Abstract
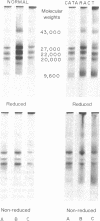
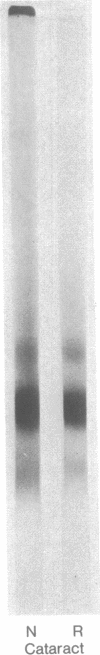
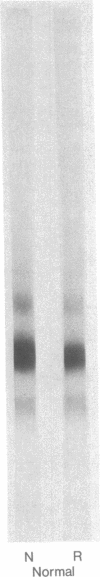
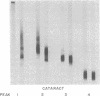
A major high molecular weight disulfide-linked protein has been isolated from cataractous lenses. It is only present in the water-insoluble protein fractions. This species has not been found in normal lenses of comparable age. Upon reduction of this fraction, polypeptides having molecular weights of approximately 60,000, 43,000, and 20,000 as well as a noncharacterized heterogeneous species are released. Similar sized polypeptides have been found in various noncovalently linked aggregates in both normal and cataractous lenses. Examination of the disulfide-linked protein fraction indicates that approximately 70% of the sulfhydryl groups are in the oxidized state. Although little change in the sizes of the other major polypeptides in the water-insoluble fraction is observed upon reduction, these components were also found to contain an appreciable disulfide content. Such results indicate that the only major lens fraction containing disulfide-linked polypeptide is the high molecular weight species and that the disulfides present in the remaining fractions are either intrachain disulfides or link polypeptides to small peptides.
Full text
PDF

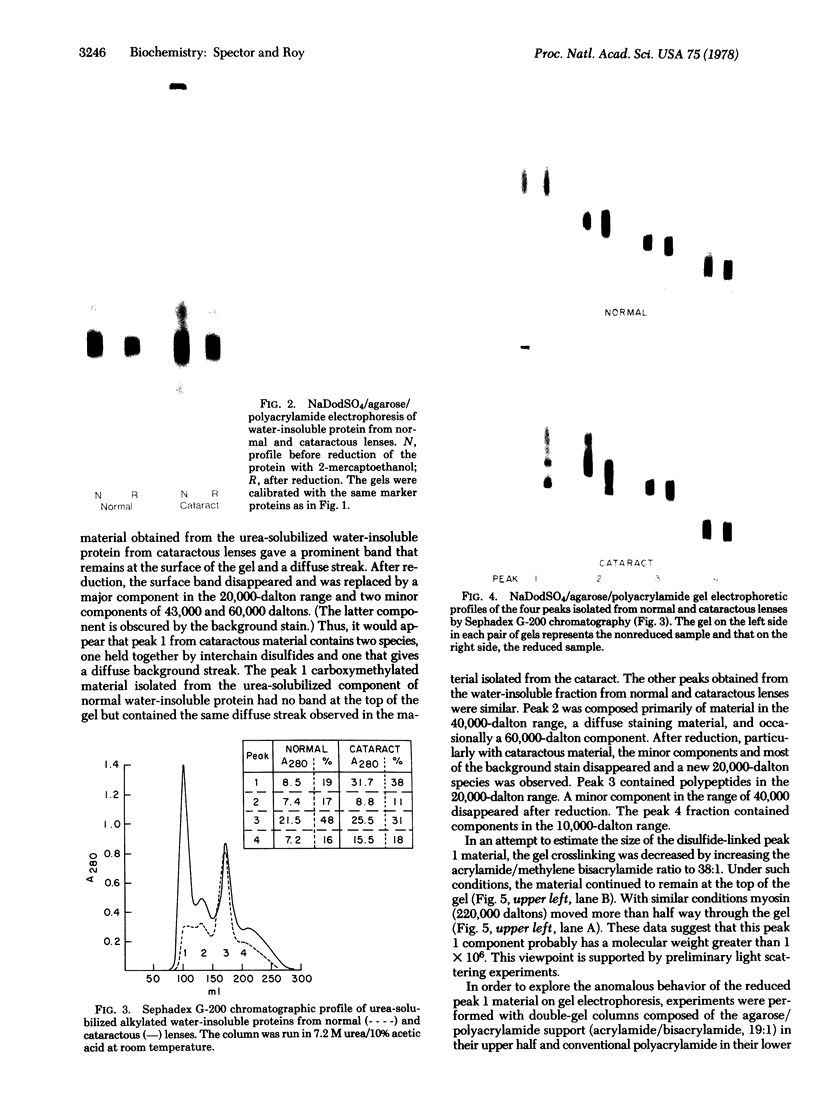
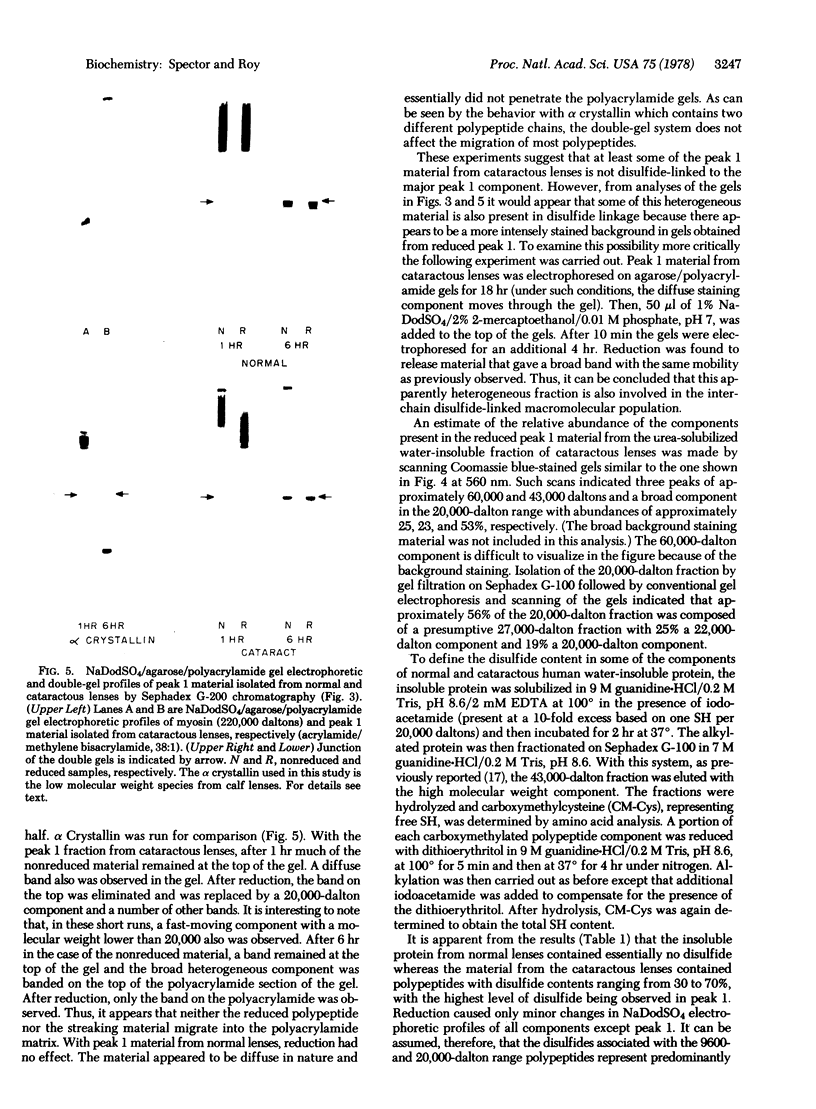

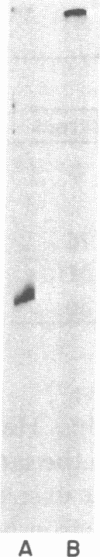
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson E. I., Spector A. The state of sulfhydryl groups in normal and cataractous human lens proteins. I. Nuclear region. Exp Eye Res. 1978 Apr;26(4):407–417. doi: 10.1016/0014-4835(78)90128-8. [DOI] [PubMed] [Google Scholar]
- Bloemendal H. The vertebrate eye lens. Science. 1977 Jul 8;197(4299):127–138. doi: 10.1126/science.877544. [DOI] [PubMed] [Google Scholar]
- Clark R., Zigman S., Lerman S. Studies on the structural proteins of the human lens. Exp Eye Res. 1969 Apr;8(2):172–182. doi: 10.1016/s0014-4835(69)80029-1. [DOI] [PubMed] [Google Scholar]
- DISCHE Z., ZIL H. Studies on the oxidation of cysteine to cystine in lens proteins during cataract formation. Am J Ophthalmol. 1951 May;34(5 2):104–113. doi: 10.1016/0002-9394(51)90013-x. [DOI] [PubMed] [Google Scholar]
- Harding J. J. Disulphide cross-linked protein of high molecular weight in human cataractous lens. Exp Eye Res. 1973 Nov 25;17(4):377–383. doi: 10.1016/0014-4835(73)90247-9. [DOI] [PubMed] [Google Scholar]
- Harding J. J. The nature and origin of the urea-insoluble protein of human lens. Exp Eye Res. 1972 Jan;13(1):33–40. doi: 10.1016/0014-4835(72)90122-4. [DOI] [PubMed] [Google Scholar]
- MACH H. UNTERSUCHUNGEN VON LINSENEIWEISS UND MIKROELEKTROPHORESE VON WASSERLOESLICHEM EIWEISS IM ALTERSSTAR. Klin Monbl Augenheilkd. 1963 Dec;143:689–710. [PubMed] [Google Scholar]
- Maisel H. The effect of urea on the lens intracellular matrix and soluble lens protein. Exp Eye Res. 1977 Dec;25(6):595–601. doi: 10.1016/0014-4835(77)90138-5. [DOI] [PubMed] [Google Scholar]
- Manski W., Behrens M., Martinez C. Immunochemical studies on albuminoid. Exp Eye Res. 1968 Jan;7(1):164–171. doi: 10.1016/s0014-4835(68)80041-7. [DOI] [PubMed] [Google Scholar]
- Masters P. M., Bada J. L., Zigler J. S., Jr Aspartic acid racemization in heavy molecular weight crystallins and water insoluble protein from normal human lenses and cataracts. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1204–1208. doi: 10.1073/pnas.75.3.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville D. M., Jr, Glossmann H. Plasma membrane protein subunit composition. A comparative study by discontinuous electrophoresis in sodium dodecyl sulfate. J Biol Chem. 1971 Oct 25;246(20):6335–6338. [PubMed] [Google Scholar]
- Pirie A. Color and solubility of the proteins of human cataracts. Invest Ophthalmol. 1968 Dec;7(6):634–650. [PubMed] [Google Scholar]
- Roy D., Spector A. High molecular weight protein from human lenses. Exp Eye Res. 1976 Mar;22(3):273–279. doi: 10.1016/0014-4835(76)90055-5. [DOI] [PubMed] [Google Scholar]
- Roy D., Spector A. Human alpha-crystallin: characterization of the protein isolated from the periphery of cataractous lenses. Biochemistry. 1976 Mar 9;15(5):1180–1188. doi: 10.1021/bi00650a034. [DOI] [PubMed] [Google Scholar]
- Roy D., Spector A. Human insoluble lens protein. I. Separation and partial characterization of polypeptides. Exp Eye Res. 1978 Apr;26(4):429–443. doi: 10.1016/0014-4835(78)90130-6. [DOI] [PubMed] [Google Scholar]
- Satoh K. Age-related changes in the structural proteins of human lens. Exp Eye Res. 1972 Jul;14(1):53–57. doi: 10.1016/0014-4835(72)90142-x. [DOI] [PubMed] [Google Scholar]
- Spector A., Li S., Sigelman J. Age-dependent changes in the molecular size of human lens proteins and their relationship to light scatter. Invest Ophthalmol. 1974 Oct;13(10):795–798. [PubMed] [Google Scholar]
- Spector A., Roy D., Stauffer J. Isolation and characterization of an age-dependent polypeptide from human lens with non-tryptophan fluorescence. Exp Eye Res. 1975 Jul;21(1):9–24. doi: 10.1016/0014-4835(75)90053-6. [DOI] [PubMed] [Google Scholar]
- Truscott R. J., Augusteyn R. C. Oxidative changes in human lens proteins during senile nuclear cataract formation. Biochim Biophys Acta. 1977 May 27;492(1):43–52. doi: 10.1016/0005-2795(77)90212-4. [DOI] [PubMed] [Google Scholar]
- Wannemacher C. F., Spector A. Protein synthesis in the core of calf lens. Exp Eye Res. 1968 Oct;7(4):623–625. doi: 10.1016/s0014-4835(68)80018-1. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]