditioned medium (M)] of cells treated with OA or DHA]. Results shown are representative of three separate experiments.
We next turned to the intracellular fate of the aggregated apoB. From previous studies, including coimmunoprecipitation analysis, we know that this material eventually becomes associated with autophagosomes (5). We hypothesized that like some other proteins, these aggregates first accumulate in cytosolic structures called aggresomes, which can be transported by the microtubule network to the juxta-nuclear region, where they are packaged into autophagosomes (16). In DHA-treated cells, this would mean that apoB and its associated lipoprotein particles would be first extruded from the Golgi and would then aggregate and be trafficked in a non-vesicular structure to the juxta-nuclear region, where they would fuse with autophagosome precursors. Alternatively, autophagosomes (perhaps originating from Golgi membranes) would first form around apoB and its associated particles, without the requirement of aggresome formation/transport, and then be clustered in the juxta-nuclear region by microtubule-dependent transport before fusion with lysosomes. To investigate these possibilities, we performed immunofluorescence studies.
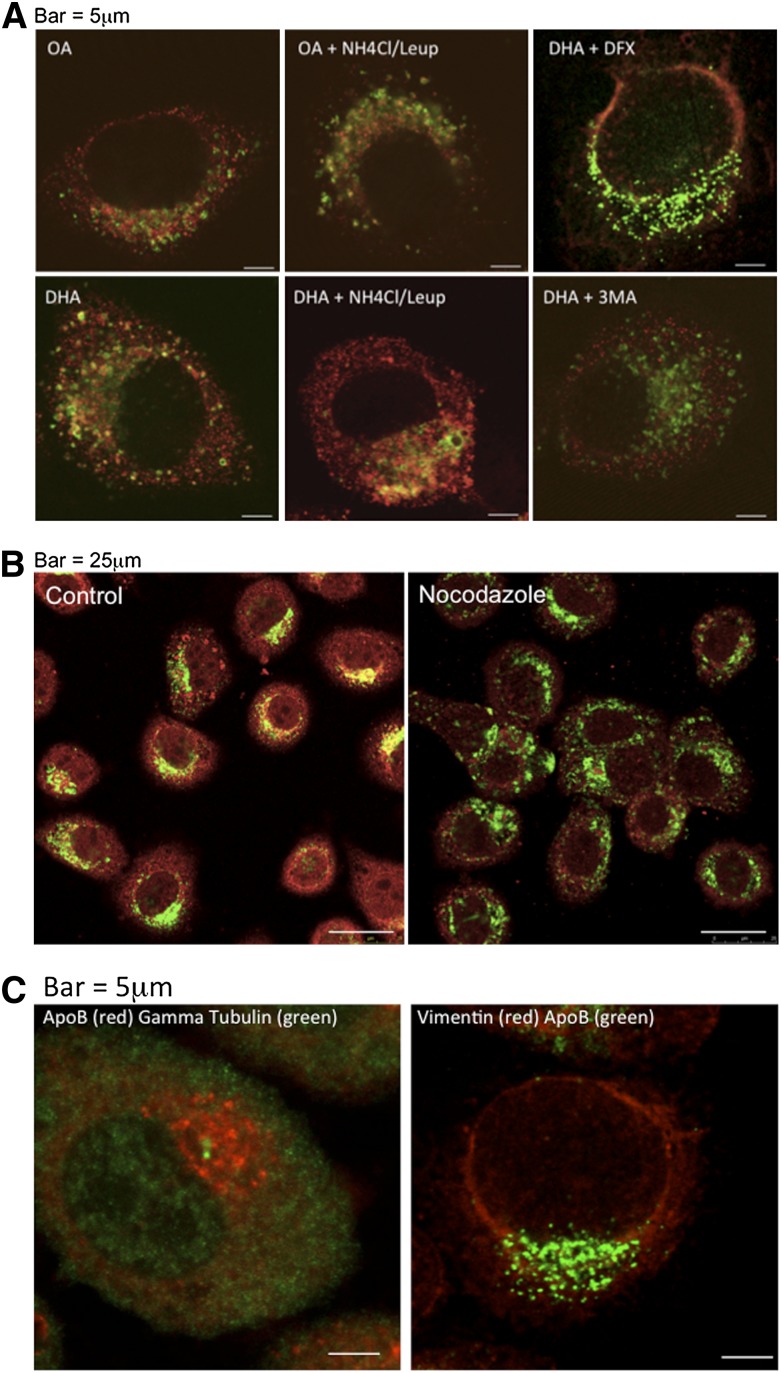
First, we confirmed that the lysosomes, the site of aggregate degradation in either scenario, were associated with apoB in DHA-treated cells. More immunostained apoB colocalized with LAMP1 (a standard marker of lysosomes) in the presence of DHA compared with hepatic cells treated with BSA, OA, or any of the conditions in the presence of DFX (Fig. 6A and supplementary Fig. I). The colocalization was also increased by cotreating the cells with NH4Cl-leupeptin, a standard condition used to block the degradation of proteins in lysosomes. In contrast, there was the absence of colocalization in cells pretreated with 3-methyl adenine (3-MA), which prevents autophagosome formation, before incubation with DHA (Fig. 6A), or, as noted above, in cells cotreated with DFX (Fig. 6A and supplementary Fig. I), which prevents DHA-induced lipid peroxidation and apoB aggregation.
Fig. 6.
ApoB in DHA-treated cells colocalizes with lysosomes in a microtubule-dependent process that does not involve aggresome formation. A: McA cells were treated for 4 h with OA or DHA with or without ammonium chloride and leupeptin (NH4Cl/Leup) to prevent acidification of the lysosomes and subsequent protein degradation or with DHA in the presence of DFX or the autophagy inhibitor 3-methyl adenine (3-MA). The cells were then fixed, permeabilized, and immunostained for apoB (Alexa Fluor 594, red) and LAMP1 (Alexa Fluor 488, green). B: McA cells were treated for 2 h with DHA to allow aggregate accumulation and then for 2 h without (B, left panel) or with (B, right panel) nocodazole (which disrupts microtubules) in the continued presence of DHA. After permeabilization, the cells were immunostained for apoB (Alexa Fluor 594, red) and LAMP1 (Alexa Fluor 488, green). C: McA cells were treated for 4 h with DHA, and immunostained for apoB (Alexa Fluor 594, red) and gamma tubulin (Alexa Fluor 488, green) (C, left panel) and for apoB (Alexa Fluor 488, green) and vimentin (Texas Red) (C, right panel). Results shown are representative of three separate experiments.
To assess the involvement of the microtubule network, which would be expected to play a role in the autophagy of apoB and its associated lipoproteins in either scenario described above, we pretreated the cells with DHA for 2 h before adding the microtubule dispersing agent nocodazole. There was an obvious decrease in apoB-LAMP1 colocalization in the treated cells (Fig. 6B). To differentiate between microtubule-mediated transport of an aggresome vs. an autophagosome, we looked for colocalization of apoB with γ-tubulin and vimentin, proteins known to associate with aggresomes (17, 18). Immunostained apoB did not colocalize with either marker (Fig. 6C). Taken with our finding that apoB aggregation in DHA-treated cells requires trafficking from the Golgi (5), the data favor the idea that aggregation occurs not as part of a cytosolic aggresomal process but after the incorporation into autophagosomes of apoB-lipoproteins that failed to mature in the Golgi.
DISCUSSION
Although it is well established that fish oil fatty acids (DHA, EPA) reduce plasma levels of triglycerides and apoB associated with VLDL, there are multiple mechanisms put forward to explain these effects. Clinical kinetic studies have shown that diets rich in fish oil decrease hepatic production of VLDL-apoB100 (e.g., Ref. 19). Based on our previous studies (reviewed in Ref. 20), one such mechanism for this is the stimulation by fish oil fatty acids of apoB degradation by autophagy. In the present set of studies, we have extended the understanding of fish oil-stimulated degradation of apoB by showing that: 1) DHA treatment impairs the full lipidation of pre-VLDL particles in the Golgi, 2) this impairment depends on lipid peroxidation and precedes apoB aggregation, and 3) apoB associated with the immature particles destined for degradation does not undergo aggresome formation in the cytosol before capture by autophagosomes.
The experimental approach we took was based on previous reports that showed in rat hepatic cells the sequential formation of HDL-density pre-VLDL particles in the ER and their conversion to mature VLDL-density particles in the Golgi (e.g., Refs. 11, 12, 15). In the current set of studies, we replicated these findings with OA, but in DHA-treated cells, there was the initial formation in the ER of pre-VLDL particles but little conversion in the Golgi to mature (i.e., fully lipidated) lipoproteins (consistent with Ref. 21). Instead, there was apoB aggregation and degradation. The deficiency of VLDL maturation in DHA-treated cells is unlikely to be a consequence of inadequate lipid synthesis, which is at least that stimulated by OA (e.g., Refs. 4, 21, 22). Because blocking Golgi exit prevented the loss of pre-VLDL particles (Fig. 3) and the formation of apoB aggregates (5), aggregation and degradation of apoB associated with pre-VLDL in DHA-treated cells appears to occur post-Golgi. This is consistent with the lack of aggregates in microsomes and with the data shown in Fig. 6. In general, the data do not support the hypothesis that the loss of pre-VLDL without its quantitative secretion or conversion to mature VLDL followed apoB-aggregation/degradation. Instead, the findings that inhibiting apoB aggregation did not restore VLDL maturation (Fig. 3) and that removal of the 20°C block resulted in formation of aggregates (5) suggest that aggregation and degradation are consequences of derangements in apoB and pre-VLDL metabolism.
The data in Fig. 6 further argue that the large aggregates that form in DHA-treated cells do not represent a process in which apoB is extruded from the Golgi into the cytosol and first assembled into an aggresome before being taken up by autophagosomes. Rather, more likely is the transfer directly of apoB associated with immature lipoprotein particles to autophagosomes, with subsequent aggregation in the lower pH environment. One possible factor involved in the transfer of “failed” VLDL intermediates is the autophagy-related protein Atg9, which has been shown to promote the recruitment of components of autophagosome isolation membranes from the Golgi apparatus (23, 24). In experiments in which we knocked down Atg9 mRNA, however, we found only a minor effect (<20%) on the degradation of apoB aggregates in DHA-treated cells (U. Andreo, E. Fisher, unpublished observations). It will be an important goal of future experiments to implicate other candidate factors.
The identifying feature of apoB to be targeted for autophagy in DHA-treated cells may be its modification by lipid peroxides during its residence in the secretory pathway. One obvious circumstance favoring apoB modification by lipid peroxides is their incorporation into the lipid fraction of nascent lipoprotein particles, which would place them in intimate contact with the protein. This is supported by finding more (∼1.5×) thiobarbituric acid reactive substances (a measure of lipid peroxidation) in lipoproteins isolated from Golgi microsomes from DHA-treated cells than in those isolated from OA-treated cells (V. Maitin, E. Fisher; unpublished observations). Other opportunities for fish oil fatty acids to disrupt apoB/pre-VLDL metabolism are afforded by the effects of the type of fatty acid on the composition of cellular membranes (plasma and endo) and the functions of transmembrane proteins. For example, it has been shown that phospholipids become enriched in EPA in microsomal membranes in EPA-treated McA cells (21). Such enrichment may disrupt the necessary association of pre-VLDL with endomembranes, which serves to lipidate apoB-lipoproteins in hepatic cells (e.g., Ref. 12, 15, 25) or may impair the utilization of triglycerides that are derived from phospholipid remodeling for VLDL lipidation (21).
In summary, the present data suggest a model in which pre-VLDL particles that “pass” ER-quality control criteria but then “fail” similar scrutiny in the Golgi if they do not properly mature are targeted for degradation by autophagy. Besides our results for DHA, further support for this scenario comes from a number of other reports. For example, Boren at al. (12) and Stillemark-Billton et al. (26) found that HDL-density apoB particles (i.e., pre-VLDL) were degraded intracellularly unless further lipidated, although the degradative process was not recognized. In a more recent study, it was shown that a naturally occurring mutation of apoB (apoB A31P) associated with hypolipidemia was trafficked from the ER to the Golgi, where it failed to be lipidated, and was subsequently degraded by autophagy (27). In another example, Brown and Gibbons (28) reported that insulin, which is known to induce autophagic degradation of apoB (29), interferes with VLDL particle maturation. It has been previously thought that the major quality control of secretory proteins is exerted in the ER. For the majority of proteins, this is probably true, but in the case of VLDL (and perhaps other cargoes that undergo significant assembly/maturation events post-ER), mechanisms to exert quality control late in the secretory pathway are also needed. Thus, the continued investigation of VLDL secretory control will not only inform the field of lipoprotein metabolism, but will more generally expand the understanding of the cellular regulation of protein secretion.
Supplementary Material
Acknowledgments
The authors thank Drs. Ana Maria Cuervo (Albert Einstein College of Medicine) and Kevin Jon Williams (Temple University) for helpful discussions and David Yaffee (NYU School of Medicine) for help in some of the experimental work.
Footnotes
Abbreviations:
- apoB
- apolipoprotein B100
- DFX
- desferrioxamine
- ER
- endoplasmic reticulum
- HS
- horse serum
- OA
- oleic acid
- [35S]Met/Cys
- [35S]methionine/cysteine
- TCA
- richloroacetic acid
This work was supported by National Institutes of Health grant R01 HL58541 (E.A.F.) and by a post-doctoral fellowship from the American Heart Association (U.A.).
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of one figure and supplementary Methods.
REFERENCES
- 1.Olofsson S. O., Boren J. 2005. Apolipoprotein B: a clinically important apolipoprotein which assembles atherogenic lipoproteins and promotes the development of atherosclerosis. J. Intern. Med. 258: 395–410 [DOI] [PubMed] [Google Scholar]
- 2.Ginsberg H. N., Fisher E. A. 2009. The ever-expanding role of degradation in the regulation of apolipoprotein B metabolism. J. Lipid Res. 50(Suppl): S162–S166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fisher E. A., Pan M., Chen X., Wu X., Wang H., Jamil H., Sparks J. D., Williams K. J. 2001. The triple threat to nascent apolipoprotein B. Evidence for multiple, distinct degradative pathways. J. Biol. Chem. 276: 27855–27863 [DOI] [PubMed] [Google Scholar]
- 4.Wang H., Chen X., Fisher E. A. 1993. N-3 fatty acids stimulate intracellular degradation of apoprotein B in rat hepatocytes. J. Clin. Invest. 91: 1380–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pan M., Maitin V., Parathath S., Andreo U., Lin S. X., St Germain C., Yao Z., Maxfield F. R., Williams K. J., Fisher E. A. 2008. Presecretory oxidation, aggregation, and autophagic destruction of apoprotein-B: a pathway for late-stage quality control. Proc. Natl. Acad. Sci. USA. 105: 5862–5867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pan M., Cederbaum A. I., Zhang Y. L., Ginsberg H. N., Williams K. J., Fisher E. A. 2004. Lipid peroxidation and oxidant stress regulate hepatic apolipoprotein B degradation and VLDL production. J. Clin. Invest. 113: 1277–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andreo U., Elkind J., Blachford C., Cederbaum A. I., Fisher E. A. 2011. Role of superoxide radical anion in the mechanism of apoB100 degradation induced by DHA in hepatic cells. FASEB J. 25: 3554–3560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilkinson J., Higgins J. A., Fitzsimmons C., Bowyer D. E. 1998. Dietary fish oils modify the assembly of VLDL and expression of the LDL receptor in rabbit liver. Arterioscler. Thromb. Vasc. Biol. 18: 1490–1497 [DOI] [PubMed] [Google Scholar]
- 9.Kendrick J. S., Higgins J. A. 1999. Dietary fish oils inhibit early events in the assembly of very low density lipoproteins and target apoB for degradation within the rough endoplasmic reticulum of hamster hepatocytes. J. Lipid Res. 40: 504–514 [PubMed] [Google Scholar]
- 10.Gusarova V., Brodsky J. L., Fisher E. A. 2003. Apolipoprotein B100 exit from the endoplasmic reticulum (ER) is COPII-dependent, and its lipidation to very low density lipoprotein occurs post-ER. J. Biol. Chem. 278: 48051–48058 [DOI] [PubMed] [Google Scholar]
- 11.Gusarova V., Seo J., Sullivan M. L., Watkins S. C., Brodsky J. L., Fisher E. A. 2007. Golgi-associated maturation of very low density lipoproteins involves conformational changes in apolipoprotein B, but is not dependent on apolipoprotein E. J. Biol. Chem. 282: 19453–19462 [DOI] [PubMed] [Google Scholar]
- 12.Boren J., Rustaeus S., Olofsson S. O. 1994. Studies on the assembly of apolipoprotein B-100- and B-48-containing very low density lipoproteins in McA-RH7777 cells. J. Biol. Chem. 269: 25879–25888 [PubMed] [Google Scholar]
- 13.Rustaeus S., Lindberg K., Stillemark P., Claesson C., Asp L., Larsson T., Boren J., Olofsson S. O. 1999. Assembly of very low density lipoprotein: a two-step process of apolipoprotein B core lipidation. J. Nutr. 129: 463S–466S [DOI] [PubMed] [Google Scholar]
- 14.Griffiths G., Fuller S. D., Back R., Hollinshead M., Pfeiffer S., Simons K. 1989. The dynamic nature of the Golgi complex. J. Cell Biol. 108: 277–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tran K., Thorne-Tjomsland G., DeLong C. J., Cui Z., Shan J., Burton L., Jamieson J. C., Yao Z. 2002. Intracellular assembly of very low density lipoproteins containing apolipoprotein B100 in rat hepatoma McA-RH7777 cells. J. Biol. Chem. 277: 31187–31200 [DOI] [PubMed] [Google Scholar]
- 16.Wong E. S., Tan J. M., Soong W. E., Hussein K., Nukina N., Dawson V. L., Dawson T. M., Cuervo A. M., Lim K. L. 2008. Autophagy-mediated clearance of aggresomes is not a universal phenomenon. Hum. Mol. Genet. 17: 2570–2582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waelter S., Boeddrich A., Lurz R., Scherzinger E., Lueder G., Lehrach H., Wanker E. E. 2001. Accumulation of mutant huntingtin fragments in aggresome-like inclusion bodies as a result of insufficient protein degradation. Mol. Biol. Cell. 12: 1393–1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagaoka U., Kim K., Jana N. R., Doi H., Maruyama M., Mitsui K., Oyama F., Nukina N. 2004. Increased expression of p62 in expanded polyglutamine-expressing cells and its association with polyglutamine inclusions. J. Neurochem. 91: 57–68 [DOI] [PubMed] [Google Scholar]
- 19.Watts G. F., Chan D. C., Ooi E. M., Nestel P. J., Beilin L. J., Barrett P. H. 2006. Fish oils, phytosterols and weight loss in the regulation of lipoprotein transport in the metabolic syndrome: lessons from stable isotope tracer studies. Clin. Exp. Pharmacol. Physiol. 33: 877–882 [DOI] [PubMed] [Google Scholar]
- 20.Brodsky J. L., Fisher E. A. 2008. The many intersecting pathways underlying apolipoprotein B secretion and degradation. Trends Endocrinol. Metab. 19: 254–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tran K., Sun F., Cui Z., Thorne-Tjomsland G., St Germain C., Lapierre L. R., McLeod R. S., Jamieson J. C., Yao Z. 2006. Attenuated secretion of very low density lipoproteins from McA-RH7777 cells treated with eicosapentaenoic acid is associated with impaired utilization of triacylglycerol synthesized via phospholipid remodeling. Biochim. Biophys. Acta. 1761: 463–473 [DOI] [PubMed] [Google Scholar]
- 22.Lang C. A., Davis R. A. 1990. Fish oil fatty acids impair VLDL assembly and/or secretion by cultured rat hepatocytes. J. Lipid Res. 31: 2079–2086 [PubMed] [Google Scholar]
- 23.Webber J. L., Young A. R., Tooze S. A. 2007. Atg9 trafficking in Mammalian cells. Autophagy. 3: 54–56 [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto H., Kakuta S., Watanabe T. M., Kitamura A., Sekito T., Kondo-Kakuta C., Ichikawa R., Kinjo M., Ohsumi Y. 2012. Atg9 vesicles are an important membrane source during early steps of autophagosome formation. J. Cell Biol. 198: 219–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pariyarath R., Wang H., Aitchison J. D., Ginsberg H. N., Welch W. J., Johnson A. E., Fisher E. A. 2001. Co-translational interactions of apoprotein B with the ribosome and translocon during lipoprotein assembly or targeting to the proteasome. J. Biol. Chem. 276: 541–550 [DOI] [PubMed] [Google Scholar]
- 26.Stillemark-Billton P., Beck C., Boren J., Olofsson S. O. 2005. Relation of the size and intracellular sorting of apoB to the formation of VLDL 1 and VLDL 2. J. Lipid Res. 46: 104–114 [DOI] [PubMed] [Google Scholar]
- 27.Zhong S., Magnolo A. L., Sundaram M., Zhou H., Yao E. F., Di Leo E., Loria P., Wang S., Bamji-Mirza M., Wang L., et al. 2010. Nonsynonymous mutations within APOB in human familial hypobetalipoproteinemia: evidence for feedback inhibition of lipogenesis and postendoplasmic reticulum degradation of apolipoprotein B. J. Biol. Chem. 285: 6453–6464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown A. M., Gibbons G. F. 2001. Insulin inhibits the maturation phase of VLDL assembly via a phosphoinositide 3-kinase-mediated event. Arterioscler. Thromb. Vasc. Biol. 21: 1656–1661 [DOI] [PubMed] [Google Scholar]
- 29.Andreo U., Guo L., Chirieac D. V., Tuyama A. C., Montenont E., Brodsky J. L., Fisher E. A. 2013. Insulin-stimulated degradation of apolipoprotein B100: roles of class II phosphatidylinositol-3-kinase and autophagy. PLoS ONE. 8: e57590. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.