Abstract
The 1918 influenza pandemic caused over 40 million deaths worldwide with 675,000 deaths in the US alone. Studies in several experimental animal models showed that 1918 influenza virus infection resulted in severe lung pathology associated with dysregulated immune and cell death responses. To determine if reactive oxygen species produced by host inflammatory responses play a central role in promoting severity of lung pathology, we treated 1918 influenza virus infected mice with the catalytic catalase/superoxide dismutase mimetic, salen-manganese complex EUK-207 beginning 3 days post-infection. Post-exposure treatment of mice infected with a lethal dose of the 1918 influenza virus with EUK-207 resulted in significantly increased survival and reduced lung pathology without a reduction in viral titers. In vitro studies also showed that EUK-207 treatment did not affect 1918 influenza viral replication. Immunohistochemical analysis showed a reduction in the detection of the apoptosis marker cleaved caspase-3 and the oxidative stress marker 8-oxo-2'-deoxyguanosine in lungs of EUK-207 treated animals compared to vehicle controls. High-throughput sequencing and RNA expression microarray analysis revealed that treatment resulted in decreased expression of inflammatory response genes and increased lung metabolic and repair responses. These results directly demonstrate that 1918 influenza virus infection leads to an immunopathogenic immune response with excessive inflammatory and cell death responses that can be limited by treatment with the catalytic antioxidant, EUK-207.
Introduction
The influenza pandemic of 1918-19 was one of the most catastrophic epidemics in history and resulted in 40-60 million deaths worldwide and 675,000 deaths in the U.S. alone [1, 2]. Contemporary and modern histopathological studies demonstrated severe lung pathology associated with primary viral infections and secondary bacterial infections [3]. Modern viral sequence determination and characterization allowed for the reverse genetics reconstruction of the 1918 H1N1 influenza virus [4, 5], and experimental infection of mice demonstrated that the virus was highly pathogenic without need for prior adaptation. In both mouse and nonhuman primate models, 1918 influenza virus infection resulted in high levels of viral replication, severe necrotizing bronchitis, bronchiolitis, and a mixed cellularity, neutrophil-predominant alveolitis and acute edema [6, 7]. Analysis of the host response mounted in the lungsjof mfce during 1918 virus infection revealed dramatically dysregulated immune responses that were elicited within 1 day post-infection (dpi) and persisted unabated until death. These responses included significant activation of antiviral, pro-inflammatory, reactive oxygen species (ROS) and cell death responses [6]. Similar studies in ferrets and cynomolgus macaques demonstrated that the 1918 virus was highly lethal in both species and with severe lung pathology similar to that seen in mice [7, 8]. Expression microarray analysis of bronchial tissue from infected macaques revealed that infection was associated with suppression of type I IFN and other antiviral responses and marked expression of pro-inflammatory cytokines and chemokines [7]. Together these studies revealed that the reconstructed 1918 pandemic influenza virus was highly pathogenic in several animal models and is associated with the over-activation of pro-inflammatory responses suggesting that a key component of virulence was driven by immunopathogenic responses.
Central to the inflammatory response is the activation of immune cell-mediated killing that can occur via several distinct mechanisms, including the production of ROS by neutrophils [9, 10]. The superoxide burst of these cells is catalyzed by the NADPH-oxidase system [11, 12]. The generation of hydrogen peroxide and other ROS in situ leads to oxidation of cellular proteins, lipids, and DNA, resulting in cellular dysfunction or death [13,14]. The production of ROS is also associated with other types of injurious conditions, including ischemia and reperfusion injury, chemical toxicity, radiation damage, and many degenerative diseases. Previous studies have shown that ROS and reactive nitrogen species (RNS) play a role in influenza virus pathogenesis and can be targets for therapeutic intervention [15-17]. Because of the central role ROS play in causing cell death and tissue damage during numerous pathogenic responses, drugs with antioxidant properties have been developed to scavenge ROS and, thereby, limit cellular damage. One such family of antioxidants is salen manganese complexes [18, 19]. Salen-manganese complexes are ROS scavengers whose catalytic and pharmacological properties have been studied for nearly 20 years [18-20]. These synthetic compounds act as mimetics of the antioxidant enzymes superoxide dismutase and catalase, neutralizing superoxide and hydrogen peroxide, respectively. In addition, the compounds can neutralize reactive nitrogen species through mechanisms analogous to their catalase activity [21]. The salen-manganese complexes are true catalysts in that one molecule can scavenge many ROS molecules. This characteristic distinguishes them from scavengers such as vitamin C, vitamin E, and N-acetyl cysteine which remove ROS by mass action. Salen manganese complexes, including EUK-207 and its earlier prototypes, have shown beneficial effects in many in vivo systems, including models for stroke and other forms of excitotoxic and ischemic injury [22-24]. They not only protect against extracellular ROS, but also intracellularly, as demonstrated, for example, by their ability to suppress mitochondrial oxidative injury in several experimental models [25].
Thus, we hypothesized that excessive cell death responses and severe lung pathology during 1918 virus infection resulted, at least in part, from the excess production of ROS by the large number of neutrophils recruited to the lungs of animals during infection. This was supported by our previous finding of increased expression of genes associated with oxidative stress and cell death in infected mice [6], although that association did not prove a causal role for inflammatory responses or ROS in pathogenesis. Here, we measured the effects of treating mice infected with a lethal dose of 1918 influenza virus with EUK-207, a compound with catalase and superoxide dismutase activities known to have antioxidant and other beneficial effects in vivo [26-29]. We found that mice infected with a 4× mouse LD50 of the 1918 H1N1 influenza virus and treated post-exposure with 30 μg/day of EUK-207 from 3-10 days post-infection (dpi) showed significantly increased survival and dramatically reduced lung pathology, including reduced staining of the apoptosis marker cleaved caspase 3 and oxidative stress marker 8-Oxo-2'-deoxyguanosine. Further, analysis of host gene expression responses showed that EUK-207 treatment was associated with reduced host inflammatory and cell death responses and the activation of lung repair responses as compared to infected but vehicle-treated animals. EUK-207 treatment did not result in a significant change in viral replication either in vivo or in vitro. Deep sequence analysis of viral transcriptome showed no fixed sequence differences between EUK-207- and vehicle-treated groups, demonstrating that treatment of infected mice with EUK-207 did not result in selection of drug-resistant viruses after 7 days of treatment. This study demonstrated that host inflammatory responses play a significant role in the severity of 1918 influenza virus infection, and that treatment with a catalytic ROS scavenger can greatly lessen lung damage, an effect correlated with increased survival.
Materials and Methods
Rescue, growth, titration of viruses and viral replication kinetics
1918 H1N1 influenza viruses were rescued using published protocols [30, 31]. The rescued 1918 virus contained the HA gene of A/South Carolina/1/1918 and M, NA, NS, PA, PB1 and PB2 genes of A/Brevig Mission/1/1918 [4] or the PB1 of A/Green Wing Teal/Ohio/175/1986 [32]. Both 1918 viruses display identical virulence and pathology in mice [32]. Viruses were passaged on MDCK cells in the presence of 1.0 μg/ml TPCK-treated trypsin in DMEM. Viruses were harvested between 48-72h after infection, centrifuged for 10 min at 1500 rpm and the supernatants were frozen at −80 C. For titrations, virus stocks were serially diluted in DMEM and added to MDCK cells grown to confluence in 12 well polystyrene plates. Each dilution was made in duplicate. Following incubation for 1h at 37C, cells were washed once with 1x PBS and overlaid with 1.5 ml of agar gel (1% agar in MEM). After 2-3 days agar was removed and the cells stained with crystal violet solution [2% ethanol (v/v); 1% crystal violet (w/v)]. Virus titer was calculated by the method of Reed and Muench [33]. All work with the reconstructed 1918 influenza virus was performed in enhanced BSL-3 and ABSL-3 laboratories at the NIH in accordance with the Biosafety in Microbiological and Biomedical Laboratories (BMBL), 5th Edition, and the Select Agent Program at the Centers for Disease Control and Prevention and the NIH and under the supervision of the Biosurety Program of the NIH Department of Health and Safety.
Mouse infection studies
Groups of 10 to 25, 8-9 week old female BALB/c mice (Jackson Labs, Bar Harbor, ME) were lightly anesthetized in a chamber with isoflurane supplemented with O2 (1.5 L/min) and intranasally (i.n.) inoculated with 5×102 PFUr(4× LD50) of the fully reconstructed 1918 H1N1 influenza virus in a total volume of 50 μl. Where indicated sub-lethal infections were performed by i.n. inoculation of 100 PFU of 1918 virus. The dose of EUK-207 used for treatment was 30 μg/day, (1.5 mg/kg/day for an average pre-infection weight of 20g per mouse), and was administered by intraperitoneal injection daily on 3 to 10 dpi (n=69 mice total). Initial dose response experiments used concentrations of between 30-600 μg/day and established that the most efficacious dose was 30 μg /day (supplementary Fig. S1). Control animals were injected intraperitoneally daily on 3 to 10 dpi with 200 μl sterile water (n=50 mice total). Body weights were measured daily for 14 dpi and mice were humanely euthanized if they lost more than 25% of their starting body weight. For each time point, lungs from 3 animals were collected for RNA isolation and histopathology. EUK-207 was custom-synthesized (Dalton Chemical Laboratories, Toronto, ON) as described previously [34]. The structure and catalytic and other properties of EUK-207, a cyclic salen Mn complex, have been described [26, 35]. For tissue oxidative damage measurements performed by western blot for protein carbonylation or immunohistochemisty for 3-NT or 8-OHdG, described below, mice were euthanized by cervical dislocation following anesthesia with Ketamine/xylazine. All experimental animal work was performed in accordance with United States Public Health Service (PHS) Policy on Humane Care and Use of Laboratory Animals in an enhanced ABSL-3 laboratory at the National Institute of Allergy anmlmectious Diseases (NIAID) of the National Institutes of Health (NIH) following approval of animal safety protocols by the NIAID Animal Care and Use Committee and in accordance with the Select Agent Program at the Centers for Disease Control and Prevention.
Cell culture kinetics
For EUK-207 treatment kinetics, MDCK cells were grown as described above and infected with 1918 influenza virus at an MOI of 0.01. Cells were then continuously treated with 1.5 μg/ml EUK-207 and supernatants were collected at 12h, 24h, 48h and 72h post-infection for determination of viral replication by plaque assay and compared to mock-treated, infected cells. This dose of EUK-207 approximated the in vivo treatment dose of the mouse treatment experiments. (Mice averaging 20g on day 0 were treated daily with 30 μg EUK-207 Thus, the estimated concentration of EUK-207 in a treated mouse was approximately 1.5 μg/ml.)
RNA isolation
Lungs were collected at 6 and 8 dpi with influenza and total RNA was isolated from whole mouse lungs of 3 individual mice in each group by homogenization of (10% w/v) in Trizol (InvitrogenLfollowed by chloroform extraction and isopropanol precipitation. RNA quality was assessed using a BioAnalyzer (Agilent Technologies, CA). For expression microarray and next-generation sequencing analysis of pooled RNA samples, equal amount of total RNA from each mouse were pooled for experimental or control groups. For expression microarray analysis of individual animals, equal masses of RNA isolated from lungs of vehicle or EUK-207-treated animals were compared to a pool of RNA isolated from lungs of mock-infected mice.
Next generation sequencing
mRNA selection, library preparation and sequencing were performed according to Illumina mRNA-seq sample preparation kit specifications. Briefly, mRNA was selected using oligo(dT) probes and then fragmented using heat treatment. cDNA was synthesized using random primers, modified and enriched for attachment to the Illumina flowcell. The cDNA fragment sizes of the libraries were ~250bp. We sequenced on two flowcells of 6 lanes each with 36-cycle paired-end runs. Each flowcell contained 3 lanes of EUK-207-treated samples and 3 lanes of vehicle-treated samples. In total of 12 lanes, more than 635 million reads of a total of >22 GB of sequence were generated. All unfiltered paired-end sequences have been deposited as a series with the accession number GSE#### at NCBI's GEO database.
Reads were mapped to bowtie indexed mouse genome (UCSC version mm9) downloaded from Center for Bioinformatics and Computational Biology, University of Maryland website (http://bowtie-bio.sourceforge.net/index.shtml) using Tophat (release 1.0.14) (http://tophat.cbcb.umd.edu/) [36]. Overall gene expression level in terms of Fragments Per Kilobase of exon per Million fragments mapped (FPKM) [37] that is similar to Reads Per Kilobase per Million reads mapped [38], were calculated by Cufflink package (release 0.8.2) (http://cufflinks.cbcb.umd.edu/) [37]. Differential expressed genes between EUK-207-and vehicle-treated sample were identified by Cuffdiff function from Cufflink package (release 0.8.2) (http://cufflinks.cbcb.umd.edu/) [37]. The finial set of differential expressed genes was the overlapped data set identified in both runs by Cuffdiff. Reads were also mapped 1918 influenza virus genome using Bowtie (version 0.12.7) [39]. We used the default options supplied with these software packages in our analyses except for Tophat mapping using -r 250 --mate-std-dev 30 and Cufflinks/Cuffdiff calling using - m250 -s 30.
Expression microarray analysis
Gene expression profiling experiments were performed using Agilent Mouse Whole Genome 44K microarrays. Fluorescent probes were prepared using Agilent QuickAmp Labeling Kit according to the manufacturer's instructions. For each microarray experiment, mRNA isolated from the lungs of an individual animal inoculated 500 PFU 1918 influenza virus and either vehicle- or EUK-207-treated compared to a pool of mRNA isolated from the lungs of mock animals (n=9). Each RNA sample (2-3 biological replicates per condition) was labeled and hybridized to individual arrays. Spot quantitation was performed using Agilent's Feature Extractor software and all data was then entered into a custom-designed database, SlimArray, and then uploaded into GeneData Analyst 2.1 (GeneData, WA) and Spotfire Decision Suite 8.1 (Spotfire, Somerville, MA). Data normalization was performed in GeneData Analyst using central tendency followed by relative normalization using pooled RNA from mock infected mouse lung as reference. Babelomics suite and Genomatix Genome Analyzer were used for gene ontology analysis [40].
Pathology and immunohistochemistry
Tissue samples including 10% neutral buffered formalin fixed, inflated lungs, mediastinal lymph nodes, and spleen were dehydrated, embedded in paraffin. 5 μm sections on positively charged slides were stained with hematoxylin and eosin for histopathologic examination. Similar sets of slides were labeled for influenza A antigen distribution and the presence of the apoptosis marker cleaved caspase-3 and the oxidative stress markers 3-nitrotyrosine (3-NT) and 8-Oxo-2'-deoxyguanosine (8-OHdG). Briefly, slides were deparaffinized, rehydrated to water, steam antigen retrieved in pH 6 citrate buffer, blocked with 3% H2O2, primary antibody goat polyclonal anti-influenza A H1N1 (ab20841, Abcam, Cambridge, MA) at a dilution of 1:500 applied at room temperature for 1h, detected with VECTASTAIN Elite ABC Kit Goat IgG (PK-6105 Vector Labs, Burlingame, CA), visualized with 3, 3-diaminobenzidine (SK-4100, Vector Labs) and hematoxylin counterstained. Similar technique was applied to a second full set of slides prior to application of primary antibody cleaved caspase-3 (Asp175) (5A1E) rabbit monoclonal (#9664; Cell Signaling Technology, Danvers, MA) at a 1:200 dilution for 1h at room temperature, followed by similar detection and visualization technique (PK-6101 and SK-4100, Vector Labs). Similar techniques were applied to slides stained for the oxidative stress markers 3-NT and 8-OHdG. For 3-NT staining, the primary antibody, rabbit anti-nitrotyrosine IgG (EMD Millipore, Billerica, MA) at a dilution of 1:200, was applied at room temperature for 1 hr, then detected with VECTASTAIN Elite ABC Kit Rabbit IgG (PK-4001, Vector Labs) with goat serum used as blocking agent. For 8-OHdG staining, the primary antibody, mouse anti-8-OHdG mcAb (JaICA, Fukuroi, Shizuoka, Japan) at a concentration of 1 μg/ml, was applied at room temperature for 30 min, then detectedlfrth a VECTASTAIN Elite ABC Kit employing a mouse on mouse (MOM) blocking protocol (Vector Labs MOM kit, PK-2200). Uninfected control mouse and no primary antibody reagent control slides were used in all protocols as well as a blocking peptide control for the anti-cleaved caspase-3 stains (cleaved caspase-3 (Asp175) blocking peptide #1050, Cell Signaling) and soluble 3-nitrotyrosine (Sigma Chemicals, St. Louis, MO) for the 3-NT stains. All slides were digitally scanned on an Aperio Scanscope XT (Aperio, Vista, CA) to enable whole slide analysis and figure preparation.
Protein carbonyl analysis
Protein carbonylation was quantitated as described previously [41]. Fluorescence was measured with an Odyssey infrared scanner (LI-COR, Lincoln, NE).
FACS analysis
Whole lung collected at 6 and 8 dpi was macerated and digested in RPMI (Invitrogen, Grand Island, NY) containing 2ug/mL dispase II (Roche USA, San Francisco, CA). Cells were then pushed through a 100μm nylon filter to form a single cell suspension, then washed, pelleted, resuspended in RPMI with 5% FBS and adjusted to 106 cells prior to staining. Non-fjpejcinc binding was blocked using rat anti-mouse CD16/CD32 (553142, BD Pharmigen, Sparks, MD). The following combinations of specific anti-mouse monoclonal antibodies at a working dilution of 1ug/mL (eBiosciences, SanUDpgQ, CA unless indicated otherwise) were used as follows: CD4+ cells: allophycocyanin (APC)-CD3 (clone 145-2C11) and phycoerythrin (PE)-CD4 (clone GK1.5); CD8+ cells: APC-CD3 and Alexa Fluor 488 (A488)-CD8 (clone, 53-6.7); Ly6c+ cells: APC-CD45 (clone 104) and PE-Ly6c/6g (clone RB6-8C5, BD Pharmigen); CD45+/CD11c+ cells: APC-CD45 and A488-CD11c (clone N418); CD45+/B220+/CD19+ cells: APC-CD45 (clone 104), A488-B220 (clone RA3-6B2) and PE-CD19 (clone ebio1D3). Isotype controls were run for gating parameters. Overall cell populations were identified using forward and side scatter on a BD FACS Canto II (Becton Dickinson) using BD FACSDIVA software, version 6.1. Subsequent data analysis of fluorescent cell markers was done using Flowjo, version 5.0 software.
Results
Mouse infection and treatment studies
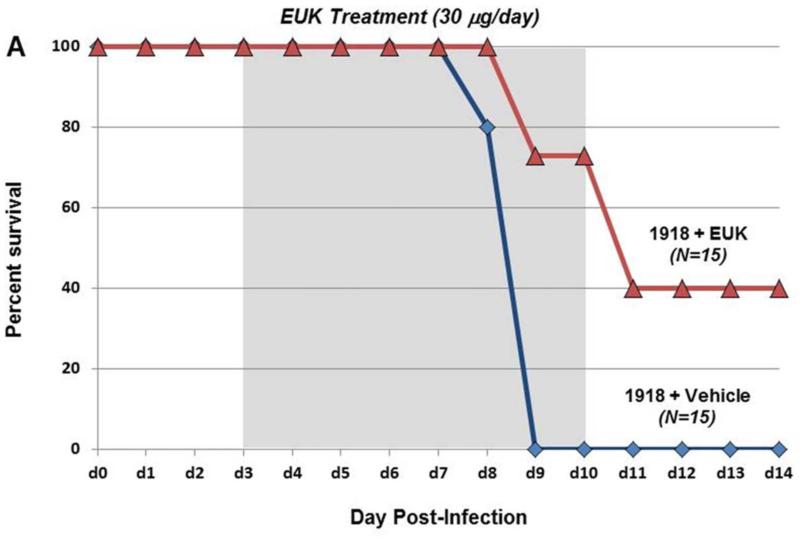
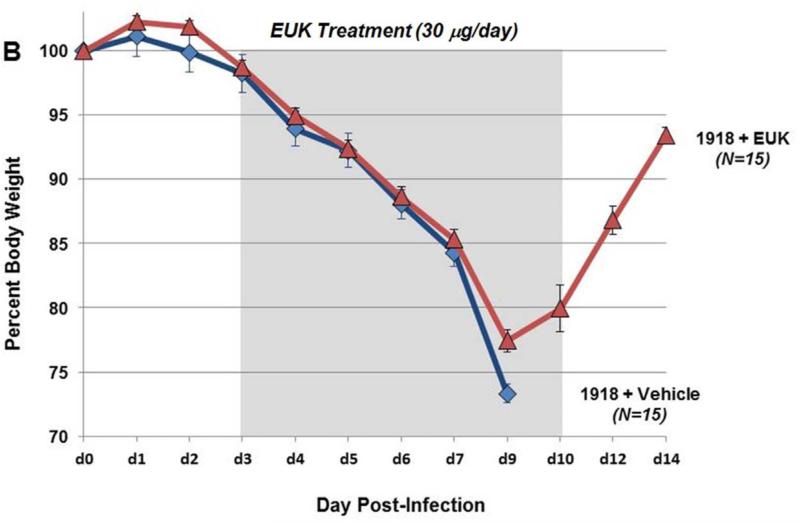
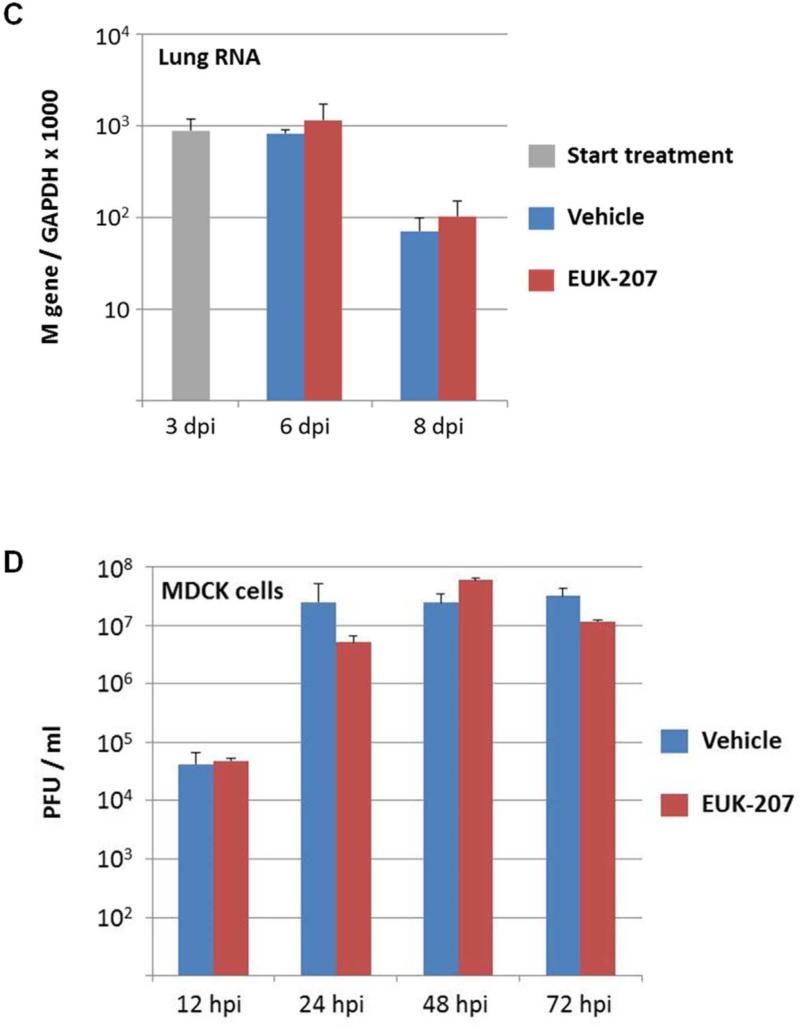
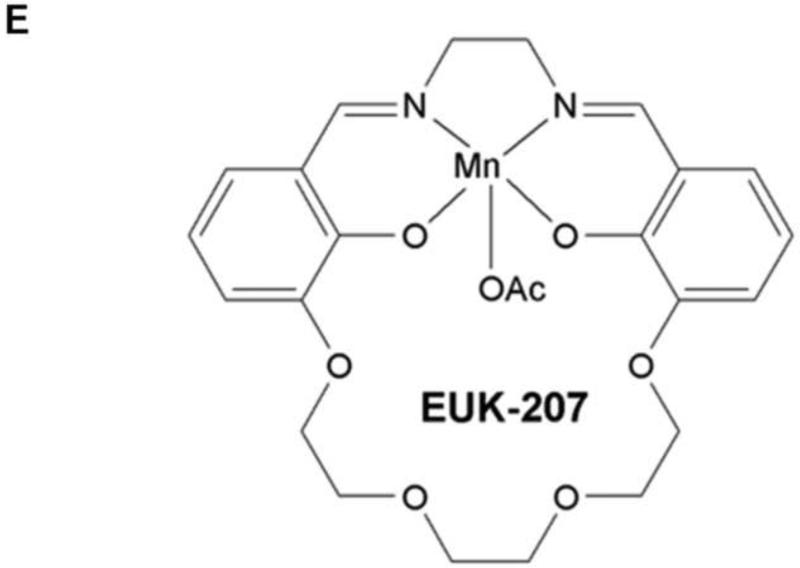
To test whether therapeutic reduction of ROS could affect influenza disease severity and outcome, groups of mice (10 to 25 per experiment) were infected with a lethal dose of the 1918 H1N1 influenza virus (500 PFU; 4× mouse LD50) and were treated with 30 μg EUK-207 by daily intraperitoneal injection from 3 to 10 dpi. Mice were weighed each day from 0 to 14 dpi and were humanely euthanized if ≥25% of starting weight was lost. EUK-207 treatment alone showed no effect on weight gain in uninfected mice even at the highest dose used (600 μg/day; data not shown). In initial titration experiments, we tested a range of EUK-207 treatment doses of 30 μg, 180 μg, 300 μg and 600 μg per day. We observed decreasing protective effects on survival with increasing doses with 600 μg/day being statistically similar to vehicle treated animals (data not shown). (supplementary Fig. S1). Since daily administration of 30 μg EUK-207 showed most effective results, this dose was chosen for all subsequent experiments. In three independent experiments, mice infected with 4× LD50 of the 1918 virus that were treated daily with 30 μg EUK-207 on 3 to 10 dpi showed percent survival ranging from 32.5% to 40% (Fig. 1 A), with an average of 36% survival (n=69 mice) across all experiments. Vehicle-treated mice showed 0% survival (n=50 mice) across all experiments. Drug- and vehicle-treated mice showed similar weight loss kinetics from 1-8 dpi, but from 9 dpi the EUK-207-treated animals had lower average weight loss and began to recover rapidly. Surviving treated animals gained up to 5% body weight every other day from 9 to 14 dpi (Fig. 1B). Quantitative RT-PCR on total RNA isolated at 3 (onset of treatment), 6 and 8 dpi showed similar levels of influenza matrix 1 gene mRNA expression in the EUK-207- and vehicle-treated groups (Fig. 1C). To ensure that the surviving EUK-207-treated mice had recovered from a 1918 virus infection, a small group of survivors were subsequently challenged with 10× LD50 of the 1918 virus several weeks later and showed no weight loss or mortality (data not shown). To validate that treatment with this dose of EUK-207 did not inhibit viral replication, we performed a kinetics tissue culture experiment in MDCK cells using a similar concentration of EUK-207, as described in Materials and Methods. As shown in Fig. 1D, addition of 1.5 μg/ml EUK-207 to culture media of MDCK cells infected with 0.01 MOI of 1918 influenza virus did not significantly affect viral replication from 12h to 72h post-infection compared to control untreated cells. The chemical structure of EUK-207 is shown in Panel 1E.
Fig. 1. EUK-207 treatment increases survival in 1918 influenza virus infected mice.
(A) Survival curves for EUK-207- and vehicle treatment during 1918 influenza virus infection. Administration of vehicle and drug by IP injection occurred on 3 to 10 dpi. (B) Weight loss during 1918 influenza virus infection in vehicle and EUK-207-treated mice. Data presented are from single experiment with 15 animals per group infected with 500 PFU (4x LD50) of the 1918 influenza virus, and are representative of 3 independent experiments with total N=69 for EUK-207-treated groups and N=55 for vehicle-treated groups. (C) Measurement of influenza M gene mRNA by qRT-PCR relative to GAPDH mRNA at 3 (onset of treatment), 6 and 8 dpi. Data are presented as average expression values ± standard deviation. (D) Viral replicatior kinetics in MDCK cells in the presence and absence of EUK-207 treatment. MDCK cells were infected with an MOI=0.01 and were cultured continuously in the presence or absence of 1.5 μg/ml EUK-207, as described in Materials and Methods. At 12h, 24h, 48h and 72h post-infection virus present in the supernatant was quantified by plaque assay. Kinetics were performed in triplicate and data are presented as mean ± standard deviation. (E) Chemical structure of EUK-207. The axial ligand, “OAc”, is an acetoxy group.
Histopathological analyses
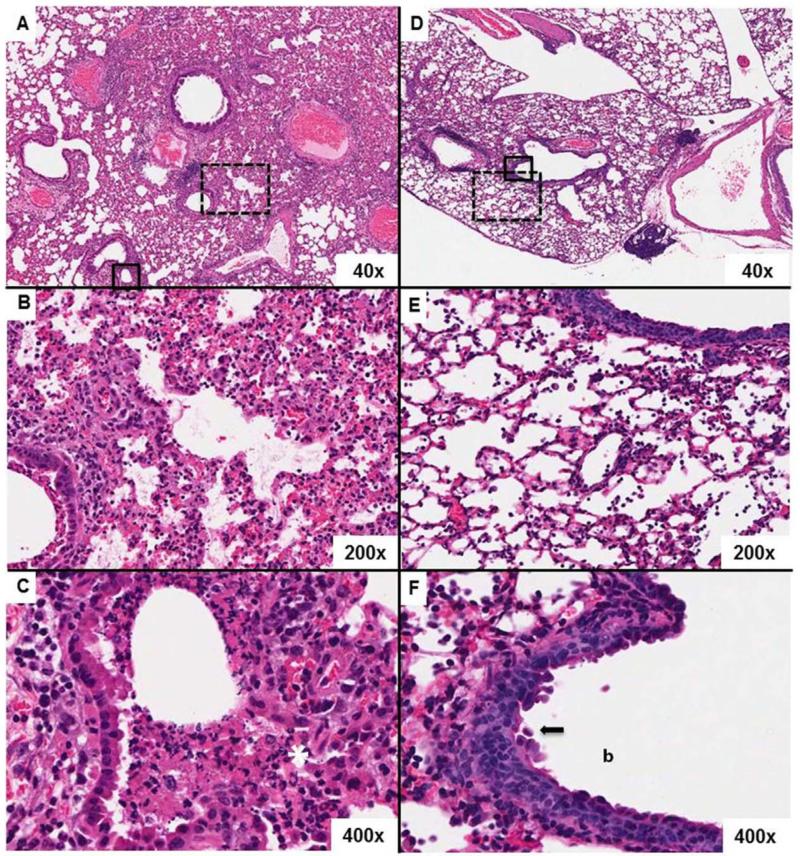
To determine the effect of EUK-207 treatment on lung pathology, necropsies and blinded histopathologic analyses were performed on respiratory tissues of mice treated either with drug and vehicle treatment or water-treated vehicle alone at 6 and 8 dpi (corresponding to days 3 and 5 days of treatment). Tissues from 4 mice were examined in each group and time point by two pathologists. Hematoxylin and eosin (H&E) stains of the respiratory tracts of vehicle-treated mice at 6 dpi showed widespread, multifocal, moderate-to-severe histopathology characterized by an acute necrotizing bronchiolitis and a predominantly neutrophilic alveolitis. In comparison, the lungs from the 6 dpi EUK-207-treated mice showed similar but less widespread, multifocal, mild-to-moderate histopathologic changes. Sections from the vehicle-treated mice at 8 dpi were characterized by acute necrotizing bronchiolitis with widespread and severe alveolitis (Fig. 2 A-C). As in 6 dpi mice, the alveolitis had a mixed inflammatory cell infiltrate with prominent numbers of neutrophils. Acute pulmonary edema with fibrin was observed multifocally. In contrast, sections from 8 dpi EUK-207-treated mice showed in aggregate a more moderate pathology with less alveolitis (Fig 2 D-F). There were a greater number of regions of the lung lacking inflammatory changes. Additionally, reparative changes in the bronchiolar epithelium of the EUK-207-treated animals were frequently observed (Fig 2 F). Sections from one 8 dpi EUK-207-treated mouse, however, showed a more severe pathology similar to the patterns observed in the vehicle-treated mice.
Fig. 2. EUK-207 treatment reduces severity of lung pathology compared to vehicle-treated mice.
Hematoxylin and eosin stains demonstrating representative histopathology differences between drug treated and untreated mice at 8 dpi. Original magnifications for each image are noted in their bottom right hand corners. A) A lung from an untreated mouse shows severe histopathologic changes. B) Severe lymphohistocytic and neutrophilic alveolitis, pulmonary edema and necrotizing bronchitis in an untreated lung. C) Close up of bronchial epithelium in an untreated lung showing transmural inflammation, white asterisk, and multifocal necrosis. The lumen is clogged with necrotic debris. D) A lung from a EUK-207-treated mouse showing less severe histopathologic changes than an untreated lung. E) Mild to moderate alveolitis with saimflmmxed cellularity infiltrate, pulmonary edema and mild bronchitis in an EUK-207-treated lung. F) Close up of bronchial epithelium in a treated lung showing marked epithelial cell jumbling in the normally pseudostratified columnar epithelium indicating epithelial reproliferation, black arrow. The bronchial lumen is denoted by the “b.” The broken line boxes in images A and D indicate the zoom regions shown in B and E, aligned vertically. The solid line boxes in same images indicate the zoom regions shown in C and F.
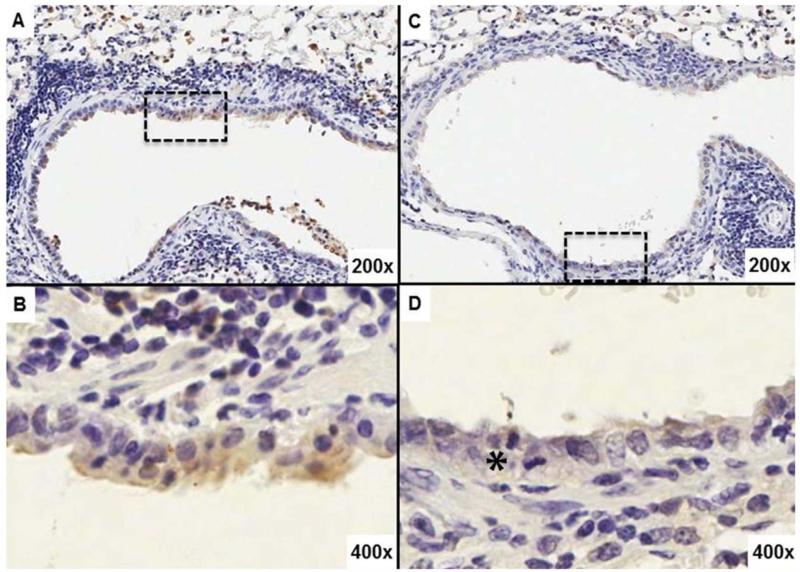
Immunohistochemical (IHC) labeling for influenza viral antigens revealed no differences in intensity or distribution of viral antigens between the EUK-207-treated and vehicle-treated groups (Fig 3) at either 6 or 8 dpi. Labeling for the apoptotic marker cleaved caspase 3 (cCASP3) stained with greater intensity and frequency in the bronchial and bronchiolar epithelia of vehicle-treated animals at 6 dpi than in the EUK-207-treated animals at the same time-point (Fig. 4A-D). However, no apparent cCASP3 IHC differences were observed in lung sections from treated or control animals at 8 dpi. Reproliferation was apparent in the sections from EUK-207 treated mice, showing frequent mitoses in the respiratory epithelium (Fig.4D).
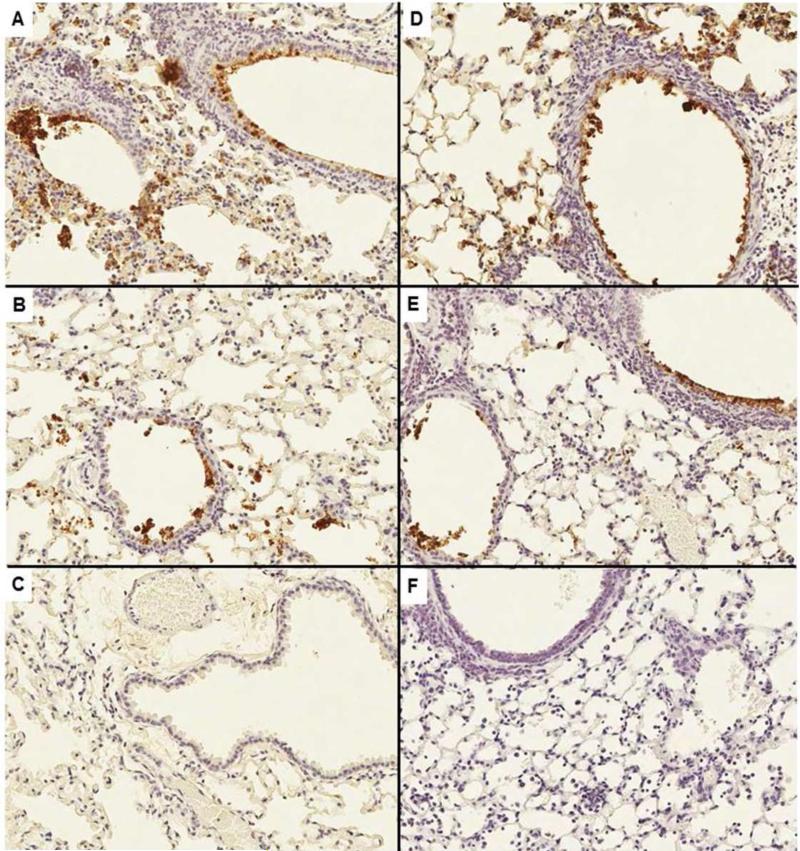
Fig. 3. EUK-207 treatment does not affect distribution of 1918 influenza virus infection in lungs of infected animals.
An immunohistochemical viral antigen positive is indicated by brown labeling of a cell's cytoplasm and/or nucleus. Cellular debris is also frequently positive. All figures were taken at an original magnification of 200×. A) Untreated mouse, 6 dpi; B) untreated mouse, 8 dpi; C) no primary antibody, negative reagent control; D) treated mouse, 6 dpi; E) treated mouse, 8 dpi; and F) uninfected mouse, biological negative control.
Fig. 4. EUK-207 treatment decreases cell death in lung of 1918 influenza virus infected animals.
Immunohistochemistry for apoptosis marker cleaved caspase 3 (cCASP3). (A) Bronchial epithelium as well as alveoli in the lung from an untreated mouse shows stronger, more frequent cytoplasmic labeling. (B) Close up of the bronchial epithelium outlined by the broken line box in A. (C) Bronchial epithelium and alveoli in a lung from a treated mouse show less labeling. (D) Close up of the bronchial epithelium outlined by the broken line box in C. The black asterisk denotes a mitotic figure. Original magnifications for each image are noted in their bottom right hand corners.
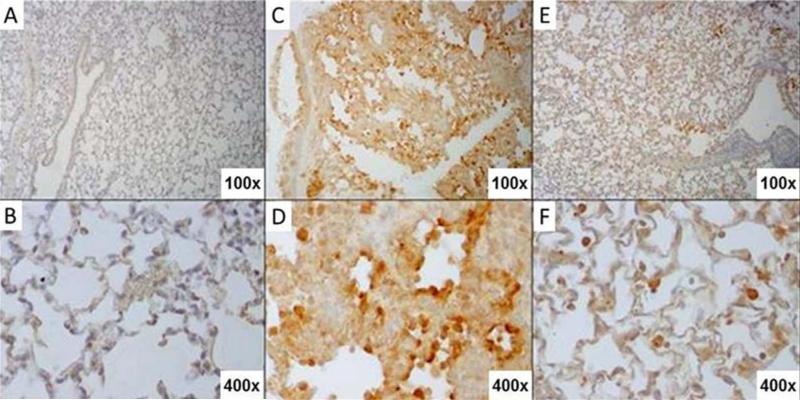
To assess effects of viral infection with or without EUK-207 treatment on generalized lung oxidative stress, samples were analyzed by IHC for 3-nitrotyrosine (3-NT) and 8-Oxo-2'-deoxyguanosine (8-OHdG) staining. Significant 3-NT staining in lungs of mock-infected control animals precluded observing qualitative differences between vehicle-treated and EUK-207-treated lung tissues (data not shown). As shown in Fig. 5, staining for 8-OHdG, however, did show differential staining in lung tissues from viral-infected as compared to mock-infected mice. Additionally, marked differences in 8-OHdG staining intensity were observed between vehicle-treated and EUK-207-treated lung tissues from the infected mice, with reduction of staining in the EUK-207-treated mice as compared to the vehicle-treated mice (Fig. 5). The 8-OHdG staining intensity showed a time-dependent relationship, increasing from 4 to 7 days post-infection in both vehicle-treated and EUK-207-treated animals (data not shown). In the vehicle-treated groups, 8-OHdG staining was most intense in respiratory epithelial cells lining bronchi and bronchioles, in alveolar epithelial cells, and in inflammatory cells within the alveoli and in interstitial and perivascular infiltrates (Fig. 5). In contrast, there was a marked reduction in overall 8-OHdG staining, but especially in alveolar epithelial cells in the EUK-207-treated group (Fig. 5). As an additional assessment of tissue oxidative stress, protein carbonyl levels in lung homogenates were quantitated as described in Methods. No significant differences at various time points after infection, with or without EUK-207, were observed (supplementary Fig. S2).
Fig. 5. EUK-207 treatment reduces oxidative damage during 1918 influenza virus infection.
Immunohistochemistry for oxidation marker 8-Oxo-2'-deoxyguanosine (8-OHdG). (A) Staining of mock infected, untreated mouse lung. (B) Close up of the alveolar epithelium from mock infected, untreated mouse lung from panel A. (C) Staining of vehicle-treated 1918 influenza virus-infected mouse lung showing prominent 8-OHdG staining in bronchial epithelium as well as alveolar epithelium. (D) Close up view of alveolar epithelium from panel C showing 8-OHdG staining. Prominently staining alveolar epithelial cells showing type II hyperplasia are shown also with staining in inflammatory cells in interstitial infiltrates. (E) Staining of EUK-207-treated 1918 influenza virus-infected mouse lung showing marked reduction in 8-OHdG staining in bronchial epithelium as well as alveolar epithelium as compared to the lung from the vehicle-treated animal in panel C. (F) Close up view of alveolar epithelium from panel E showing staining in alveolar macrophages and a marked reduction in 8OH-dG staining in alveolar epithelium. Original magnifications for each image are noted in their bottom right hand corners.
Host response analysis
To determine if EUK-207-treatment affected the population of immune cells present in the pulmonary tissues, we performed FACS analysis on forward- and side-scatter gated whole lung cell suspensions at 6 dpi and 8 dpi to quantify the percentages of CD4+ and CD8+ T cells, CDC11c+ macrophages and Ly6c+ neutrophils. There were no significant differences (all p>0.05) in these immune cell populations in lung suspensions from EUK-207-treated and vehicle-treated animals at either 6 or 8 dpi (supplementary Fig. S3). Although, we observed that in both EUK-207- and vehicle-treated animals, Ly6C+ cells were the dominant immune cell type in the lungs of infected animals at 8 dpi.
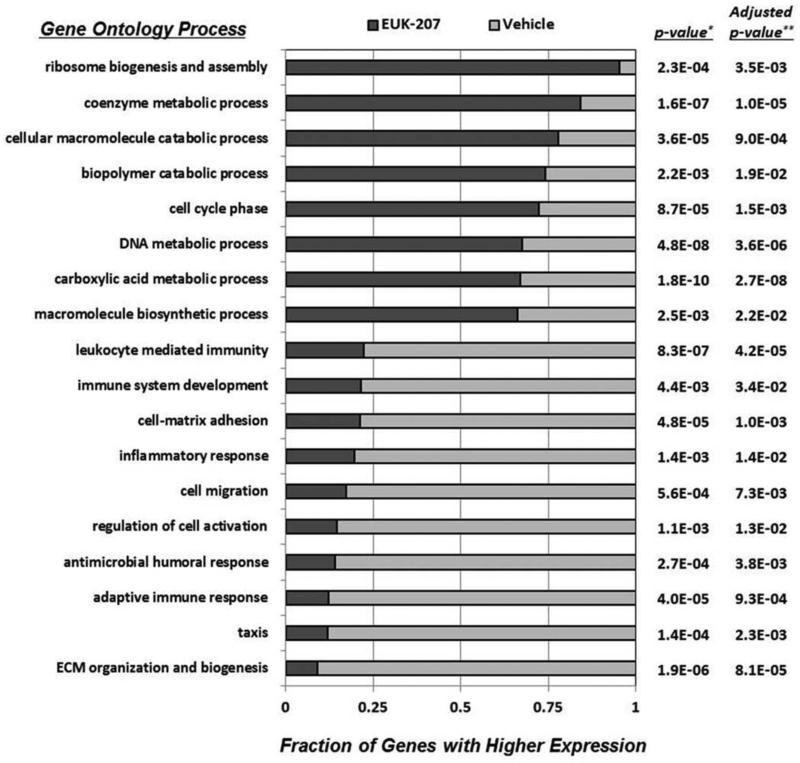
To characterize the host gene response to 1918 virus infection during EUK-207 treatment, we performed two independent high-throughput next-generation sequencing (NGS) analyses on poly(A)+ cDNA libraries prepared from EUK-207- and vehicle-treated mouse lungs at 8 dpi, as described in Materials and Methods. This time point was chosen for analysis as our histological analyses showed clear differences between EUK-207- and vehicle-treated lungs, but the EUK-207-treated animals were still in the acute phase of infection. NGS resulted in 635 million reads of a total of >22 GB of sequence data generated. Of these reads, 7,187 genes were identified in both experiments as being differentially expressed in the lungs of EUK-207- or vehicle-treated mice. Restriction of our analysis to genes that were ≥1.5-fold differentially regulated in EUK-207- and vehicle-treated groups resulted in the identification of 869 genes preferentially up-regulated in EUK-207-treated animals and 515 genes preferentially up-regulated in vehicle-treated animals. As shown in Fig. 6, functional analysis by gene ontology of these >1.5× differentially regulated genes revealed that EUK-207-treatment increased biological processes associated with ribosomal biogenesis, metabolism, cell proliferation and differentiation, TCA cycle and macromolecule biosynthesis. In marked contrast, the population of genes that were more abundant in the vehicle-treated lungs was associated predominantly with immune responses and inflammation. To confirm these data, we performed qRT-PCR on 33 selected genes, whose result supported that from our NGS (supplementary Fig. S4).
Fig. 6. EUK-207 treatment results in increased expression of lung repair related genes and decreased expression of inflammatory response and cell death related genes.
Gene ontology comparison of genes identified using next-generation sequencing (NGS) that were induced in pooled mRNA isolated from lungs of EUK-207-treated mice or in pooled mRNA isolated from lungs of vehicle-treated animals at 8 dpi. * Fishers exact test, ** Fishers exact test with FDR method ofBenjamini and Hochberg.
Follow-up bioinformatics analyst to characterize the upstream transcription factors controlling the expression of differentially regulated genes in the EUK-207- and vehicle-treated groups were performed using oPOSSUM [42]. These results showed that of the genes with >2× fold expression in the EUK-207-treated group compared to vehicle-treated, the transcription factors identified that control expression of these genes with the 20 lowest Fisher exact test scores were most often associated with tissue development and proliferation (supplementary Fig. S5). In contrast, this analysis revealed that the transcription factors controlling the genes that were >2× higher expression in the vehicle-treated group were most often associated with immune responses, cell stress, hypoxia, ROS damage and cell stress, including Sp1, Arnt, and several members of the NFkB family (supplementary Fig. S6).
The gene expression data obtained by NGS were from pooled samples and provide an overall comparison of the host gene expression response in the lungs of EUK-207- and vehicle-treated animals. However, given the approximate 40% survival rates from EUK-207 treatment, we wanted to determine whether individual treated animals showed a range of responses, as would be predicted. To accomplish this, traditional expression analysis was performed by expression microarray on RNA prepared from individual EUK-207- and vehicle-treated animals infected with a lethal dose of 1918 influenza virus (500 PFU; 4× mouse LD50) at 8 dpi compared to a pool of mock-infected animals. To check for consistency with the NGS data, gene ontology analysis that was performed on averaged expression data from the EUK-207-treated compared to vehicle-treated group. This analysis showed overall functional similarity with increased cell death, inflammation and stress responses in vehicle-treated animals and increased cellular metabolism and increased generation of energy precursors in EUK-207-treated animals. Further examination of the two gene expression data sets, we were able to identify approximately 1,100 genes that showed similar EUK-to-vehicle expression ratios by both NGS and microarray, and functional analysis showed increased expression of cell death and immune related genes in vehicle-treated groups (supplementary Fig. S7 and Table S1). These included many proinflammatory chemokines and cytokines, e.g. Il1a, Il1b, Cxcl1, Cxcl2 and Cxcl5; cell death response genes, e.g. Tnfrsf1b, Tnfsf14 and Tradd; and acute phase response genes, e.g. 116, Gzma, Gzmb, and Saal-4. Analysis of variance (ANOVA) and subsequent pathways analysis performed on expression microarray data from individual EUK-207- and vehicle-treated animals confirmed these expression differences in the immune response, cell differential and tissue repair responses between the drug and vehicle-treated groups (supplementary Fig. S8).
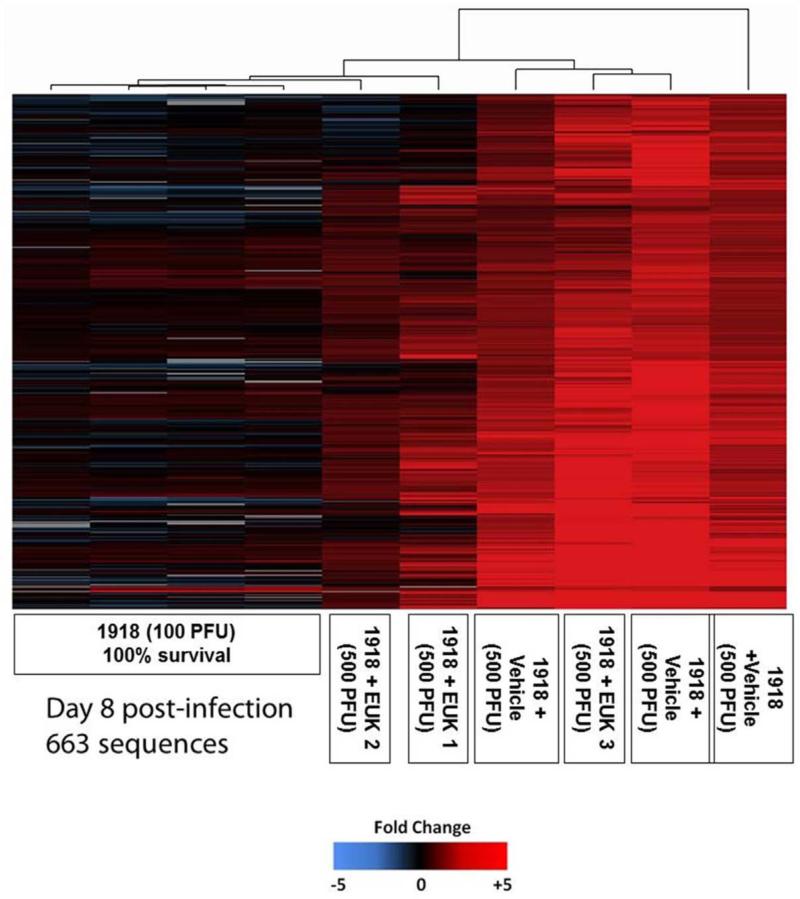
To serve as a control for sick animals that fully recover from infection expression microarray analysis was also performed on RNA isolated from lungs of mice infected with a sub-lethal dose (100 PFU; 0.67 LD50) of the 1918 influenza virus at 8 dpi. Mice infected with 100 PFU of 1918 influenza virus showed substantial weight loss and viral replication, but with 100% survival [43]. When the gene expression patterns of individual animals were examined, the vehicle-treated mice infected with 500 PFU showed a high degree of similarity (supplementary Fig. 9). In contrast, the EUK-207-treated infected animals showed a range of gene expression patterns with some being more similar to vehicle-treated, lethal infections and other patterns more similar to sub-lethal 1918 infected animals (compared EUK1 and 2 with EUK 3 in Fig. S8). Using these data, a set of genes induced during fatal, vehicle-treated infections was identified, as shown in Fig. 7. Gene ontology analysis of these biomarker genes showed that this population of genes was largely associated with regulation of transcription (25.9%), apoptosis (14.2%), RNA polymerase II transcription (9.3%), regulation of programmed cell death (8.8%), and protein amino acid phosphorylation (8.8%) (supplementary Fig. S10).
Fig. 7. Identification of RNA biomarkers in vehicle-treated, fatal 1918 influenza virus infected animals.
Two-dimensional hierarchical clustering diagram of genes preferentially induced in individual vehicle-treated animals at 8 dpi, but not in lungs of individual mice infected with 100 PFU of 1918 influenza virus at 8 dpi. Also shown is the expression of this population of genes in individual EUK-207-treated animals. These animals show a range of expression that was either similar to lethal or non-lethal 1918 influenza virus infections. Genes shown in red were up-regulated and genes shown in blue were down-regulated and genes shown in black showed no change in expression compared to a pool RNA isolated from mock infected mice.
Viral sequence analysis
To determine if EUK-207 treatment exerted a selective pressure on intrahost viral evolution, we performed mRNA_Seq high-throughput sequencing on EUK-207- and vehicle-treated mouse lungs at 8 dpi. This resulted in a total number of 174,198 reads that were mapped to the 1918 influenza virus genome, with an average base coverage of 477.5. In concordance with our qRT-PCR analysis of influenza M gene expression and immunohistochemistry for viral antigen, we observed a very similar FPKM value of reads mapped to influenza viral sequences with a vehicle to EUK-207-treated group ratio of 0.992, indicating similar levels of viral gene expression. Single nucleotide polymorphism (SNP) analysis of viral mRNA showed 2 consistent synonymous changes (one in PB2 and the other in M). SNP analysis also identified 1 quasi-species non-synonymous change (25% of sequences) in PA observed at nucleotide 1090 in EUK-207-treated samples; 2 quasi-species non-synonymous changes in vehicle-treated samples at nucleotide 1036 of PA (52% of sequences) and at nucleotide 2107 in PB1 (28% of sequences). However, no fixed mutations in the viral genome were identified between the EUK-207- and vehicle-treated groups.
Discussion
Studies from our group have shown neutrophilic alveolitis in the lung autopsy samples from 1918 influenza virus human deaths [44]. Studies in various animal models have confirmed these human findings by showing a marked infiltration of neutrophils into the lungs of infected animals [4, 6, 8]. The results from our FACS experiments also substantiate previously reported increases in neutrophils identified by histopathology and further support that these immune cells are playing an important role in the excess respiratory tissue damage associated with lethal 1918 virus infections. A key component of neutrophil and macrophage cell killing activity is the production of ROS via the NADPH-oxidase system [9, 10]. Oxidative damage has been shown to be an important mediator of lung damage during acute lung injury caused by a variety of injury [45]. Moreover, a study by Snelgrove, et al. showed that loss of phagocyte NADPH-oxidase decreased lung pathology and improved resolution of influenza virus infection in knock-out mice [46]. Thus, the development of agents to intervene in deleterious ROS-generating responses during influenza virus infection has been suggested as an important area for new drug treatment modalities [45, 47], Redox signaling is now clearly established as an important mechanism of cellular regulation [48, 49]. Viral infection-induced dysregulation of these pathways may also contribute to disease severity. We speculate that the incremental loss of protective effects and survival benefit seen with higher dosages of EUK-207 treatment is likely be due to the need for limited ROS as a component of the immune response and/or interference with redox signaling pathways involved in host defense responses.
Cells can protect themselves against oxidative damage using endogenous mechanisms, such as those mediated by superoxide dismutases and catalase, which catalytically destroy superoxide ion and hydrogen peroxide, respectively. However, if the production of ROS is sufficiently high to overcome endogenous protective responses, tissues undergo oxidative stress. This process leads to oxidation of protein, lipids and DNA, which can in turn lead to cell death. Thus, treatments that increase the ROS scavenging potential of cells and tissues can limit ROS-mediated damage and reduce cell death [18, 19]. Salen-manganese complexes are synthetic manganese-containing compounds with catalytic superoxide dismutase and catalase activities [19] and the ability to reduce ROS-mediated tissue injury in a variety of in vivo experimental disease and injury models [26-28, 50-53]. In recent studies, EUK-207 was found to cause functional improvement as well as reducCiLoxidative damage in experimental models for radiation injury to the lung [28] and other tissues [50] and cardiac injury caused by ischemia-reperfusion [54]. In several previously reported injury models, there was a substantial increase in indicators for oxidative stress such as 8-OHdG [26, 28] and cysteine oxidation (50), and EUK-207 treatment substantially inhibited such oxidative modifications while concomitantly protecting tissues. The present study showed a reduction in lung oxidative stress, demonstrated by decreased 8-OHd staining associated with EUK-207 treatment during viral infection. Significantly, EUK-207 treatment markedly reduced 8-OHdG staining in alveolar epithelial cells compared to vehicle-treated animals late in infection. Unlike 8-OHdG, the other markers for oxidative injury tested, 3-NT and protein carbonylation, showed no discernible differences during the course of infection. Such an observation is not surprising since it is increasingly recognized that the mediators as well as targets of oxidative stress vary depending on the stimulus [55]. Overall, our results indicate that a modest increase in broadly detectable lung oxidative stress is occurring in the infected mice, particularly in alveolar epithelial cells, as a feature of the pathological consequences of virus infection. These observations are in agreement with recent studies that showed inducible nitric oxide contributes to lung pathology during 1918 virus infection in mice [56].
While the dose of EUK-207 (30 μg/day) is quite low, as compared to the doses required in other studies to substantially attenuate damage and inhibit biochemical indicators of tissue oxidative stress, our data indicate, that this low EUK-207 dose can reduce overall 8-OHdG staining, particularly in the alveoli and substantially modulates virus-induced host responses, as indicated by the marked changes in gene expression and improvements in histopathology, leading to increased survival of infected mice. At higher EUK-207 doses (180 to 600 μg/day), more consistent with pharmacologically active EUK-207 dosing regimens in other studies [28, 29], survival benefits were lessened (supplemental Fig 1). We hypothesize that, while these higher EUK-207 doses are comparable to those that broadly attenuate indicators of oxidative stress and tissue injury in other experimental models, in this influenza infection model an excessive antioxidant dose might impair beneficial host defense processes, or otherwise interfere with redox signaling in a deleterious way, such that mice are not protected from infection-induced lung damage. (It should be noted that all EUK-207 doses employed in this study are well below those that cause toxicity in uninfected mice.) Clearly, in a system as complex as this, the potential effects of ROS are also complex, as are the potential ramifications of introducing an antioxidant intervention. And, as with any other study, it is always possible that the pharmacological agent has some effects not directly due to its antioxidant properties.
Inflammatory responses, particularly hypercytokinemia, have been implicated in severe influenza viral disease [6, 7, 57-63]. Several studies have examined the effects of immunosuppressive drugs, such as corticosteroids and COX-2 inhibitors, but with limited success [64-66]. Recently, Oldstone and colleagues have reported that treatment with an immunomodulator, which targets sphingosine-1-phosphate (S2P) receptors, limited immune-mediated tissue damage and increase survival in 2009 pandemic H1N1 influenza virus infected mice [67-69]. Given the similar levels of viral replication, overlapping early weight loss and the lack of a statistical difference in immune cell populations by FACS, we conclude EUK-207 is likely blocking specific deleterious effects of ROS over-production by immune cells, rather than working either as a traditional antiviral or immunomodulatory drug. We hypothesize that the similar weight loss patterns of the EUK-207- and vehicle-treated animals at 0 to 8 dpi can be explained by the effects of high levels of expression of acute inflammatory cytokines leading to cachexia and dehydration. By 10 dpi, the surviving EUK-207-treated mice appeared to be able to control viral replication as these animals rapidly gained weight, fully recovered and were fully protected from a subsequent 10x LD50 viral challenge. In support of this, Snelgrove et al. observed similar weight loss between influenza virus infected wild-type and NADPH-oxidase knock-out mice, even though the knock-out mice showed reduced lung pathology [46].
Conclusions
An exaggerated immune response has been predicted to play an important role in disease severity of pathogenic influenza virus infections [6, 7, 59-61, 70, 71]. Previous studies from our group and others have shown that lethal infection of experimental animal models with 1918 influenza virus is associated with aberrant immune responses that were thought to be immunopathogenic and tdfclay central role in disease severity [6, 7]. This was supported by previous studies that treatment targeting ROS and RNS responses can alter the pathogenesis of influenza viruses [15]. In the current study, we have shown that treatment with a catalytic ROS scavenger, EUK-207, reduced severity of lung pathology and increased survival in a lethal 1918 challenge model. Although several previous studies from our group and other have shown an association between 1918 influenza virus infection and strong inflammatory responses this is the first demonstration that limiting the damage caused by the host immune and ROS responses can increase survival during an otherwise lethal 1918 influenza virus infection.
While EUK-207 treatment can reduce effects of oxidative damage in the lungs of 1918 influenza virus infected mice, the exact molecular mechanisms of how EUK-207 protected mice from pulmonary damage from 1918 influenza virus infection are yet to be determined. However, the antioxidant effects of salen manganese complexes, including EUK-207, have been clearly demonstrated in numerous other pathological systems where increased oxidative stress was dramatic [18, 19, 28, 29, 50, 51]. Further, the present study showed that treatment with EUK-207 caused a marked reduction in severity of lung pathology and substantially reduced cell death responses at both the RNA and protein levels, as well as inhibiting alveolar staining for an oxidative stress indicator, 8-OHdG. Taken together, we believe these data support a model where EUK-207 treatment reduces damage to the infected lung, likely by limiting the most relevant cytotoxic effects of ROS, thereby limiting excess cell death responses and allowing for increased lung repair responses. This hypothesis is supported by reduced staining for cCASP3 and host RNA expression data showing a reduction in inflammatory and cell death related responses and increased metabolic, tissue repair and development pathways in EUK-207-treated, relative to vehicle-treated mice.
This study shows conclusively that the aberrant inflammatory and cell death responses reported to be associated with 1918 influenza virus infections are key to both severity of lung pathology and mortality. These results suggest that further development of treatments targeting ROS responses, in a controlled manner, in conjunction with treatment with other antiviral and immunomodulatory drugs could reduce disease severity during severe influenza virus infections. Studies are planned to characterize a combinatorial approach of salen-manganese complexes with neuraminidase inhibitors (NAI to examine this hypothesis. Further, because EUK-207 targeted host responses, there was little selective pressure for the virus to develop resistance, which has been reported to occur quickly after antiviral drug treatment in humans [72-75]. It will be important to characterize the effects of EUK-207 treatment on other seasonal and pandemic influenza viruses, as well as highly pathogenic avian influenza (HPAI) viruses. Overall, this study demonstrates the importance of immunopathogenic responses as key contributors to disease severity during 1918 influenza virus infection indicating that such host responses are excellent potential candidates for therapeutic intervention. Since EUK-207 treatment modulates the response of the host and not the virus, these findings may have broader implications for infectious organisms other than influenza, though this remains to be investigated
Supplementary Material
Highlights.
EUK-207 treatment increased survival from a lethal 1918 influenza virus infection.
Treatment was associated with greatly decreased severity of lung pathology.
Treatment resulted in decreased pulmonary oxidative damage.
Treatment was associated with reduced inflammatory responses and increased lung repair responses.
Acknowledgements
This work was supported by the Intramural Research Programs of the National Institutes of Health, National Institute of Allergy and Infectious Diseases, and the National Heart, Lung, and Blood Institute. K.A.W., R.E.K. and A.O. were supported by Defense Threat Reduction Agency contract HDTRA-1-08-C-0023. Development of EUK-207 was funded in part by GM57770 (SRD).
Abbreviations used
- ANOVA
analysis of normal variance
- cCASP3
cleaved caspase 3
- 3-NT
3-nitrotyrosine
- 8-OHdG
8-Oxo-2'-deoxyguanosine
- dpi
day post-infection
- FPKM
fragments per kilobase of exon per million fragments mapped
- LD50
lethal dose 50%
- NGS
next-generation sequencing
- PFU
plaque forming units
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Johnson NP, Mueller J. Updating the accounts: global mortality of the 1918-1920 “Spanish” influenza pandemic. Bull Hist Med. 2002;76:1–05-115. doi: 10.1353/bhm.2002.0022. [DOI] [PubMed] [Google Scholar]
- 2.Taubenberger JK, Morens DM. 1918 Influenza: the mother of all pandemics. Emerg Infect Dis. 2006;12:1–5-22. doi: 10.3201/eid1201.050979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morens DM, Taubenberger JK, Fauci AS. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis. 2008;198:9–62-970. doi: 10.1086/591708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tumpey TM, Basler CF, Aguilar PV, Zeng H, Solorzano A, Swayne DE, Cox NJ, Katz JM, Taubenberger JK, Palese P, Garcia-Sastre A. Characterization of the reconstructed 1918 Spanish influenza pandemic virus. Science. 2005;310:7–7-80. doi: 10.1126/science.1119392. [DOI] [PubMed] [Google Scholar]
- 5.Taubenberger JK, Reid AH, Lourens RM, Wang R, Jin G, Fanning TG. Characterization of the 1918 influenza virus polymerase genes. Nature. 2005;437:8–89-893. doi: 10.1038/nature04230. [DOI] [PubMed] [Google Scholar]
- 6.Kash JC, Tumpey TM, Proll SC, Carter V, Perwitasari O, Thomas MJ, Basler CF, Palese P, Taubenberger JK, Garcia-Sastre A, Swayne DE, Katze MG. Genomic analysis of increased host immune and cell death responses induced by 1918 influenza virus. Nature. 2006;443:5–78-581. doi: 10.1038/nature05181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kobasa D, Jones SM, Shinya K, Kash JC, Copps J, Ebihara H, Hatta Y, Kim JH, Halfmann P, Hatta M, Feldmann F, Alimonti JB, Fernando L, Li Y, Katze MG, Feldmann H, Kawaoka Y. Aberrant innate immune response in lethal infection of macaques with the 1918 influenza virus. Nature. 2007;445:3–19-323. doi: 10.1038/nature05495. [DOI] [PubMed] [Google Scholar]
- 8.Memoli MJ, Tumpey TM, Jagger BW, Dugan VG, Sheng ZM, Qi L, Kash JC, Taubenberger JK. An early ‘classical’ swine H1N1 influenza virus shows similar pathogenicity to the 1918 pandemic virus in ferrets and mice. Virology. 2009;393:3–38-345. doi: 10.1016/j.virol.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheson BD, Curnette JT, Babior BM. The oxidative killing mechanisms of the neutrophil. Prog Clin Immunol. 1977;3:1–-65. [PubMed] [Google Scholar]
- 10.Segel GB, Halterman MW, Lichtman MA. The paradox of the neutrophil's role in tissue injury. J Leukoc Biol. 2011;89:3–59-372. doi: 10.1189/jlb.0910538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeLeo FR, Allen LA, Apicella M, Nauseef WM. NADPH oxidase activation and assembly during phagocytosis. J Immunol. 1999;163:6–732-6740. [PubMed] [Google Scholar]
- 12.DeLeo FR, Quinn MT. Assembly of the phagocyte NADPH oxidase: molecular interaction of oxidase proteins. J Leukoc Biol. 1996;60:6–77-691. doi: 10.1002/jlb.60.6.677. [DOI] [PubMed] [Google Scholar]
- 13.Stadtman ER, Levine RL. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids. 2003;25:2–07-218. doi: 10.1007/s00726-003-0011-2. [DOI] [PubMed] [Google Scholar]
- 14.Stadtman ER. Protein oxidation in aging and age-related diseases. Ann N Y Acad Sci. 2001;928:2–2-38. doi: 10.1111/j.1749-6632.2001.tb05632.x. [DOI] [PubMed] [Google Scholar]
- 15.Akaike T, Ando M, Oda T, Doi T, Ijiri S, Araki S, Maeda H. Dependence on O2- generation by xanthine oxidase of pathogenesis of influenza virus infection in mice. J Clin Invest. 1990;85:7–39-745. doi: 10.1172/JCI114499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akaike T, Noguchi Y, Ijiri S, Setoguchi K, Suga M, Zheng YM, Dietzschold B, Maeda H. Pathogenesis of influenza virus-induced pneumonia: involvement of both nitric oxide and oxygen radicals. Proc Natl Acad Sci U S A. 1996;93:2–448-2453. doi: 10.1073/pnas.93.6.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maeda H, Akaike T. Oxygen free radicals as pathogenic molecules in viral diseases. Proc Soc Exp Biol Med. 1991;198:7–21-727. doi: 10.3181/00379727-198-43309c. [DOI] [PubMed] [Google Scholar]
- 18.Doctrow SR, Huffman K, Marcus CB, Musleh W, Bruce A, Baudry M, Malfroy B. Salen-manganese complexes: combined superoxide dismutase/catalase mimics with broad pharmacological efficacy. Adv Pharmacol. 1997;38:2–47-269. doi: 10.1016/s1054-3589(08)60987-4. [DOI] [PubMed] [Google Scholar]
- 19.Doctrow SR, Huffman K, Marcus CB, Tocco G, Malfroy E, Adinolfi CA, Kruk H, Baker K, Lazarowych N, Mascarenhas J, Malfroy B. Salen-manganese complexes as catalytic scavengers of hydrogen peroxide and cytoprotective agents: structure-activityrelationship studies. J Med Chem. 2002;45:4–549-4558. doi: 10.1021/jm020207y. [DOI] [PubMed] [Google Scholar]
- 20.Baudry M, Etienne S, Bruce A, Palucki M, Jacobsen E, Malfroy B. Salen-manganese complexes are superoxide dismutase-mimics. Biochem Biophys Res Commun. 1993;192:9–64-968. doi: 10.1006/bbrc.1993.1509. [DOI] [PubMed] [Google Scholar]
- 21.Sharpe MA, Ollosson R, Stewart VC, Clark JB. Oxidation of nitric oxide by oxomanganese-salen complexes: a new mechanism for cellular protection by superoxide dismutase/catalase mimetics. Biochem J. 2002;366:9–7-107. doi: 10.1042/BJ20020154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baker K, Marcus CB, Huffman K, Kruk H, Malfroy B, Doctrow SR. Synthetic combined superoxide dismutase/catalase mimetics are protective as a delayed treatment in a rat stroke model: a key role for reactive oxygen species in ischemic brain injury. J Pharmacol Exp Ther. 1998;284:2–15-221. [PubMed] [Google Scholar]
- 23.Rong Y, Doctrow SR, Tocco G, Baudry M. EUK-134, a synthetic superoxide dismutase and catalase mimetic, prevents oxidative stress and attenuates kainate-induced neuropathology. Proc Natl Acad Sci U S A. 1999;96:9–897-9902. doi: 10.1073/pnas.96.17.9897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gianello P, Saliez A, Bufkens X, Pettinger R, Misseleyn D, Hori S, Malfroy B. EUK-134, a synthetic superoxide dismutase and catalase mimetic, protects rat kidneys from ischemia-reperfusion-induced damage. Transplantation. 1996;62:1–664-1666. doi: 10.1097/00007890-199612150-00022. [DOI] [PubMed] [Google Scholar]
- 25.Doctrow SR, Liesa M, Melov S, Shirihai OS, Tofilon PJ. Salen Mn complexes are superoxide dismutase and catalase mimetics that protect the mitochondria. Current Inorganic Chemistry. 2012;2:3–25-334. [Google Scholar]
- 26.Liu R, Liu IY, Bi X, Thompson RF, Doctrow SR, Malfroy B, Baudry M. Reversal of age-related learning deficits and brain oxidative stress in mice with superoxide dismutase/catalase mimetics. Proc Natl Acad Sci U S A. 2003;100:8–526-8531. doi: 10.1073/pnas.1332809100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clausen A, Doctrow S, Baudry M. Prevention of cognitive deficits and brain oxidative stress with superoxide dismutase/catalase mimetics in aged mice. Neurobiol Aging. 2010;31:4–25-433. doi: 10.1016/j.neurobiolaging.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahmood J, Jelveh S, Calveley V, Zaidi A, Doctrow SR, Hill RP. Mitigation of radiation-induced lung injury by genistein and EUK-207. Int J Radiat Biol. 2011;87:8–89-901. doi: 10.3109/09553002.2011.583315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doctrow SR, Baudry M, Huffman K, Malfroy B, Melov S. Salen Mn complexes: multifunctional catalytic antioxidants protective in models for neurodegenerative disease and aging. In: Sessler JL, Doctrow SR, McMurry TJ, Lippard SJ, editors. Medicinal Inorganic Chemistry. American Chemical Society and Oxford University Press; New York, NY: 2005. [Google Scholar]
- 30.Fodor E, Devenish L, Engelhardt OG, Palese P, Brownlee GG, Garcia-Sastre A. Rescue of influenza A virus from recombinant DNA. J Virol. 1999;73:9–679-9682. doi: 10.1128/jvi.73.11.9679-9682.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neumann G, Watanabe T, Ito H, Watanabe S, Goto H, Gao P, Hughes M, Perez DR, Donis R, Hoffmann E, Hobom G, Kawaoka Y. Generation of influenza A viruses entirely from cloned cDNAs. Proc Natl Acad Sci U S A. 1999;96:9–345-9350. doi: 10.1073/pnas.96.16.9345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qi L, Davis AS, Jagger BW, Schwartzman LM, Dunham EJ, Kash JC, Taubenberger JK. Analysis by single-gene reassortment demonstrates that the 1918 influenza virus is functionally compatible with a low-pathogenicity avian influenza virus in mice. J Virol. 2012;86:9–211-9220. doi: 10.1128/JVI.00887-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reed LJ, Muench H. A simple method of estimating fifty percent endpoints. American Journal of Hygiene. 1938;27:4–93-497. [Google Scholar]
- 34.Malfroy-Camine B, Doctrow SR. Cyclic salen-metal compounds as scavengers for oxygen radicals and useful as antioxidants in the treatment and prevention of diseases. Office, U. S. P. a. T.; United States: 2006. [Google Scholar]
- 35.Rosenthal RA, Doctrow SR, Callaway WB. Superoxide dismutase mimics. Antioxid Redox Signal. 2011;14:1–173. doi: 10.1089/ars.2010.3758. author reply 1174-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trapnell C, Pachter L, Salzberg SL. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics. 2009;25:1–105-1111. doi: 10.1093/bioinformatics/btp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, Salzberg SL, Wold BJ, Pachter L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol. 2010;28:5–11-515. doi: 10.1038/nbt.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods. 2008;5:6–21-628. doi: 10.1038/nmeth.1226. [DOI] [PubMed] [Google Scholar]
- 39.Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10:R–25. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Medina I, Carbonell J, Pulido L, Madeira SC, Goetz S, Conesa A, Tarraga J, Pascual-Montano A, Nogales-Cadenas R, Santoyo J, Garcia F, Marba M, Montaner D, Dopazo J. Babelomics: an integrative platform for the analysis of transcriptomics, proteomics and genomic data with advanced functional profiling. Nucleic Acids Res. 2010;38:W–210-213. doi: 10.1093/nar/gkq388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wehr NB, Levine RL. Quantification of protein carbonylation. Methods Mol Biol. 2013;965:2–65-281. doi: 10.1007/978-1-62703-239-1_18. [DOI] [PubMed] [Google Scholar]
- 42.Ho Sui SJ, Mortimer JR, Arenillas DJ, Brumm J, Walsh CJ, Kennedy BP, Wasserman WW. oPOSSUM: identification of over-represented transcription factor binding sites in co-expressed genes. Nucleic Acids Res. 2005;33:3–154-3164. doi: 10.1093/nar/gki624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jagger BW, Wise HM, Kash JC, Walters KA, Wills NM, Xiao YL, Dunfee RL, Schwartzman LM, Ozinsky A, Bell GL, Dalton RM, Lo A, Efstathiou S, Atkins JF, Firth AE, Taubenberger JK, Digard P. An overlapping protein-coding region in influenza A virus segment 3 modulates the host response. Science. 2012;337:1–99-204. doi: 10.1126/science.1222213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sheng ZM, Chertow DS, Ambroggio X, McCall S, Przygodzki RM, Cunningham RE, Maximova OA, Kash JC, Morens DM, Taubenberger JK. Autopsy series of 68 cases dying before and during the 1918 influenza pandemic peak. Proc Natl Acad Sci U S A. 2011;108:1–6416-16421. doi: 10.1073/pnas.1111179108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Imai Y, Kuba K, Neely GG, Yaghubian-Malhami R, Perkmann T, van Loo G, Ermolaeva M, Veldhuizen R, Leung YH, Wang H, Liu H, Sun Y, Pasparakis M, Kopf M, Mech C, Bavari S, Peiris JS, Slutsky AS, Akira S, Hultqvist M, Holmdahl R, Nicholls J, Jiang C, Binder CJ, Penninger JM. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell. 2008;133:2–35-249. doi: 10.1016/j.cell.2008.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Snelgrove RJ, Edwards L, Rae AJ, Hussell T. An absence of reactive oxygen species improves the resolution of lung influenza infection. Eur J Immunol. 2006;36:1–364-1373. doi: 10.1002/eji.200635977. [DOI] [PubMed] [Google Scholar]
- 47.Vlahos R, Stambas J, Selemidis S. Suppressing production of reactive oxygen species (ROS) for influenza A virus therapy. Trends Pharmacol Sci. 2012;33:3–-8. doi: 10.1016/j.tips.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 48.Janssen-Heininger YM, Mossman BT, Heintz NH, Forman HJ, Kalyanaraman B, Finkel T, Stamler JS, Rhee SG, van der Vliet A. Redox-based regulation of signal transduction: principles, pitfalls, and promises. Free Radic Biol Med. 2008;45:1–-17. doi: 10.1016/j.freeradbiomed.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bae YS, Oh H, Rhee SG, Yoo YD. Regulation of reactive oxygen species generation in cell signaling. Mol Cells. 2011;32:4–91-509. doi: 10.1007/s10059-011-0276-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rosenthal RA, Fish B, Hill RP, Huffman KD, Lazarova Z, Mahmood J, Medhora M, Molthen R, Moulder JE, Sonis ST, Tofilon PJ, Doctrow SR. Salen Mn complexes mitigate radiation injury in normal tissues. Anticancer Agents Med Chem. 2011;11:3–59-372. doi: 10.2174/187152011795677490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vorotnikova E, Rosenthal RA, Tries M, Doctrow SR, Braunhut SJ. Novel synthetic SOD/catalase mimetics can mitigate capillary endothelial cell apoptosis caused by ionizing radiation. Radiat Res. 2010;173:7–48-759. doi: 10.1667/RR1948.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Melov S, Wolf N, Strozyk D, Doctrow SR, Bush AI. Mice transgenic for Alzheimer disease beta-amyloid develop lens cataracts that are rescued by antioxidant treatment. Free Radic Biol Med. 2005;38:2–58-261. doi: 10.1016/j.freeradbiomed.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 53.Jung C, Rong Y, Doctrow S, Baudry M, Malfroy B, Xu Z. Synthetic superoxide dismutase/catalase mimetics reduce oxidative stress and prolong survival in a mouse amyotrophic lateral sclerosis model. Neurosci Lett. 2001;304:1–57-160. doi: 10.1016/s0304-3940(01)01784-0. [DOI] [PubMed] [Google Scholar]
- 54.Liesa M, Luptak I, Qin F, Hyde BB, Sahin E, Siwik DA, Zhu Z, Pimentel DR, Xu XJ, Ruderman NB, Huffman KD, Doctrow SR, Richey L, Colucci WS, Shirihai OS. Mitochondrial transporter ATP binding cassette mitochondrial erythroid is a novel gene required for cardiac recovery after ischemia/reperfusion. Circulation. 2011;124:8–06-813. doi: 10.1161/CIRCULATIONAHA.110.003418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kadiiska MB, Gladen BC, Baird DD, Germolec D, Graham LB, Parker CE, Nyska A, Wachsman JT, Ames BN, Basu S, Brot N, Fitzgerald GA, Floyd RA, George M, Heinecke JW, Hatch GE, Hensley K, Lawson JA, Marnett LJ, Morrow JD, Murray DM, Plastaras J, Roberts LJ, 2nd, Rokach J, Shigenaga MK, Sohal RS, Sun J, Tice RR, Van Thiel DH, Wellner D, Walter PB, Tomer KB, Mason RP, Barrett JC. Biomarkers of oxidative stress study II: are oxidation products of lipids, proteins, and DNA markers of CCl4 poisoning? Free Radic Biol Med. 2005;38:6–98-710. doi: 10.1016/j.freeradbiomed.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 56.Perrone LA, Belser JA, Wadford DA, Katz JM, Tumpey TM. Inducible nitric oxide contributes to viral pathogenesis following highly pathogenic influenza virus infection in mice. J Infect Dis. 2013;207:1–576-1584. doi: 10.1093/infdis/jit062. [DOI] [PubMed] [Google Scholar]
- 57.Kash JC. Applications of high-throughput genomics to antiviral research: evasion of antiviral responses and activation of inflammation during fulminant RNA virus infection. Antiviral Res. 2009;83:1–0-20. doi: 10.1016/j.antiviral.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Doherty PC, Turner SJ, Webby RG, Thomas PG. Influenza and the challenge for immunology. Nat Immunol. 2006;7:4–49-455. doi: 10.1038/ni1343. [DOI] [PubMed] [Google Scholar]
- 59.Lipatov AS, Andreansky S, Webby RJ, Hulse DJ, Rehg JE, Krauss S, Perez DR, Doherty PC, Webster RG, Sangster MY. Pathogenesis of Hong Kong H5N1 influenza virus NS gene reassortants in mice: the role of cytokines and B- and T-cell responses. J Gen Virol. 2005;86:1–121-1130. doi: 10.1099/vir.0.80663-0. [DOI] [PubMed] [Google Scholar]
- 60.Seo SH, Hoffmann E, Webster RG. The NS1 gene of H5N1 influenza viruses circumvents the host anti-viral cytokine responses. Virus Res. 2004;103:1–07-113. doi: 10.1016/j.virusres.2004.02.022. [DOI] [PubMed] [Google Scholar]
- 61.Seo SH, Hoffmann E, Webster RG. Lethal H5N1 influenza viruses escape host anti-viral cytokine responses. Nat Med. 2002;8:9–50-954. doi: 10.1038/nm757. [DOI] [PubMed] [Google Scholar]
- 62.Hilleman MR. Realities and enigmas of human viral influenza: pathogenesis, epidemiology and control. Vaccine. 2002;20:3–068-3087. doi: 10.1016/s0264-410x(02)00254-2. [DOI] [PubMed] [Google Scholar]
- 63.Taubenberger JK, Kash JC. Insights on influenza pathogenesis from the grave. Virus Res. 2011;162:2–-7. doi: 10.1016/j.virusres.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Carter MJ. A rationale for using steroids in the treatment of severe cases of H5N1 avian influenza. J Med Microbiol. 2007;56:8–75-883. doi: 10.1099/jmm.0.47124-0. [DOI] [PubMed] [Google Scholar]
- 65.Falagas ME, Vouloumanou EK, Baskouta E, Rafailidis PI, Polyzos K, Rello J. Treatment options for 2009 H1N1 influenza: evaluation of the published evidence. Int J Antimicrob Agents. 2010;35:4–21-430. doi: 10.1016/j.ijantimicag.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 66.Zheng BJ, Chan KW, Lin YP, Zhao GY, Chan C, Zhang HJ, Chen HL, Wong SS, Lau SK, Woo PC, Chan KH, Jin DY, Yuen KY. Delayed antiviral plus immunomodulator treatment still reduces mortality in mice infected by high inoculum of influenza A/H5N1 virus. Proc Natl Acad Sci U S A. 2008;105:8–091-8096. doi: 10.1073/pnas.0711942105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Walsh KB, Teijaro JR, Rosen H, Oldstone MB. Quelling the storm: utilization of sphingosine-1-phosphate receptor signaling to ameliorate influenza virus-induced cytokine storm. Immunol Res. 2011;51:1–5-25. doi: 10.1007/s12026-011-8240-z. [DOI] [PubMed] [Google Scholar]
- 68.Walsh KB, Teijaro JR, Wilker PR, Jatzek A, Fremgen DM, Das SC, Watanabe T, Hatta M, Shinya K, Suresh M, Kawaoka Y, Rosen H, Oldstone MB. Suppression of cytokine storm with a sphingosine analog provides protection against pathogenic influenza virus. Proc Natl Acad Sci U S A. 2011;108:1–2018-12023. doi: 10.1073/pnas.1107024108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Oldstone M, Hodder P, Crisp M, Roberts E, Guerrero M, Urbano M, Velaparthi S, Zhao J, Rosen H, Schaeffer MT, Brown S, Ferguson J. Probe Development Efforts to Identify Novel Antagonists of the Sphingosine 1-phosphate Receptor 4 (S1P4) 2010. [PubMed]
- 70.Szretter KJ, Gangappa S, Lu X, Smith C, Shieh WJ, Zaki SR, Sambhara S, Tumpey TM, Katz JM. Role of Host Cytokine Responses in the Pathogenesis of Avian H5N1 Influenza Viruses in Mice. J Virol. 2007;81:2–736-2744. doi: 10.1128/JVI.02336-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kaffi JC, Basler CF, Garcia-Sastre A, Carter V, Billharz R, Swayne DE, Przygodzki RM, Taubenberger JK, Katze MG, Tumpey TM. Global host immune response: pathogenesis and transcriptional profiling of type A influenza viruses expressing the hemagglutinin and neuraminidase genes from the 1918 pandemic virus. J Virol. 2004;78:9–499-9511. doi: 10.1128/JVI.78.17.9499-9511.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hurt AC, Leang SK, Speers DJ, Barr IG, Maurer-Stroh S. Mutations I117V and I117M and oseltamivir sensitivity of pandemic (H1N1) 2009 viruses. Emerg Infect Dis. 2012;18:1–09-112. doi: 10.3201/eid1801.111079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Memoli MJ, Hrabal RJ, Hassantoufighi A, Eichelberger MC, Taubenberger JK. Rapid selection of oseltamivir- and peramivir-resistant pandemic H1N1 virus during therapy in 2 immunocompromised hosts. Clin Infect Dis. 2010;50:1–252-1255. doi: 10.1086/651605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Memoli MJ, Hrabal RJ, Hassantoufighi A, Jagger BW, Sheng ZM, Eichelberger MC, Taubenberger JK. Rapid selection of a transmissible multidrug-resistant influenza A/H3N2 virus in an immunocompromised host. J Infect Dis. 2010;201:1–397-1403. doi: 10.1086/651610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Storms AD, Gubareva LV, Su S, Wheeling JT, Okomo-Adhiambo M, Pan CY, Reisdorf E, St George K, Myers R, Wotton JT, Robinson S, Leader B, Thompson M, Shannon M, Klimov A, Fry AM. Oseltamivir-Resistant Pandemic (H1N1) 2009 Virus Infections, United States, 2010-11. Emerg Infect Dis. 2012;18:3–08-311. doi: 10.3201/eid1802.111466. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.