Abstract
Increasing dietary protein within a physiologic range stimulates intestinal calcium absorption, but it is not known if specific amino acids or dietary protein as a whole are responsible for this effect. Therefore, we selectively supplemented a low-protein (0.7 g/kg) diet with either the calcium-sensing receptor-activating amino acids (CaSR-AAAs) L-tryptophan, L-phenylalanine, and L-histidine, or the dibasic amino acids (DAAs) L-arginine and L-lysine, to achieve intakes comparable to the content of a high-protein diet (2.1 g/kg) and measured intestinal calcium absorption. Fourteen young women took part in a placebo-controlled, double-blind, crossover feeding trial in which each participant ingested a 6-d low-protein diet supplemented with CaSR-AAAs, DAAs, or methylcellulose capsules (control) after an 11-d adjustment period. All participants ingested all 3 diets in random order. Intestinal calcium absorption was measured between days 5 and 6 using dual-stable calcium isotopes (42Ca, 43Ca, and 44Ca). There was no difference in calcium absorption between the diet supplemented with CaSR-AAAs (22.9 ± 2.0%) and the control diet (22.3 ± 1.4%) (P = 0.64). However, calcium absorption tended to be greater during the DAA supplementation period (25.2 ± 1.4%) compared with the control diet period (22.3 ± 1.4%) (P < 0.10). Larger and longer clinical trials are needed to clarify the possible benefit of arginine and lysine on calcium absorption.
Introduction
For nearly 90 y, we have known that dietary protein affects calcium metabolism (1). In a meta-analysis of 26 clinical intervention trials in adults in which dietary protein was manipulated and urinary calcium was measured, there was a strong linear correlation between the two (2) such that, on average, for every 40-g increment in dietary protein, urinary calcium increased by 50 mg. It was widely assumed that the skeleton was the principal source of the additional calcium. Higher protein diets, particularly those including animal sources, generate a fixed metabolic acid load because of the metabolism of sulfur-containing amino acids. The diet-induced acid load is believed to be incompletely accommodated by renal mechanisms, therefore, requiring release of buffer from bone. The liberation of alkali from bone requires osteoclast-mediated bone resorption, which could over a prolonged period of time reduce skeletal mass (3–6). In support of this hypothesis, balance studies in the 1970s reported no change in intestinal calcium absorption as dietary protein increased (7–13). However, more recent studies using dual-stable calcium isotopes found that in the short term, dietary protein significantly affects intestinal calcium absorption (14, 15). In particular, Kerstetter et al. (14) demonstrated that increasing dietary protein from 1.0 to 2.1 g/kg results in an increase in urinary calcium that is not accompanied by evidence for increased bone resorption. The increment in urinary calcium observed by these investigators was nearly quantitatively explained by a parallel improvement in intestinal calcium absorption. Recent work using an experimental rat model found that dietary protein had a similar effect on intestinal calcium absorption (16). Initial rates of calcium uptake were increased in brush border membrane vesicles isolated from rats acclimated to a high-protein diet (40%) compared with brush border membrane vesicles from rats consuming a low-protein diet (5%), suggesting that protein augments intestinal calcium absorption at least in part by increasing transcellular calcium uptake. The constituents of protein-containing foods that are responsible for its effect on intestinal calcium absorption remain unclear. One obvious candidate is amino acids.
Amino acids could affect calcium absorption by altering cellular metabolism in enterocytes or act as extracellular agonists via cell surface receptors or acceptors. Regarding the latter possibility, amino acids are known to be allosteric activators of the calcium-sensing receptor (CaSR)8, which is expressed throughout the gastrointestinal tract (17). Phenylalanine, tryptophan, and histidine are the most potent activators of the CaSR (18, 19). Conigrave et al. (18) first reported amino acid–induced activation of the CaSR at physiologic extracellular calcium concentrations in human embryonic kidney cells that were engineered to overexpress the receptor. Busque et al. (20) reported that L-phenylalanine and L-tryptophan at concentrations comparable to those seen postprandially can stereoselectively allosterically activate the CaSR on gastric parietal cells both in vivo and ex vivo. Work by Dawson-Hughes et al. (21) provides further evidence to support the hypothesis that the CaSR could mediate dietary protein–induced increases in intestinal calcium absorption. They demonstrated that supplementing a low-protein diet with phenylalanine and histidine increased urinary calcium, although they did not directly measure intestinal calcium absorption. Another group of amino acids that appear to have effects on intestinal calcium absorption are the dibasic amino acids (DAAs). In particular, dietary lysine supplementation was reported to increase calcium absorption in individuals with osteoporosis (22). The mechanism by which lysine supplementation induces changes in calcium absorption efficiency is unclear and is likely independent of CaSR activation because lysine is minimally effective as a CaSR agonist (18).
Despite these data, whether specific amino acids or dietary protein as a whole are responsible for the protein-induced increases in calcium absorption remains unresolved. The current human intervention trial was designed to determine whether supplementing a low-protein diet (0.7 g/kg) with the CaSR-activating amino acids (CaSR-AAAs) tryptophan, phenylalanine, and histidine, or the DAAs arginine and lysine, augments intestinal calcium absorption to an extent comparable to that seen when dietary protein is increased from low to high.
Methods
Participants
Sixty-five women (20–40 y old) with a BMI (kg/m2) ranging from 18 to 28 were screened for eligibility. Participants were recruited through flyers and advertisements in the Yale Bulletin between the years 2010 and 2012. Recruitment was limited to non-Hispanic Caucasian or Asian adults because these 2 ethnic groups are at highest risk of osteoporosis (23). Potential participants were excluded if they were taking medications known to affect calcium metabolism (antiosteoporotic medications, glucocorticoids, nonsteroidal anti-inflammatory medications, and birth control pills); were pregnant; reported excessive body weight change during the past 6 mo; followed intensive physical exercise regimens; smoked; had an eating disorder or food allergies, or followed medically prescribed diets; or had renal, gastrointestinal, or bone disease, or amenorrhea. Participants were asked to stop taking all multivitamin or mineral supplements during the entire study. Throughout the study, participants continued their usual activities at home, school, or work except on days 5 and 6 of the 3 experimental periods, at which time they were admitted to the Hospital Research Unit of the Yale Center for Clinical Investigation for measurement of calcium absorption. The study was approved by human investigation committees at Yale University and the University of Connecticut. Informed consent was obtained from each participant.
Experimental design
Diets.
The study protocol included 3 cycles, each consisting of an 11-d adjustment diet followed by 6 d of an experimental diet. The 3 experimental low-protein diets (control, CaSR-AAA–supplemented, and DAA-supplemented) were provided in random order. The experimental and adjustment diets were similar to those described in previous reports (14, 15, 24). The Yale Center for Clinical Investigation’s research dietitian and her staff prepared all meals for every study participant during the feeding portion of the study (last 3 d of the adjustment diet and 6 d of the experimental diet). For the first 8 d of each adjustment period, participants were instructed by the research dietitian to self-select their diets to contain a dietary protein intake of 1.2 g/kg, 2300 mg (100 mmol) of sodium, and 800 mg (20 mmol) of calcium. The low-protein experimental diet (0.7 g/kg) consisted of a variety of foods common to the western diet and contained 800 mg (20 mmol) calcium, 2300 mg (100 mmol) sodium, 800–1,200 mg (26–39 mmol) phosphorus, and 13 g of fiber. For each study participant, food items remained the same on all 3 experimental diets. Each participant began with an energy intake of 30–36 kcal/kg (125–150 kJ/kg), which was adjusted in 200–300 kcal/kg (840–1260 kJ/kg) increments (with simple sugars and fats) during the experimental period to maintain body weight within 1% of initial weight. The macronutrient and mineral composition of the experimental diets was calculated from USDA Handbook No. 8 and manufacturer’s information. The primary sources of calcium in the experimental diets were dairy foods and a chewable calcium carbonate (Tums; GlaxoSmithKline). Caffeine-containing beverages were limited to 1 a day and alcohol was not permitted. Distilled water was consumed ad libitum. After the final urine collection on day 6, participants resumed their usual unstructured diet and subsequently participated in the second and third dietary cycles.
Amino acid supplements.
L-amino acids were purchased from Ajinomoto Pharmaceuticals (Ajinomoto Food Ingredients, LLC) and dispensed in capsules by the Yale Investigational Drug Pharmacy. The total milligrams of supplemental amino acids for a given day were divided among the 3 meals in amounts proportional to the total protein in each meal. The amount of each amino acid added to the low-protein diet was the amount contained in the 1.4 g/kg increment in dietary protein required to increase protein intake from low (0.7 g/kg) to high (2.1 g/kg) (i.e., 2.1–0.7 = 1.4). Depending on a participant’s body weight, this required between 5 and 7 capsules with each meal. The average amino acid content (mg) per gram of total protein in the low- and high-protein diets (shown in Table 1) was used as the basis for determining amino acid supplementation (14, 15).
TABLE 1.
Amino acid concentrations in the low- and high-protein diets1
| Amino Acid | Functional group | Low-protein diet | High-protein diet | Combined diets2 |
| mg/g dietary protein | mg/g dietary protein | mg/g dietary protein | ||
| His | CaSR-AAA | 25.3 ± 0.6 | 28.2 ± 0.2 | 27 |
| Phe | CaSR-AAA | 75.4 ± 0.8 | 76.5 ± 0.6 | 76 |
| Trp | CaSR-AAA | 11.3 ± 0.2 | 11.3 ± 0.0 | 11 |
| Arg | DAA | 46.5 ± 1.2 | 56.8 ± 0.4 | 52 |
| Lys | DAA | 58.9 ± 1.1 | 74.9 ± 0.8 | 67 |
Values are means ± SEMs. CaSR-AAA, calcium-sensing receptor-activating amino acid; DAA, dibasic amino acid.
The amino acid concentrations of the low- and high-protein diets were used to calculate amino acid supplementation concentrations.
A 60-kg female is used in the following example illustrating how amino acid supplementation was calculated for the study. The baseline low-protein diet for a 60-kg female participant would contain 42 g of protein (0.7 g/kg × 60 kg) consumed over 3 meals. The participant would require 126 g of protein (2.1 g/kg × 60 kg) on the high-protein study diet. The increment in dietary protein required to increase her protein intake from 0.7 g/kg to 2.1 g/kg is 84 grams. This participant’s 3 experimental diets would be as follows: 1) 42 g of protein plus placebo (methylcellulose); 2) 42 g of protein plus 2268 mg of histidine (27 × 84), 6384 mg of phenylalanine (76 × 84), and 924 mg of tryptophan (11 × 84); and 3) 42 g of protein plus 4368 mg of arginine (52 × 84) and 5628 mg of lysine (67 × 84). The italicized values were obtained by averaging the amino acid content of the low- and high-protein diets previously used by Kerstetter et al. (14, 15) and represent the milligram of each amino acid per gram of dietary protein (last column of Table 1).
Biochemical sample collection.
Between days 0 and 1 and days 4 and 5, participants collected 24-h timed urine excretions for measurement of calcium, phosphorous, and sodium. On day 1 and 5 of the experimental diet, fasting blood was drawn for measurements of serum 1,25-dihydroxyvitamin D, parathyroid hormone (PTH), and creatinine. Glomerular filtration rate (GFR) was estimated from serum creatinine, age, sex, and race using the National Kidney Foundation online calculator Modification of Diet in Renal Disease study equation (25). Serum concentrations of 25-hydroxyvitamin D were determined and corrected (if needed) in each study participant prior to beginning each adjustment diet to ensure a vitamin D–sufficient status. If the serum 25-hydroxyvitamin D concentrations were <67 nmol/L but >50 nmol/L, participants were administered a one-time correction dose of 50,000 IU of vitamin D2. Participants with a serum 25-hydroxyvitamin D of <50 nmol/L were given 2 correction doses of 50,000 IU of vitamin D2 separated by 5 d. Serum 25-hydroxyvitamin D was retested at least 2 wk after supplementation. Participants were not permitted to start their adjustment diet until their serum 25-hydroxyvitamin D was at least 67 nmol/L.
Measurement of intestinal calcium absorption.
Intestinal calcium absorption was measured using dual-stable calcium isotopes as previously described (14). In brief, 0.125 mg of 44Ca/kg body weight was administered orally in 3 divided doses with each meal in proportion to the calcium content of the meal. The isotope was equilibrated in milk for 18–24 h prior to administration. Immediately after the breakfast meal on day 5, 0.02 mg of 42Ca/kg (for experimental diets 1 and 3) or 0.004 mg/kg of 43Ca (for experimental diet 2) was administered intravenously, after which urine was collected for the next 34 h in three urine pools (8 h, 12 h, and 14 h). The intravenous isotope was changed for every other experimental diet to ensure that there was no carryover of isotope from one diet to the next.
Calcium isotope ratios were measured using a Thermo Scientific Triton TI magnetic sector thermal ionization mass spectrometer (TIMS; Thermo Fisher Scientific). A ratio was made between each administered calcium tracer (42Ca, 43Ca, and 44Ca) and another naturally occurring calcium isotope (i.e., 48Ca). Fractional calcium absorption was determined as the ratio of the cumulative oral tracer recovery to the cumulative IV tracer recovery in the 34-h urine collection obtained after dosing. True calcium absorption was calculated as the product of fractional calcium absorption and the calculated calcium intake (14).
Assays.
Twenty-four-hour urinary calcium, urinary phosphorous, and serum creatinine were measured on an AlfaWasserman ACE analyzer (Alfa Wassermann Diagnostic Technologies). Urinary sodium was measured in the clinical chemistry laboratory of the Yale–New Haven Hospital. Intact PTH was measured by radioimmunoassay (Total Intact PTH Assay; Scantibodies Laboratory). Vitamin D metabolites were measured by radioimmunoassays (Diasorin).
Statistical analyses
The estimated number of participants required to detect a change in calcium absorption is based on the hypothesis that adding CaSR-AAAs to a low-protein diet will increase intestinal calcium absorption to an extent similar to that observed when the participant’s dietary protein intake is increased from 0.7 to 2.1 g/kg. Because it is possible that amino acids besides those that activate the CaSR also affect intestinal calcium absorption, we estimated the effect size for the CaSR-AAAs to be ∼70% of that seen with the 2.1 g/kg diet. We reported that increasing dietary protein from 0.7 to 2.1 g/kg increased calcium absorption from 19 ± 5.0% to 26 ± 8.0% (an increment of 7 ± 6.5%, P < 0.01) (15). To be conservative, we estimated an effect size ∼30% less than that (a 5 ± 6.5% increase in calcium absorption). Using this effect size and SD, a sample size of 14 provides a power of 0.80 with P = 0.05 (calculated using GraphPad StatMate, version 2.0a for Macintosh; GraphPad Software).
Analyses were performed using SPSS version 12.0 for Windows (SPSS). The graphical summary was generated using Prism software (version 4.0, 2004; GraphPad Software). All values are presented as means ± SEMs. The Shapiro–Wilks test was used to test data for normality. All data were normally distributed except for serum 25-hydroxyvitamin D and 24-h urinary calcium at baseline and intact PTH at baseline and day 5 of all 3 interventions. A repeated measures ANOVA or Friedman test (for non-normally distributed data) was used to assess differences in baseline measures between the 3 experimental diets. A paired t test or Wilcoxon signed ranked test (for non-normally distributed data) was used to assess differences between each amino acid supplementation group and the control diet at day 5 and differences between baseline (day 1) and day 5 of each experimental diet. A comprehensive assessment of the primary outcome variable, intestinal calcium absorption, was performed by calculating mean effect sizes for the 2 interventions. This was done by the following calculation: mean absorption on the amino acid–supplemented diet for the entire study group minus the mean absorption on the control diet for all study participants divided by the SD of the difference between the control diet and each amino acid–supplemented diet (26). A probability level of P ≤ 0.05 indicated statistical significance, and P > 0.05 but < 0.10 indicated a trend.
Results
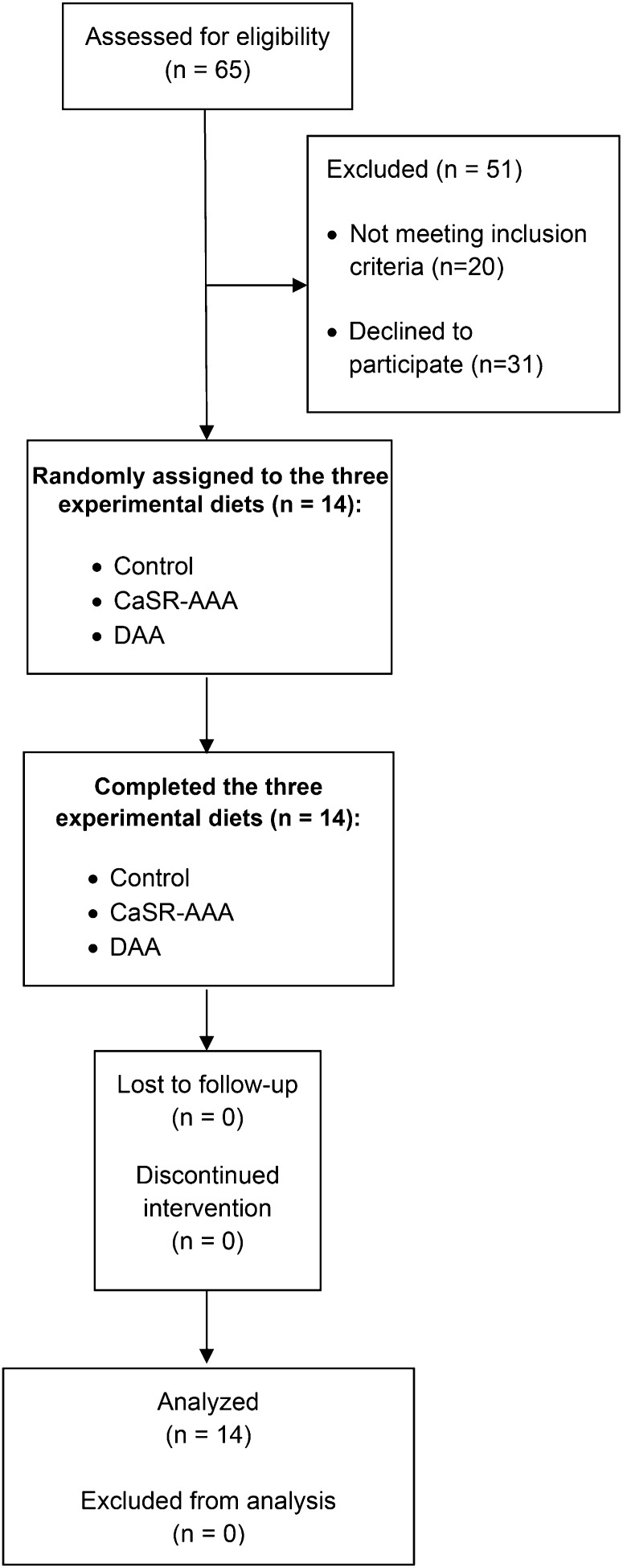
Figure 1 summarizes study participant recruitment, enrollment, and random assignment. Of the 65 participants screened for eligibility, 51 participants did not meet the inclusion criteria or were not interested in participating after receiving a detailed explanation of the study. Fourteen healthy premenopausal women with a mean age of 27.8 ± 1.2 and a BMI of 23.7 ± 0.9 were enrolled and randomly assigned.
FIGURE 1.
Flow diagram displaying recruitment, enrollment, and random assignment of study participants. Fourteen healthy young women took part in a crossover-design feeding study in which they received 3 experimental diets in random order (control, CaSR-AAA–supplemented, and DAA-supplemented; n = 14 for each experimental diet). CaSR-AAA, calcium-sensing receptor-activating amino acid; DAA, dibasic amino acid.
Serum and urine metabolites.
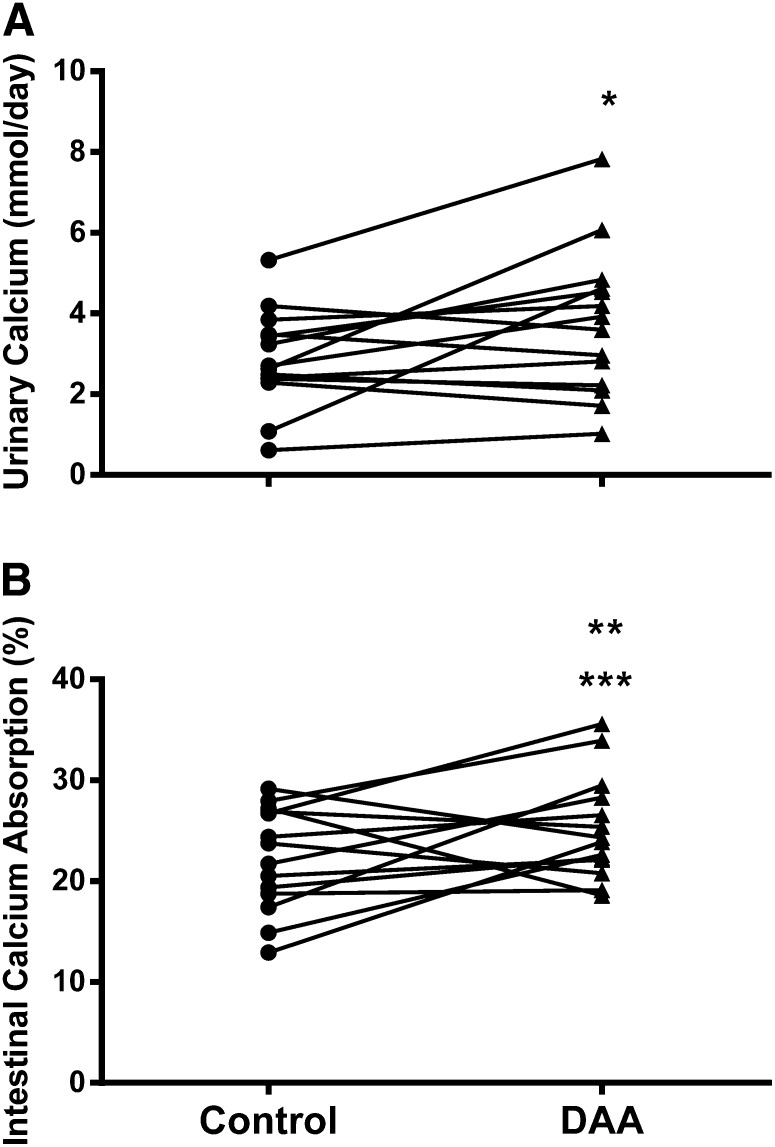
Serum 25-hydroxyvitamin D was within normal limits at the start of each participant’s adjustment diet and was not significantly different between the 3 dietary interventions (control: 88.1 ± 4.8 nmol/L; CaSR-AAA: 88.1 ± 4.6 nmol/L; DAA: 87.5 ± 4.3 nmol/L, P = 0.67) (27). Baseline measures of serum and 24-h urine metabolites did not differ significantly between the 3 diets (Table 2). Changes observed in calcium-related metabolites during the 3 experimental diets are presented in Table 2. No significant differences were observed in 1,25-dihydroxyvitamin D or intact PTH between baseline and day 5 of each intervention. Analyses of the day-5 values for PTH and 1,25-dihydroxyvitamin D revealed no significant differences among the 3 interventions. Urinary sodium and GFR remained stable with no significant differences observed at any time point for the duration of the study. As expected, GFR was above 60 mL/(min · 1.73 m2) in all participants throughout the entire study period. On day 5, urinary calcium was significantly lower than the value at baseline during the control diet period (37.4%, z = –2.67, P = 0.008) but not during the CaSR-AAA or DAA diet period. On day 5, urinary calcium was significantly greater with the DAA supplementation than the control diet (P = 0.039) (Table 2, Fig. 2A). Twenty-four–hour urinary phosphorus excretion on day 5 of the 3 study diet periods was lower than at baseline (P ≤ 0.047).
TABLE 2.
Effect of CaSR-AAA and DAA supplementation on calcium-related metabolites and calcium absorption in young women1
| Control |
CaSR-AAA supplementation |
DAA supplementation |
||||
| Day 1 | Day 5 | Day 1 | Day 5 | Day 1 | Day 5 | |
| Participants, n | 14 | 14 | 14 | 14 | 14 | 14 |
| Serum metabolites | ||||||
| 1,25-Dihydroxyvitamin D, pmol/L | 151 ± 13 | 163 ± 8 | 162 ± 12 | 155 ± 12 | 172 ± 14 | 166 ± 13 |
| Intact PTH, nmol/L | 36 ± 6 | 31 ± 6 | 31 ± 7 | 30 ± 6 | 32 ± 9 | 31 ± 7 |
| GFR, mL/(min · 1.73 m2) | 91 ± 5 | 97 ± 6 | 90 ± 5 | 91 ± 6 | 93 ± 5 | 94 ± 6 |
| 24-h urine metabolites, mmol | ||||||
| Sodium | 90.8 ± 12.6 | 74.9 ± 6.7 | 80.9 ± 10.7 | 86.4 ± 8.2 | 80.2 ± 7.5 | 90.1 ± 9.7 |
| Calcium | 4.6 ± 1.1 | 2.9 ± 0.3a | 3.9 ± 0.6 | 3.3 ± 0.3 | 4.2 ± 0.6 | 3.8 ± 0.5b |
| Phosphorus | 18.4 ± 1.8 | 11.4 ± 1.2a | 15.6 ± 1.7 | 12.0 ± 1.2a | 18.2 ± 1.4 | 12.7 ± 1.3a |
| Calcium absorption, % | ||||||
| Intestinal calcium absorption | 22.3 ± 1.4 | 22.9 ± 2.0 | 25.2 ± 1.4c | |||
Values are means ± SEMs. aDifferent from corresponding baseline, P < 0.05; bdifferent from control, day 5, P < 0.05; cdifferent from control, day 5, 0.05 < P < 0.10. CaSR-AAA, calcium-sensing receptor-activating amino acid; DAA, dibasic amino acid; GFR, glomerular filtration rate; PTH, parathyroid hormone.
FIGURE 2.
Urinary calcium excretion (A) and intestinal calcium absorption (B) by 14 healthy young women on day 5 of the control and DAA-supplemented low-protein diet periods. *Different from control, P < 0.05; **different from control, 0.05 < P < 0.10; ***effect size = 0.54 ± 0.1. DAA, dibasic amino acid.
Calcium absorption.
As shown in Table 2, there was no significant difference in calcium absorption between the diet supplemented with CaSR-AAAs and the control diet at day 5 (effect size = 0.12 ± 0.09, P = 0.64). However, there was a nonsignificant trend toward increased calcium absorption with DAA supplementation compared with the control diet (P = 0.094), with 10 of the 14 participants evidencing higher absorption. Because dietary calcium was fixed at 20 mmol/d, intestinal calcium absorption with consumption of the low-protein diet supplemented with DAAs was 0.6 ± 0.3 mmol/d higher than with consumption of the control diet. Calculation of effect size revealed that this observed difference in mean intestinal calcium absorption is considered to be a medium effect (0.54 ± 0.1) (28). As seen in Fig. 2, the increase in intestinal calcium absorption mirrored the change in urinary calcium observed during the 2 diets. The mean difference in urinary calcium at day 5 between the control and DAA-supplemented diets was 0.9 ± 0.4 mmol/d.
Discussion
We employed dual-stable calcium isotopes to evaluate the effect of a low-protein diet supplemented with either DAAs or CaSR-AAAs on intestinal calcium absorption. The intestinal handling of calcium was not influenced by the addition of CaSR-AAAs to a low-protein diet. Short-term supplementation with DAAs resulted in a significant increase in urinary calcium and augmented calcium absorption without significant changes in PTH or 1,25-dihydroxyvitamin D during the 3 diet interventions, suggesting that the change in calcium excretion is due to improved intestinal calcium absorption and not an induced renal calcium leak.
It is generally assumed that the dietary protein-induced increases in urinary calcium results from the release of skeletal buffer and calcium in response to the metabolic load imposed by sulfur-containing amino acids (3–6). Our data provide evidence for amino acid-induced increases in urinary calcium independent of changes in dietary sulfur content because arginine and lysine do not contain sulfur. Therefore, the observed increase in urinary calcium is very likely attributable to the rise in intestinal calcium absorption, rather than an increase in skeletal catabolism.
Our laboratory previously reported increased intestinal calcium absorption during a high-normal protein diet (2.1 g/kg) consisting of both animal and vegetable protein when compared with both low (0.7g/kg) and medium (1.0 g/kg) protein intakes (14, 15). In these prior studies, dual-stable calcium isotopes were also used to assess calcium absorption, calcium was fixed at 19.8–20 mmol/d, and nutrients known to affect calcium metabolism were tightly controlled. Under these study conditions, when dietary protein was increased from 0.7 to 2.1 g/kg, intestinal calcium absorption rose by 36.8% (15). The current study supplemented the same low-protein diet with the amount of amino acids contained in the 1.4-g/kg increment in dietary protein that was required to increase protein intake from 0.7 to 2.1 g/kg. Intestinal calcium absorption was 13% higher with DAA supplementation compared with the low-protein control diet. The change in calcium absorption in the present study reflects roughly a third of the change in absorption observed when dietary protein was increased by 1.4 g/kg using mixed food sources. Thus, the addition of DAAs to the low-protein diet did not increase intestinal calcium absorption to the same extent as when dietary protein sources were manipulated. However, DAA supplementation resulted in a quantifiable change in intestinal calcium absorption, which can explain 67% of the 0.9-mmol change in urinary calcium. Since intestinal calcium absorption declines with aging and after menopause, even a modest improvement in intestinal calcium absorption could have long-term physiological significance. Thus, most estrogen-deficient postmenopausal women are in negative-calcium balance, and an intervention that improves calcium homeostasis could potentially protect against long-term deleterious effects on skeletal integrity.
Research in rat models supports a DAA effect on calcium economy (29, 30). Over 50 y ago, Wasserman et al. (29) measured 45Ca in the femur of young male rats after the enteral administration of an individual amino acid bolus. Of the 10 essential and 8 nonessential amino acids studied, L-lysine followed by L-arginine resulted in the greatest accumulation of 45Ca in bone. Compared with the control rats, administration of L-lysine and L-arginine caused 1.7-fold and 1.6-fold increases in 45Ca accretion in bone, respectively. In subsequent work, these investigators evaluated the interaction between lysine and other nutrients known to effect calcium metabolism in vitamin D–deficient rats (30). Treatment with L-lysine or vitamin D enhanced calcium absorption more than seen with control treatment, but not to the same extent as when the two were provided in combination. The authors hypothesized that L-lysine and vitamin D may be acting via different cellular pathways to increase intestinal calcium absorption. Taken together, the studies by Wasserman et al. and the present study support the notion that additional studies to clarify the role of DAAs in calcium metabolism are warranted. At present, it is unclear whether DAAs exert an effect on the transcellular, paracellular, or both calcium-transport mechanisms.
The current study also evaluated a second group of amino acids (phenylalanine, tryptophan, and histidine) because they allosterically activate the CaSR. We found no CaSR-AAA effect on calcium absorption. In contrast, we found an increase in urinary calcium from the DAA supplementation compared with the control. It is unlikely that the slight DAA effect we observed on intestinal calcium absorption is mediated by the CaSR. Conigrave et al. (18) demonstrated that arginine and lysine were not very effective in activating the CaSR in human embryonic kidney-293 cells that were stably expressing the receptor.
Dawson-Hughes et al. (21) examined the effects of selectively supplementing a low-protein diet with either the CaSR-AAAs phenylalanine and histidine, or the BCAAs isoleucine and leucine, on urinary calcium. There was no significant difference in urinary calcium between baseline and day 11 of amino acid supplementation for either study group or between the 2 study groups at the end of the supplementation period. These findings are consistent with the results of the present study, in which we found no significant change in urinary calcium with CaSR-AAA supplementation. In a subsequent analysis, mean change in 24-h urine calcium was also compared between to the 2 groups (21). The addition of CaSR-AAAs to a baseline low-protein intake of 0.5 g/kg resulted in an 11 ± 9 mg increase in calcium excretion from baseline, which was significantly different from the 20 ± 9 mg decline in urinary calcium observed with BCAA supplementation. In contrast to the study by Dawson-Hughes et al., our baseline dietary protein intake was higher and we did not observe an increase in urinary calcium with either amino acid supplementation. In our study rather than change from baseline, we analyzed differences after 5 d of each of the 3 interventions since we felt that this would most accurately reflect the impact of the intervention. The dietary intervention used by Dawson-Hughes et al. differed from the current study in that it contained 0.2 g/kg less dietary protein, 16.1 mg/kg more histidine, and 52.5 mg/kg less phenylalanine. Differences in study design, study duration, and amino acid supplementation may have contributed to the discrepancy in directional change in urinary calcium between the present study and the report by Dawson-Hughes et al.
A BCAA intervention was not included in the present study. Two human clinical trials found no effect from BCAA supplementation on urinary calcium or calcium absorption (21, 22). Civitelli et al. (22) supplemented osteoporotic patients with 3 different amino acids, including the BCAA L-valine, which did not induce a rise in intestinal calcium absorption. Similarly, Dawson-Hughes et al. (21) reported a blunted calciuric response in participants consuming a diet rich in leucine and isoleucine compared with those with high CaSR-AAA intakes. There are obviously numerous other combinations of amino acids that need to be explored in relation to calcium homeostasis, which is beyond the scope of this study.
Not all investigators have observed a dietary protein effect on calcium absorption. Hunt et al. (31) and Ceglia et al. (32) reported no differences in calcium absorption between different amounts of dietary protein when calcium intakes met or surpassed the RDA (31, 32) and dietary phosphorous was not fixed (32). Higher calcium and phosphorus intakes could have masked any potential regulatory actions by dietary protein on calcium absorption. It would be of interest to examine the effect of graded calcium intakes in conjunction with different quantities or combinations of amino acid supplements on calcium economy to determine whether this has a protective effect on calcium homeostasis in low dietary calcium environments.
The current study had a number of strengths including a tightly controlled crossover design with all meals provided by the metabolic kitchen at the Yale Center for Clinical Investigation. Good dietary compliance was evident by consistency in urinary sodium. We targeted young Asian and Caucasian women because these ethnic groups are at highest risk of osteoporosis in later adult life (23). A recent analysis of NHANES (2003–2006) reported that women between the ages of 19 and 40 who do not use calcium supplements are not meeting the current recommendations for calcium (33). Thus, young Asian and Caucasian women would likely benefit from a nutrient based, low-risk therapeutic option, such as dietary protein/amino acid supplementation, to maximize calcium absorption. Moreover, protein intake has decreased in women from the 1970s to 2006 (34). We chose to study a group of individuals who, as they age, are at greatest risk of osteoporosis and, depending on dietary protein intake, could benefit from protein/amino acid supplementation to optimize intestinal calcium absorption.
The study also had some limitations. Our sample size was small because of the time and expense involved in stable calcium isotopic studies. It may be that larger studies of longer duration will be needed to further clarify the impact of DAAs on calcium economy. Some participants required vitamin D supplementation prior to starting their adjustment diets. However, the number of study participants requiring vitamin D supplementation was nearly equal for each dietary intervention (DAA: 7 participants, CaSR-AAA: 8 participants, control: 8 participants), making it unlikely that this confounded our results. Time of menstrual cycle was not controlled for when scheduling each study participant’s calcium absorption assessments. In initial studies from our group examining the effect of increasing protein intake on calcium absorption using the same dual-stable calcium isotope methodology as in our current report, we did not control for time of menstrual cycle in premenopausal women (14, 15). We observed a quantitatively comparable effect from dietary protein in men and postmenopausal women in whom cyclical changes in reproductive hormone levels do not occur when compared with premenopausal women. Thus, although we cannot exclude a contribution by variations in estrogen levels to our observed results, given the above, this seems unlikely to have made a major contribution.
In summary, calcium absorption was assessed by dual-stable calcium isotopes in healthy premenopausal women consuming a low-protein diet supplemented with CaSR-AAAs or DAAs for 6 days. The addition of CaSR-AAAs to a low-protein diet did not result in a significant rise or decline in calcium absorption. Urinary calcium was significantly higher with arginine and lysine supplementation, which could potentially be explained by a change in calcium absorption efficiency.
Supplementary Material
Acknowledgments
The authors thank Tera R. Kent for her assistance with mass spectrometry analyses. J.D.B., J.E.K., and K.L.I. designed the research; J.D.B., R.R.S., and D.M.C. conducted the research; K.O.O. oversaw isotope analyses and provided dual-stable calcium isotopes; C.A.S. performed biochemical analyses; T.B.H.-M. and J.D.B. performed statistical analyses; J.D.B. wrote the paper; and J.E.K. and K.L.I. had primary responsibility for the final content. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: CaSR, calcium-sensing receptor; CaSR-AAA, CaSR-activating amino acid; DAA, dibasic amino acid; GFR, glomerular filtration rate; PTH, parathyroid hormone.
Literature Cited
- 1.Sherman HC. Calcium requirement of maintenance in man. J Biol Chem. 1920;44:21–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kerstetter JE, O'Brien KO, Insogna KL. Low protein intake: the impact on calcium and bone homeostasis in humans. J Nutr. 2003;133:855S–61S. [DOI] [PubMed] [Google Scholar]
- 3.Bushinsky DA. Acid-base imbalance and the skeleton. Eur J Nutr. 2001;40:238–44. [DOI] [PubMed] [Google Scholar]
- 4.Remer T. Influence of diet on acid-base balance. Semin Dial. 2000;13:221–6. [DOI] [PubMed] [Google Scholar]
- 5.Barzel US, Massey LK. Excess dietary protein can adversely affect bone. J Nutr. 1998;128:1051–3. [DOI] [PubMed] [Google Scholar]
- 6.Bushinsky DA, Frick KK. The effects of acid on bone. Curr Opin Nephrol Hypertens. 2000;9:369–79. [DOI] [PubMed] [Google Scholar]
- 7.Anand CR, Linkswiler HM. Effect of protein intake on calcium balance of young men given 500 mg calcium daily. J Nutr. 1974;104:695–700. [DOI] [PubMed] [Google Scholar]
- 8.Kim Y, Linkswiler HM. Effect of level of protein intake on calcium metabolism and on parathyroid and renal function in the adult human male. J Nutr. 1979;109:1399–404. [DOI] [PubMed] [Google Scholar]
- 9.Allen LH, Oddoye EA, Margen S. Protein-induced hypercalciuria: a longer term study. Am J Clin Nutr. 1979;32:741–9. [DOI] [PubMed] [Google Scholar]
- 10.Hegsted M, Linkswiler HM. Long-term effects of level of protein intake on calcium metabolism in young adult women. J Nutr. 1981;111:244–51. [DOI] [PubMed] [Google Scholar]
- 11.Schuette SA, Hegsted M, Zemel MB, Linkswiler HM. Renal acid, urinary cyclic AMP, and hydroxyproline excretion as affected by level of protein, sulfur amino acid, and phosphorus intake. J Nutr. 1981;111:2106–16. [DOI] [PubMed] [Google Scholar]
- 12.Schuette SA, Zemel MB, Linkswiler HM. Studies on the mechanism of protein-induced hypercalciuria in older men and women. J Nutr. 1980;110:305–15. [DOI] [PubMed] [Google Scholar]
- 13.Spencer H, Kramer L, DeBartolo M, Norris C, Osis D. Further studies of the effect of a high protein diet as meat on calcium metabolism. Am J Clin Nutr. 1983;37:924–9. [DOI] [PubMed] [Google Scholar]
- 14.Kerstetter JE, O'Brien KO, Caseria DM, Wall DE, Insogna KL. The impact of dietary protein on calcium absorption and kinetic measures of bone turnover in women. J Clin Endocrinol Metab. 2005;90:26–31. [DOI] [PubMed] [Google Scholar]
- 15.Kerstetter JE, O'Brien KO, Insogna KL. Dietary protein affects intestinal calcium absorption. Am J Clin Nutr. 1998;68:859–65. [DOI] [PubMed] [Google Scholar]
- 16.Gaffney-Stomberg E, Sun BH, Cucchi CE, Simpson CA, Gundberg C, Kerstetter JE, Insogna KL. The effect of dietary protein on intestinal calcium absorption in rats. Endocrinology. 2010;151:1071–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitsuma T, Rhue N, Kayama M, Mori Y, Adachi K, Yokoi Y, Ping J, Nogimori T, Hirooka Y. Distribution of calcium sensing receptor in rats: an immunohistochemical study. Endocr Regul. 1999;33:55–9. [PubMed] [Google Scholar]
- 18.Conigrave AD, Quinn SJ, Brown EM. L-amino acid sensing by the extracellular Ca2+-sensing receptor. Proc Natl Acad Sci USA. 2000;97:4814–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conigrave AD, Franks AH, Brown EM, Quinn SJ. L-amino acid sensing by the calcium-sensing receptor: a general mechanism for coupling protein and calcium metabolism? Eur J Clin Nutr. 2002;56:1072–80. [DOI] [PubMed] [Google Scholar]
- 20.Busque SM, Kerstetter JE, Geibel JP, Insogna K. L-type amino acids stimulate gastric acid secretion by activation of the calcium-sensing receptor in parietal cells. Am J Physiol Gastrointest Liver Physiol. 2005;289:G664–9. [DOI] [PubMed] [Google Scholar]
- 21.Dawson-Hughes B, Harris SS, Rasmussen HM, Dallal GE. Comparative effects of oral aromatic and branched-chain amino acids on urine calcium excretion in humans. Osteoporos Int. 2007;18:955–61. [DOI] [PubMed] [Google Scholar]
- 22.Civitelli R, Villareal DT, Agnusdei D, Nardi P, Avioli LV, Gennari C. Dietary L-lysine and calcium metabolism in humans. Nutrition. 1992;8:400–5. [PubMed] [Google Scholar]
- 23.Pothiwala P, Evans EM, Chapman-Novakofski KM. Ethnic variation in risk for osteoporosis among women: a review of biological and behavioral factors. J Womens Health (Larchmt). 2006;15:709–19. [DOI] [PubMed] [Google Scholar]
- 24.Kerstetter JE, Caseria DM, Mitnick ME, Ellison AF, Gay LF, Liskov TA, Carpenter TO, Insogna KL. Increased circulating concentrations of parathyroid hormone in healthy, young women consuming a protein-restricted diet. Am J Clin Nutr. 1997;66:1188–96. [DOI] [PubMed] [Google Scholar]
- 25.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–70. [DOI] [PubMed] [Google Scholar]
- 26.Becker BJ. Synthesizing standardized mean-change measures. Br J Math Stat Psychol. 1988;41:257–78. [Google Scholar]
- 27.Malabanan A, Veronikis IE, Holick MF. Redefining vitamin D insufficiency. Lancet. 1998;351:805–6. [DOI] [PubMed] [Google Scholar]
- 28.Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: Lawrence Earlbaum Associates; 1988. [Google Scholar]
- 29.Wasserman RH, Comar CL, Nold MM. The influence of amino acids and other organic compounds on the gastrointestinal absorption of calcium 45 and strontium 89 in the rat. J Nutr. 1956;59:371–83. [DOI] [PubMed] [Google Scholar]
- 30.Wasserman RH, Comar CL, Schooley JC, Lengemann FW. Interrelated effects of L-lysine and other dietary factors on the gastrointestinal absorption of calcium 45 in the rat and chick. J Nutr. 1957;62:367–76. [DOI] [PubMed] [Google Scholar]
- 31.Hunt JR, Johnson LK, Fariba Roughead ZK. Dietary protein and calcium interact to influence calcium retention: a controlled feeding study. Am J Clin Nutr. 2009;89:1357–65. [DOI] [PubMed] [Google Scholar]
- 32.Ceglia L, Harris SS, Abrams SA, Rasmussen HM, Dallal GE, Dawson-Hughes B. Potassium bicarbonate attenuates the urinary nitrogen excretion that accompanies an increase in dietary protein and may promote calcium absorption. J Clin Endocrinol Metab. 2009;94:645–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mangano KM, Walsh SJ, Insogna KL, Kenny AM, Kerstetter JE. Calcium intake in the United States from dietary and supplemental sources across adult age groups: new estimates from the National Health and Nutrition Examination Survey 2003–2006. J Am Diet Assoc. 2011;111:687–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Austin GL, Ogden LG, Hill JO. Trends in carbohydrate, fat, and protein intakes and association with energy intake in normal-weight, overweight, and obese individuals: 1971–2006. Am J Clin Nutr. 2011;93:836–43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.