Abstract
The link between iron intake as well as body iron stores and coronary heart disease (CHD) has been contentiously debated, and the epidemiologic evidence is inconsistent. We aimed to quantitatively summarize the literature on the association between dietary iron intake/body iron stores and CHD risk by conducting a meta-analysis of prospective cohort studies. PubMed was used to find studies published through June 2013 in peer-reviewed journals. Embase or a hand search of relevant articles was used to obtain additional articles. The pooled RRs of CHD incidence and mortality with 95% CIs were calculated by using either a random-effects or fixed-effects model, as appropriate. Twenty-one eligible studies (32 cohorts) including 292,454 participants with an average of 10.2 y of follow-up were included. Heme iron was found to be positively associated with CHD incidence (RR: 1.57; 95% CI: 1.28, 1.94), whereas total iron was inversely associated (RR: 0.85; 95% CI: 0.73, 0.999). Neither heme-iron nor total iron intakes were significantly associated with CHD mortality. Both transferrin saturation and serum iron were inversely related to CHD incidence [RR (95% CI): 0.76 (0.66, 0.88) and 0.68 (0.56, 0.82), respectively], but only transferrin saturation was inversely associated with CHD mortality (RR: 0.85; 95% CI: 0.73, 0.99). In conclusion, total iron intake and serum iron concentrations were inversely associated with CHD incidence, but heme iron intake was positively related to CHD incidence. Elevated serum transferrin saturation concentration was inversely associated with both CHD incidence and mortality. Future research is needed to establish the causal relation and to elucidate potential mechanisms.
Introduction
The iron hypothesis, first introduced by Sullivan in 1981, posits that the higher risk of coronary heart disease (CHD)4 among men and postmenopausal women is due to higher body iron stores (1). There are several proposed mechanisms to explain the unequal distribution of risk, as follows: increased oxidative stress due to iron’s role as a catalyst in the formation of hydroxyl radicals and in the creation of oxidized LDL cholesterol, alterations in endothelial function, decreased vascular reactivity, and myocardial reperfusion injury (2–4).
Although observational studies have indicated that higher iron exposure may be associated with higher risk of CHD (5–7), the epidemiologic evidence is still inconclusive; some studies have found no association (8, 9) or an inverse association (10, 11) between iron and CHD incidence or mortality. Systematic reviews (12–14) have been able to summarize the literature, but the last meta-analysis on both dietary iron and serum iron biomarkers and CHD was completed in 1999 (15), and since then, at least 16 more prospective cohort studies have been published (5, 8, 9, 11, 16–27). An update of the preexisting literature is urgently needed.
Creating further problems in the iron-CHD debate is the difficulty of quantifying iron exposure in observational studies. Investigators generally determine exposure status by estimating dietary iron intake using FFQs or by measuring different serum iron biomarkers. When dietary iron intake is used, total iron intake is subdivided into heme and nonheme iron due to the differences between the 2 types of iron in absorption and bioavailability (28). Total iron intake is estimated by determining the total iron content in the diet from all of the foods listed in the FFQ, and heme iron is then calculated by determining ∼40% of total iron intake from meat products. Nonheme iron is then approximated by subtracting heme-iron intake from total iron intake. When biomarkers are used, investigators measure serum ferritin, serum iron, total iron-binding capacity (TIBC), and transferrin saturation to estimate exposure levels. Serum ferritin is a marker of total body iron stores (29). Serum iron is a measure of circulating iron, whereas TIBC indicates the concentration of transferrin, a protein that transports iron to cells (29). Transferrin saturation, the ratio of serum iron to TIBC, is a measure of circulating iron freely available to tissues (13). These differences between heme- and nonheme-iron intake and between the serum iron biomarkers must be considered when addressing the potential iron-CHD link. In this study, we aimed to quantitatively summarize the literature on the association of dietary iron intake as well as serum iron biomarker concentrations with CHD incidence and CHD mortality by conducting a meta-analysis of prospective cohort studies.
Methods
Search strategy.
Studies were identified through a PubMed search by using the terms “iron or iron, dietary or serum iron or ferritin or transferrin saturation or transferrin iron binding capacity,” and “cardiovascular disease or coronary heart disease or myocardial infarction,” and “epidemiologic studies” and “cohort/prospective/follow-up/longitudinal studies,” and “proportional hazards models or Cox or hazard ratio or risk.” Additional information was found through Embase and a hand search of the related references. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed in this process, and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses checklist is listed in Supplemental Table 1 (30).
Study selection.
Studies were included in the meta-analysis if they were published in English through June 2013 and met all of the following criteria: 1) the study was a prospective cohort study; 2) the exposure of interest was dietary iron intake or any serum biomarker (iron, ferritin, TIBC, or transferrin saturation); 3) the outcome of interest was either CHD, myocardial infarction, or cardiovascular disease (CVD) incidence or mortality; 4) reported RRs or HRs with 95% CIs or these data could be derived from reported results; 5) participants were from the generally healthy population; and 6) the study used the lowest exposure group as the reference.
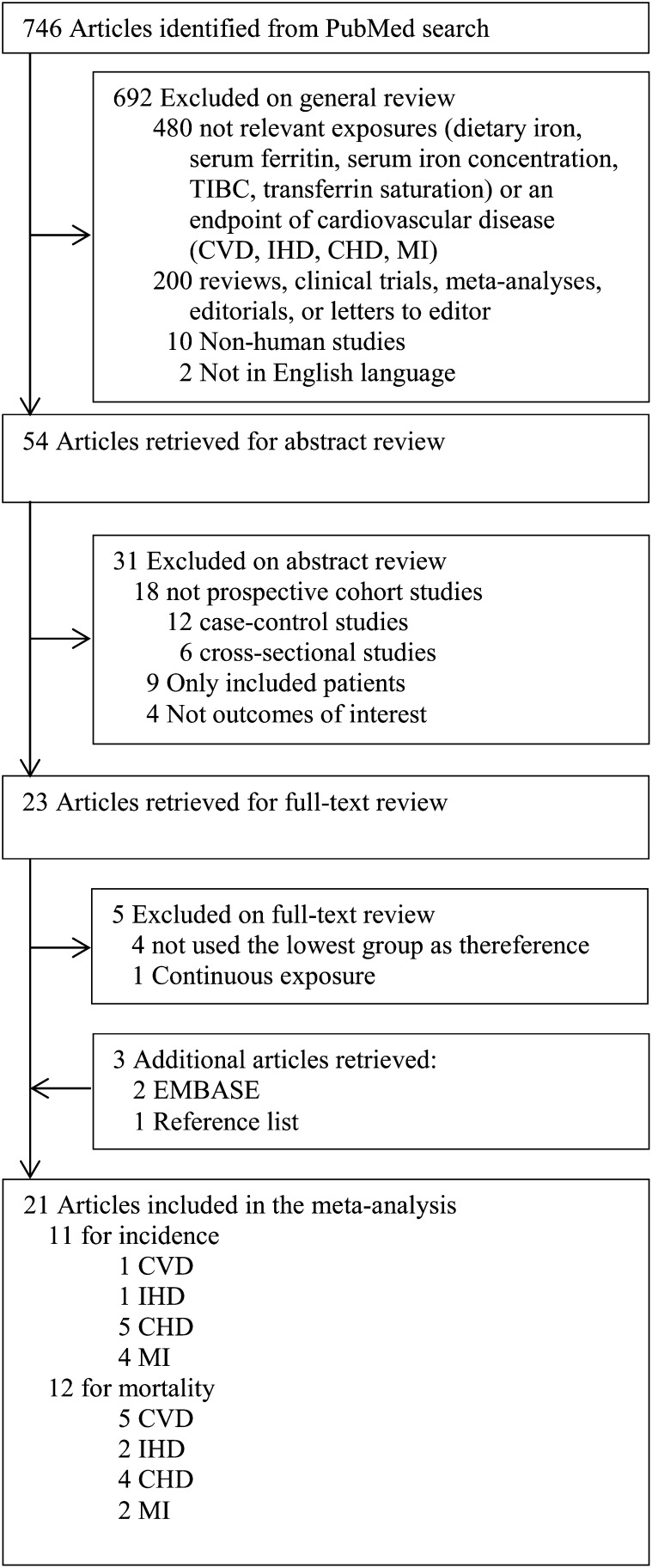
As shown in Fig. 1, 746 abstracts were found during the PubMed search. Of these, 723 articles were excluded in the general/abstract review for 1 of the following reasons: 1) did not include a relevant exposure or endpoint; 2) were clinical trials, meta-analyses, editorials, or letters to the editor; 3) were in vitro or animal studies; 4) reported a continuous exposure; 5) did not use the lowest exposure group as the reference; 6) were conducted in patients with a specific disease; or 7) were not published in English.
FIGURE 1.
Selection of studies for meta-analysis. CHD, coronary heart disease; CVD, cardiovascular disease; IHD, ischemic heart disease; MI, myocardial infarction; TIBC, total iron-binding capacity.
Twenty-three articles were retrieved from the PubMed search (5–11, 16, 18, 19, 21, 22, 24–27, 31–37), and 3 more articles were identified from Embase or by hand searching (20, 23, 38), leading to an independent full-text review of 26 articles by 2 authors (5–11, 16, 18–27, 31–38). Five were excluded: 4 articles that did not use the lowest exposure group as a reference (24–27) and 1 that reported a continuous exposure (37). Therefore, 21 identified eligible prospective cohort studies were included in this meta-analysis.
Data extraction.
Data extracted from the studies included the following: first author, publication year, study name, country, percentage of men, range or mean age of participants at baseline, duration of follow-up, number of participants and person-years of follow-up, number of cases, how the exposure was assessed, the categories of the exposure, how the outcome was assessed, the variables adjusted for in the analysis, and the RRs or HRs with corresponding 95% CIs of CHD related to different categories of dietary iron intake or serum iron biomarkers. We also reported the results for CHD incidence and CHD mortality separately. If 1 study reported results from both men and women, we treated them as separate cohorts. All procedures, including literature search, study selection, and data extraction, were performed independently by 2 reviewers (J.H. and P.X.). Discrepancies were resolved through group discussion.
Statistical analysis.
We pooled RR estimates separately for each exposure and CHD incidence or mortality combination by using a random-effects or fixed-effects model as appropriate. We evaluated the statistical heterogeneity of the RRs by the Cochran Q test and I2 statistic. Publication bias was generally assessed by using the Egger’s (when the numbers of studies pooled were ≥3) or Begg’s (when the numbers of studies pooled were <3) asymmetry tests. The average of follow-up years was calculated as the sum of person-years divided by total numbers of individuals. If unavailable, the person-years were estimated by multiplying the number of the individuals and the average (mean or median) of follow-up time. Stratified analyses by gender, region, or follow-up time were used to find potential effect modifiers. Sensitivity analyses evaluated whether the results were robust to a single study. All analyses were performed by using Stata statistical software (version 11.0; StataCorp).
Results
Characteristics of included studies.
The characteristics of 32 separate cohorts from 21 studies are shown in Table 1. In total, our meta-analysis comprised 292,454 participants with 12,721 incident cases during an average of 10.2 y of follow-up. There were 6 studies with 6 cohorts addressing the association of dietary iron intake with CHD incidence (80,162 individuals and 1663 cases) and 6 studies with 11 cohorts reporting results based on body iron store biomarkers and CHD incidence (23,389 individuals and 2596 cases). For CHD mortality, 4 studies with 5 cohorts focused on dietary iron intake (142,842 individuals and 4518 cases) and 9 studies with 16 cohorts reported results based on body iron stores (97,295 individuals and 4289 cases).
TABLE 1.
Characteristics of 21 prospective cohort studies included in the meta-analysis1
| Source, year, study, and l ocation | Men | Age at baseli ne2 | Duration of follow-up | No. of individuals/person-years | Cases | Exposure | Incidence or mortality | Outcome |
| % | y | y | n | |||||
| de Oliveira Otto et al. (18), 2012, MESA, USA | 47.3 | 61.8 ± 10.3 | 6.2 | 5285/32,767 | 279 | Dietary heme- and nonheme-iron intake | Incidence | CVD |
| Galan et al. (8), 2006, SU.VI.MAX, France | 32.5 | 48.6 ± 6.5 (35–60) | 7.5 (median) | 9917/74,774 | 187 | Serum ferritin | Incidence | IHD |
| Liao et al. (35), 1994, NHANES I, USA | 43.6 | 58.5 ± 11.0 (40–74) | 13 (0–16) | 4237/55,081 | 1151 | Serum iron, TIBC. and transferrin saturation | Incidence | CHD |
| Sempos et al. (34), 1994, NHANES I and NHEFS, USA | 44.8 | 61 (25–74) | 14.6 | 4518/65,962.8 | 842 | Transferrin saturation | Incidence | CHD |
| Gartside and Glueck (33), 1995, NHANES I, USA | NA (both genders) | 44.4 ± 16.5 (20–72) | 10 | 8251/NA | 492 | Dietary iron | Incidence | CHD |
| Fox et al. (19), 2002, Bussleton Health Study, Australia | 52.2 | 50 ± 13 (20–79) | 4 | 2031/NA | 235 | Serum ferritin and transferrin saturation | Incidence | CHD |
| van der A et al. (16), 2005, Prospect-EPIC, Netherlands | 0 | 56 (52–62) | 4.3 (median) | 16,136/69,600 | 252 | Dietary heme-, nonheme-, and total iron intake | Incidence | CHD |
| Salonen et al. (6), 1992, KIHDRFS, Finland | 100.0 | ≥42 | 3 | 1931/5793 | 51 | Serum ferritin | Incidence | MI |
| Ascherio et al. (36), 1994, HPFS, USA | 100 | 40–75 | 4 | 44,933/157,010 | 249 | Dietary heme- and total iron intake | Incidence | MI |
| Klipstein-Grobusch et al. (5), 1999, Rotterdam Study, Netherlands | NA (both genders) | ≥55 | 4 (3–7) | 4802/19,208 | 124 | Dietary heme- and total iron intake | Incidence | MI |
| Marniemi et al. (11), 2005, NA, Finland | 47.81 | 65–99 | 10 | 755/NA | 130 | Dietary iron intake and serum iron | Incidence | MI |
| Sempos et al. (34), 1994, NHANES I and NHEFS, USA | 44.8 | 61 (25–74) | 14.6 | 744/10,862.4 | 164 | Serum transferrin saturation | Mortality | CVD |
| Marniemi et al. (31), 1998, NA, Finland | 52.9 | ≥65 | 13 | 344/NA | 142 | Serum iron, ferritin, and transferrin saturation | Mortality | CVD |
| Wells et al. (23), 2004, NH2MS, USA | 45.7 | 30–75 | >12.83 | 3410/43,761 | NA | Serum transferrin saturation | Mortality | CVD |
| Lee et al. (20), 2005, IWHS, USA | 0 | 61.5 (55–69) | 15 | 34,492/NA | 1767 | Dietary heme- and nonheme-iron intake | Mortality | CVD |
| Kim et al. (22), 2012, NHANES III, USA | 50.8 | 67.1 | 15.5 | 5695/NA | 1361 | Serum ferritin and transferrin saturation | Mortality | CVD |
| van Asperen et al. (32), 1995, NA, Netherlands | 49.6 | 70.7 ± 4.9 (64–87) | 17 | 260/2845 | 50 | Serum transferrin saturation and TIBC | Mortality | IHD |
| Morkedal et al. (21), 2011, HUNT 2 study, Norway | 46.0 | 49.5 ± 16.5 | 11.4 | 60,798/608,748 | 1034 | Serum TIBC | Mortality | IHD |
| Ascherio et al. (36), 1994, HPFS, USA | 100 | 40–75 | 4 | 44,933/157,010 | 137 | Dietary heme- and total iron intake | Mortality | CHD |
| Reunanen et al. (38), 1995, NA, Finland | 49.9 | 45–64 | 13.8 | 12,188/68,194.4 | 984 | Serum iron, TIBC, and transferrin saturation | Mortality | CHD |
| Corti et al. (10), 1997, EPESE, USA | 35.2 | 78.8 (≥71) | 4.4 (median) | 3936/16,250 | 209 (CAD) | Serum iron | Mortality | CHD |
| Zhang et al. (9), 2012, JACC Study, Japan | 39.4 | 56.1 | 14.7 (median) | 58,615/859,450 | 557 | Dietary heme-, nonheme-, and total iron intake | Mortality | CHD |
| Klipstein-Grobusch et al. (5), 1999, Rotterdam Study, Netherlands | NA (both genders) | ≥55 | 4 | 4802/19,208 | 30 | Dietary heme and total iron intake | Mortality | MI |
| Morrison et al. (7), 1994, Nutrition Canada Survey, Canada | 42.7 | 35–79 | 10.2 | 9920/103,142 | 224 | Serum iron | Mortality | MI |
CAD, coronary artery disease; CHD, coronary heart disease; CVD, cardiovascular disease; EPESE, Established Populations for Epidemiologic Studies of the Elderly; EPIC, European Prospective Investigation into Cancer and Nutrition; HPFS, Health Professionals Follow-Up Study; HUNT, Nord-Trøndelag Health Study; IHD, ischemic heart disease; IWHS, Iowa Women’s Health Study; JACC, Japan Collaborative Cohort; KIHDRFS, Kuopio Ischemic Heart Disease Risk Factor Survey; MESA, Multi-Ethnic Study of Atherosclerosis; MI, myocardial infarction; NA, not available; NH2MS, NHANES II Mortality Study; NHEFS, NHANES I Epidemiologic Follow-up Study; SU.VI.MAX, Supplementation en Vitamines et Mineraux Antioxydants (Supplementation in Vitamin and Mineral Antioxidants); TIBC, total iron-binding capacity.
Values are means ± SDs (ranges of age).
Among the 21 studies included, 10 were conducted in North America, 9 in Europe, 1 in Australia, and 1 in Asia. Detailed information can be found in Supplemental Table 1.
Association of dietary iron intake with incidence of CHD.
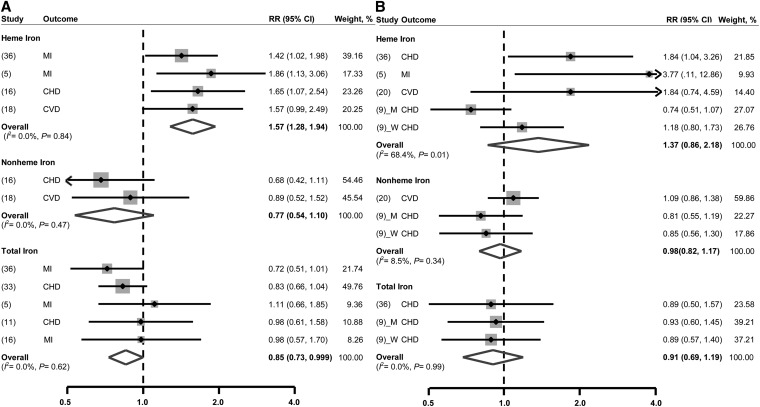
As seen in Fig. 2, the combined RR for CHD risk comparing the highest and lowest total iron intake groups was 0.85 (95% CI: 0.73, 0.999) with no heterogeneity between studies (I2 = 0%, P = 0.62). Egger’s test provided no statistical evidence of publication bias (P = 0.40).
FIGURE 2.
Multivariable-adjusted RRs and 95% CIs of risk of CHD incidence (A) and mortality (B) comparing the highest with the lowest quantile of dietary iron intake. The pooled estimates were obtained by using a fixed-effects or random-effects model depending on the heterogeneity test. The dots indicate the adjusted RRs. The size of the shaded squares is proportional to the percentage weight of each study. Horizontal lines represent the 95% CIs, and the diamonds represent the pooled RRs and 95% CIs. CHD, coronary heart disease; CVD, cardiovascular disease; M, men; MI, myocardial infarction; W, women.
When subtypes of dietary iron were considered, heme-iron intake was found to increase the risk of CHD by 57% (RR: 1.57; 95% CI: 1.28, 1.94), whereas no association was observed for nonheme-iron intake (RR: 0.77; 95% CI: 0.54, 1.10). No heterogeneity was found among studies for both iron subtypes, and there was no statistical evidence of publication bias (Egger’s test, P = 0.08 for heme-iron intake; Begg’s test, P = 0.32 for nonheme-iron intake).
Association of dietary iron intake with CHD mortality.
When CHD mortality was the outcome of interest, all measures of dietary iron intake, i.e., total, heme, and nonheme, were found to be nonsignificantly related to this outcome [pooled RRs (95% CI): 0.91 (0.69, 1.19), 1.37 (0.86, 2.18), and 0.98 (0.82, 1.17), respectively, comparing the highest with lowest levels of exposure of interest] (see Fig. 2). There was a significant medium heterogeneity between studies based on heme-iron intake (I2 = 68.4%, P = 0.01) and no heterogeneity for nonheme- and total iron intake. No evidence of publication bias was found for each pooling.
Association of serum iron stores with incidence of CHD.
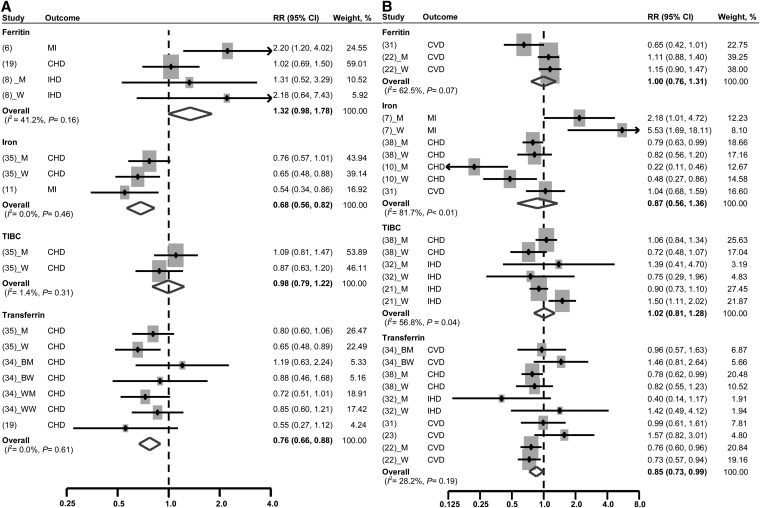
As seen in Fig. 3, the pooled RR for CHD risk, when comparing the highest with lowest serum ferritin concentrations, was 1.32 (95% CI: 0.98, 1.78) with a nonsignificant heterogeneity between studies (I2 = 41.2%, P = 0.16). No statistical evidence of publication bias was found (Egger’s test, P = 0.39).
FIGURE 3.
Multivariable-adjusted RRs and 95% CIs of risk of CHD incidence (A) and mortality (B) by comparing the highest with the lowest quantile of serum iron biomarkers. The pooled estimates were obtained by using a fixed-effects or random-effects model depending on the heterogeneity test. The dots indicate the adjusted RRs. The size of the shaded squares is proportional to the percentage weight of each study. Horizontal lines represent the 95% CIs, and the diamonds represent the pooled RRs and 95% CIs. BM, black men; BW, black women; CHD, coronary heart disease; CVD, cardiovascular disease; IHD, ischemic heart disease; M, men; MI, myocardial infarction; TIBC, total iron-binding capacity; W, women; WM, white men; WW, white women.
This marginally positive association disappeared when we used TIBC (RR: 0.98; 95% CI: 0.79, 1.22). Among studies using serum iron as a marker, the pooled association became inverse (RR: 0.68; 95% CI: 0.58, 0.82), and a similar inverse association was found between transferrin saturation and the risk of CHD (RR: 0.76; 95% CI: 0.66, 0.88). No heterogeneity between studies was found within each pooling, and there was no evidence of publication bias (data not shown).
Association of serum iron stores and CHD mortality.
The pooled RR for CHD mortality comparing the highest and lowest concentrations of serum ferritin was 1.00 (95% CI: 0.76, 1.31), without significant heterogeneity between studies (I2 = 62.5%, P = 0.07). No publication bias was found (Egger’s test, P = 0.12). When based on serum iron, the pooled RR was 0.87 (95% CI: 0.56, 1.36), with high heterogeneity between studies (I2 = 81.7%, P < 0.01) and without publication bias (Egger’s test, P = 0.67). TIBC was also not associated with CHD mortality (RR: 1.02; 95% CI: 0.81, 1.28). There was medium heterogeneity between studies (I2 = 56.8%, P = 0.04) but no publication bias (Egger’s test, P = 0.995). Alternatively, transferrin saturation was found to have an inverse association with CHD mortality, with a pooled RR of 0.85 (95% CI: 0.73, 0.99). There was no heterogeneity between studies (I2 = 28.2%, P = 0.19) and no publication bias (Egger’s test, P = 0.10).
Effect modification.
Stratified analyses showed that the associations observed were not substantially modified by gender, region (United States vs. other countries), or follow-up time except for when analyzing the relation between heme iron and CHD mortality. Heme-iron intake was found to be significantly and positively associated with CHD mortality (RR: 1.84; 95% CI: 1.13, 2.99) in the United States although not in other countries (RR: 1.17; 95% CI: 0.64, 2.13).
Sensitivity analysis.
The findings were generally consistent when using a fixed-effects model instead of a random-effects model or vice versa. Omitting 1 study each time in each pooling did not materially change the results. When we excluded the studies using CVD as the outcome, the results were again materially unchanged (data not shown).
Discussion
In this quantitative meta-analysis of prospective cohort studies, we found that total iron intake was inversely associated with the incidence of CHD, whereas heme-iron intake was positively associated with incidence. When examining body iron stores, inverse associations were observed between serum iron and transferrin saturation and CHD incidence, whereas a modest positive relation was found between serum ferritin and incidence of CHD. For CHD mortality, only transferrin saturation was found to be inversely associated, whereas no association was found with all other dietary and body iron markers.
Our findings that heme iron increases the risk of CHD are in agreement with those from a recently published meta-analysis (39). However, the authors only reported the association for heme-iron intake and risk of CHD, whereas ours included heme-, nonheme-, and total iron intake and separated CHD incidence from CHD mortality. When examining iron biomarkers, the last meta-analysis on this topic published in 1999 found no association of total dietary iron intake, transferrin saturation, and serum iron with risk of CHD (15). Statistical power was substantially increased in the current study because we included 14 additional studies (5, 8–11, 16, 18–23, 31, 33).
The observed positive association between heme iron and risk of CHD may be explained by the high bioavailability of heme iron and its role as the primary source of iron in iron-replete participants (40). Heme iron is absorbed at a much greater rate in comparison to nonheme iron (37% vs. 5%, respectively) and is less affected by iron status than nonheme iron (28, 41). Once absorbed, it may contribute as a catalyst in the oxidation of LDLs, causing tissue-damaging inflammation (2, 3), which is a potential risk factor for CHD (42, 43).
Nonheme iron was found not to be associated with CHD incidence (P = 0.15). This was most likely due to lack of power, because only 2 studies reported the association between nonheme-iron intake and CHD incidence separately. Because the majority of iron consumed is nonheme iron, investigators may have considered total dietary iron intake as primarily reflecting the effects of nonheme iron and preferred not to report the results of nonheme iron separately due to the high correlation between nonheme- and total iron intake (36).
Total iron was found to be inversely associated with CHD incidence, which was difficult to understand because, theoretically, it was combined from a positive significant association (heme-iron and CHD incidence) and 1 inverse nonsignificant association (nonheme-iron and CHD incidence). One possible explanation is that the nonsignificant inverse association between nonheme-iron intake and CHD incidence was due to lack of power, because there were only 2 studies (16, 18) composed of 21,421 participants and 545 cases in comparison to 81,038 participants and 1643 cases from 5 studies (5, 11, 16, 33, 36) reporting on total iron intake.
We found a borderline positive and significant association between serum ferritin and CHD incidence (P = 0.07). Although the underlying mechanism is not clear, excess iron storage in the blood could result in the production of free radicals that aid in the oxidation of LDL cholesterol. Also, several epidemiologic studies have found significant and positive associations between heme-iron intake and serum ferritin concentrations, which might help to support our findings by considering the established positive relation of heme-iron intake and CHD risk (36, 44, 45). More studies are needed to further clarify the relation between serum ferritin and CHD risk.
We found that both serum iron and transferrin saturation were inversely associated with CHD risk. Because these biomarkers have wide diurnal variation and low intraclass correlation (46, 47), they may not be the best markers of body iron stores, which may explain the inverse relation. It is also possible that the proposed relation between iron stores and an inflammatory response is reversely causal, which means inflammation affects body iron stores. Inflammation has been associated with increased serum ferritin as well as decreased serum iron and transferrin saturation (48–51). None of the studies included evaluated markers of inflammation such as C-reactive protein to see how they affected the analysis. However, studies examining the relation between serum ferritin and atherosclerosis still found a positive association even after adjusting for C-reactive protein or fibrinogen concentrations (52, 53). This is a limitation, and further research is needed to explore the strength and directionality of the relation between iron and inflammation.
When CHD mortality was considered, all previous associations were attenuated regardless of dietary iron intake or serum iron biomarkers used as exposures, which could be due to an increased awareness of dietary risk factors and subsequent behavioral changes among those identified as being at risk of CHD (54). One exception occurred when the relation between heme iron and CHD mortality was examined while stratifying by region (United States vs. other countries), which found that there was an increased risk of CHD in relation to heme-iron intake in the United States but not in other countries. This difference by region may be due to differences in the range of normal consumption of heme iron. Heme-iron consumption in the Japanese cohorts was low, with a median of 0.44 mg/d (9) in their highest quantile, which was similar to the intakes in the reference group of the American studies (5, 20, 36). In addition, differences in association between heme iron and CHD mortality between the 2 regions could have been attributable to residual confounders such as access to health care, diet, and physical activity, all of which could have played a role in the lower rate of CHD morality in the Japanese cohort and attenuated the observed effects of heme iron (55).
A few strengths of this meta-analysis should be mentioned. First, this meta-analysis included more than 292,000 male and female participants and almost 13,000 cases, as well as both dietary iron intake and serum markers of iron as exposures. Second, most of the studies or cohorts included in this meta-analysis had relatively large sample sizes and long follow-up periods, which potentially increased the statistical power to investigate the relation between iron and CHD risk. In addition, this meta-analysis was based on prospective cohort studies, which largely reduced the potential selection and recall bias. Although randomized controlled trials would be best for assessing a causal relation, it would be difficult to conduct a long-term, double-blinded, randomized placebo-controlled trial on iron intake and risk of CHD.
Several limitations also need to be considered when interpreting our results. First, the inherent limitations of primary studies may have affected our findings. For example, the possibility of residual confounding or bias due to systematic measurement errors or unmeasured factors cannot be ruled out. Second, our inability to standardize iron intake in all of the included studies might have confounded our results, although the likelihood should be small. Third, publication bias due to unpublished data or publications in non-English journals could affect the results of meta-analyses, although no statistically significant evidence of this bias was detected. Finally, there were insufficient data to evaluate the potential modification effect of menopausal status on the relation between iron and CHD risk. Only 3 studies reported results for postmenopausal women specifically, and they could not be pooled due to different markers of iron exposure. Another 3 studies adjusted for menopausal status in their analyses, but these studies also used different markers for iron exposure. Nevertheless, results from these studies did not appreciably differ in a systematic manner from other studies that did not adjust for menopausal status.
In conclusion, data from this meta-analysis suggest that heme-iron intake is positively associated whereas total dietary iron is negatively associated with CHD incidence but not CHD mortality. When body iron stores were examined, serum iron and transferrin saturation were significantly and inversely related to the incidence of CHD. For mortality, the inverse association still remained for transferrin saturation. We should consider the adjustment for inflammation when studying iron and CHD. Future work is needed to elucidate the causal relation and the potential mechanism.
Supplementary Material
Acknowledgments
K.H. provided funding support; P.X. and K.H. provided the study concept and design; J.H. and P.X. completed the literature search, study selection, and data extraction; performed data analysis; and prepared the tables and figures; and all authors interpreted the results and critically reviewed the manuscript for important intellectual content. All authors read and approved the final version of the manuscript.
Footnotes
Abbreviations used: CHD, coronary heart disease; CVD, cardiovascular disease; TIBC, total iron-binding capacity.
Literature Cited
- 1.Sullivan JL. Iron and the sex difference in heart disease risk. Lancet. 1981;1:1293–4. [DOI] [PubMed] [Google Scholar]
- 2.Meyers DG. The iron hypothesis: does iron play a role in atherosclerosis? Transfusion. 2000;40:1023–9. [DOI] [PubMed] [Google Scholar]
- 3.McCord JM. Iron, free radicals, and oxidative injury. Semin Hematol. 1998;35:5–12. [PubMed] [Google Scholar]
- 4.Sullivan JL. Stored iron and vascular reactivity. Arterioscler Thromb Vasc Biol. 2005;25:1532–5. [DOI] [PubMed] [Google Scholar]
- 5.Klipstein-Grobusch K, Grobbee DE, Den Breeijen JH, Boeing H, Hofman A, Witteman JCM. Dietary iron and risk of myocardial infarction in the Rotterdam Study. Am J Epidemiol. 1999;149:421–8. [DOI] [PubMed] [Google Scholar]
- 6.Salonen JT, Nyyssonen K, Korpela H, Tuomilehto J, Seppanen R, Salonen R. High stored iron levels are associated with excess risk of myocardial infarction in Eastern Finnish men. Circulation. 1992;86:803–11. [DOI] [PubMed] [Google Scholar]
- 7.Morrison HI, Semenciw RM, Mao Y, Wigle DT. Serum iron and risk of fatal acute myocardial infarction. Epidemiology. 1994;5:243–6. [DOI] [PubMed] [Google Scholar]
- 8.Galan P, Noisette N, Estaquio C, Czernichow S, Mennen L, Renversez JC, Briancon S, Favier A, Hercberg S. Serum ferritin, cardiovascular risk factors and ischaemic heart diseases: a prospective analysis in the SU.VI.MAX (SUpplementation en VItamines et Mineraux AntioXydants) cohort. Public Health Nutr. 2006;9:70–4. [DOI] [PubMed] [Google Scholar]
- 9.Zhang W, Iso H, Ohira T, Date OC, Tanabe N, Kikuchi S, Tamakoshi A. Associations of dietary iron intake with mortality from cardiovascular disease: the JACC Study. J Epidemiol. 2012;22:484–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corti MC, Guralnik JM, Salive ME, Ferrucci L, Pahor M, Wallace RB, Hennekens CH. Serum iron level, coronary artery disease, and all-cause mortality in older men and women. Am J Cardiol. 1997;79:120–7. [DOI] [PubMed] [Google Scholar]
- 11.Marniemi J, Alanen E, Impivaara O, Seppanen R, Hakala P, Rajala T, Ronnemaa T. Dietary and serum vitamins and minerals as predictors of myocardial infarction and stroke in elderly subjects. Nutr Metab Cardiovasc Dis. 2005;15:188–97. [DOI] [PubMed] [Google Scholar]
- 12.Corti MC, Gaziano JM, Hennekens CH. Iron status and risk of cardiovascular disease. Ann Epidemiol. 1997;7:62–8. [DOI] [PubMed] [Google Scholar]
- 13.Sempos CT, Looker AC, Gillum RF. Iron and heart disease: the epidemiologic data. Nutr Rev. 1996;54:73–84. [DOI] [PubMed] [Google Scholar]
- 14.Muñoz-Bravo C, Gutiérrez-Bedmar M, Gómez-Aracena J, García-Rodríguez A, Navajas JF-C. Iron: protector or risk factor for cardiovascular disease? Still controversial. Nutrients. 2013;5:2384–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Danesh J, Appleby P. Coronary heart disease and iron status: meta-analyses of prospective studies. Circulation. 1999;99:852–4. [DOI] [PubMed] [Google Scholar]
- 16.van der A DL, Peeters PHM, Grobbee DE, Marx JJM, Van Der Schouw YT. Dietary haem iron and coronary heart disease in women. Eur Heart J. 2005;26:257–62. [DOI] [PubMed] [Google Scholar]
- 17.Qi L, van Dam RM, Rexrode K, Hu FB. Heme iron from diet as a risk factor for coronary heart disease in women with type 2 diabetes. Diabetes Care. 2007;30:101–6. [DOI] [PubMed] [Google Scholar]
- 18.de Oliveira Otto MC, Alonso A, Lee DH, Delclos GL, Bertoni AG, Jiang R, Lima JA, Symanski E, Jacobs DR, Jr, Nettleton JA. Dietary intakes of zinc and heme iron from red meat, but not from other sources, are associated with greater risk of metabolic syndrome and cardiovascular disease. J Nutr. 2012;142:526–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fox CJ, Cullen DJ, Knuiman MW, Cumpston GN, Divitini ML, Rossi E, Gochee PA, Powell LW, Olynyk JK. Effects of body iron stores and haemochromatosis genotypes on coronary heart disease outcomes in the Busselton Health Study. J Cardiovasc Risk. 2002;9:287–93. [DOI] [PubMed] [Google Scholar]
- 20.Lee DH, Folsom AR, Jacobs DR., Jr Iron, zinc, and alcohol consumption and mortality from cardiovascular diseases: the Iowa Women's Health Study. Am J Clin Nutr. 2005;81:787–91. [DOI] [PubMed] [Google Scholar]
- 21.Morkedal B, Laugsand LE, Romundstad PR, Vatten LJ. Mortality from ischaemic heart disease: sex-specific effects of transferrin saturation, serum iron, and total iron binding capacity. The HUNT Study. Working Groups on Epidemiology & Prevention and Cardiac Rehabilitation and Exercise Physiology. Eur J Cardiovasc Prev Rehabil. 2011;18:687–94. [DOI] [PubMed] [Google Scholar]
- 22.Kim KS, Son HG, Hong NS, Lee DH. Associations of serum ferritin and transferrin % saturation with all-cause, cancer, and cardiovascular disease mortality: Third National Health and Nutrition Examination Survey follow-up study. J Prev Med Public Health. 2012;45:196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wells BJ, Mainous AG, III, King DE, Gill JM, Carek PJ, Geesey ME. The combined effect of transferrin saturation and low density lipoprotein on mortality. Fam Med. 2004;36:324–9. [PubMed] [Google Scholar]
- 24.Casiglia E, Tikhonoff V, Bascelli A, Giordano N, Caffi S, Andreatta E, Mazza A, Boschetti G, Grasselli C, Saugo M, et al. Dietary iron intake and cardiovascular outcome in Italian women: 10-year follow-up. J Womens Health (Larchmt). 2011;20:1565–71. [DOI] [PubMed] [Google Scholar]
- 25.Menke A, Muntner P, Fernandez-Real JM, Guallar E. The association of biomarkers of iron status with mortality in US adults. Nutr Metab Cardiovasc Dis. 2012;22:734–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Friedrich N, Milman N, Volzke H, Linneberg A, Jorgensen T. Is serum ferritin within the reference range a risk predictor of cardiovascular disease? A population-based, long-term study comprising 2874 subjects. Br J Nutr. 2009;102:594–600. [DOI] [PubMed] [Google Scholar]
- 27.Sempos CT, Looker AC, Gillum RE, McGee DL, Vuong CV, Johnson CL. Serum ferritin and death from all causes and cardiovascular disease: the NHANES II Mortality Study. Ann Epidemiol. 2000;10:441–8. [DOI] [PubMed] [Google Scholar]
- 28.Cook JD. Adaptation in iron metabolism. Am J Clin Nutr. 1990;51:301–8. [DOI] [PubMed] [Google Scholar]
- 29.Anderson PGJ, Vulpe PCD. The cellular physiology of iron. In: Yehuda S, Mostofskyron DI, editors. Iron deficiency and overload. New York: Humana Press; 2010. p. 3–29. [Google Scholar]
- 30.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marniemi J, Jarvisalo J, Toikka T, Raiha I, Ahotupa M, Sourander L. Blood vitamins, mineral elements and inflammation markers as risk factors of vascular and non-vascular disease mortality in an elderly population. Int J Epidemiol. 1998;27:799–807. [DOI] [PubMed] [Google Scholar]
- 32.van Asperen IA, Feskens EJ, Bowles CH, Kromhout D. Body iron stores and mortality due to cancer and ischaemic heart disease: a 17-year follow-up study of elderly men and women. Int J Epidemiol. 1995;24:665–70. [DOI] [PubMed] [Google Scholar]
- 33.Gartside PS, Glueck CJ. The important role of modifiable dietary and behavioral characteristics in the causation and prevention of coronary heart disease hospitalization and mortality: the prospective NHANES I follow-up study. J Am Coll Nutr. 1995;14:71–9. [DOI] [PubMed] [Google Scholar]
- 34.Sempos CT, Looker AC, Gillum RF, Makuc DM. Body iron stores and the risk of coronary heart disease. N Engl J Med. 1994;330:1119–24. [DOI] [PubMed] [Google Scholar]
- 35.Liao Y, Cooper RS, McGee DL. Iron status and coronary heart disease: negative findings from the NHANES I epidemiologic follow-up study. Am J Epidemiol. 1994;139:704–12. [DOI] [PubMed] [Google Scholar]
- 36.Ascherio A, Willett WC, Rimm EB, Giovannucci EL, Stampfer MJ. Dietary iron intake and risk of coronary disease among men. Circulation. 1994;89:969–74. [DOI] [PubMed] [Google Scholar]
- 37.Magnusson MK, Sigfusson N, Sigvaldason H, Johannesson GM, Magnusson S, Thorgeirsson G. Low iron-binding capacity as a risk factor for myocardial infarction. Circulation. 1994;89:102–8. [DOI] [PubMed] [Google Scholar]
- 38.Reunanen A, Takkunen H, Knekt P, Seppanen R, Aroma A. Body iron stores, dietary iron intake and coronary heart disease mortality. J Intern Med. 1995;238:223–30. [DOI] [PubMed] [Google Scholar]
- 39.Yang W, Li B, Dong X, Zhang X-Q, Zeng Y, Zhou J-L, Tang Y-H, Xu J-J. Is heme iron intake associated with risk of coronary heart disease? A meta-analysis of prospective studies. Eur J Nutr. 2013; May 26 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 40.Hallberg L, Hultén L, Gramatkovski E. Iron absorption from the whole diet in men: how effective is the regulation of iron absorption? Am J Clin Nutr. 1997;66:347–56. [DOI] [PubMed] [Google Scholar]
- 41.Björn-Rasmussen E, Hallberg L, Isaksson B, Arvidsson B. Food iron absorption in man: applications of the two-pool extrinsic tag method to measure heme and nonheme iron absorption from the whole diet. J Clin Invest. 1974;53:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaptoge S, Di Angelantonio E, Lowe G, Pepys MB, Thompson SG, Collins R, Danesh J. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010;375:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Willerson JT, Ridker PM. Inflammation as a cardiovascular risk factor. Circulation. 2004;109:II-2–10. [DOI] [PubMed] [Google Scholar]
- 44.Liu JM, Hankinson SE, Stampfer MJ, Rifai N, Willett WC, Ma J. Body iron stores and their determinants in healthy postmenopausal US women. Am J Clin Nutr. 2003;78:1160–7. [DOI] [PubMed] [Google Scholar]
- 45.Fleming DJ, Jacques PF, Dallal GE, Tucker KL, Wilson PW, Wood RJ. Dietary determinants of iron stores in a free-living elderly population: the Framingham Heart Study. Am J Clin Nutr. 1998;67:722–33. [DOI] [PubMed] [Google Scholar]
- 46.Al-Delaimy WK, Jansen E, Peeters P, Van der Laan J, Van Noord P, Boshuizen H, Van der Schouw Y, Jenab M, Ferrari P, Bueno-de-Mesquita H. Reliability of biomarkers of iron status, blood lipids, oxidative stress, vitamin D, C-reactive protein and fructosamine in two Dutch cohorts. Biomarkers. 2006;11:370–82. [DOI] [PubMed] [Google Scholar]
- 47.Zimmermann MB, Hurrell RF. Nutritional iron deficiency. Lancet. 2007;370:511–20. [DOI] [PubMed] [Google Scholar]
- 48.Thurnham DI, McCabe GP. Influence of infection and inflammation on biomarkers of nutritional status with an emphasis on vitamin A and iron. In: World Health Organization (WHO). Report: Priorities in the assessment of vitamin A and iron status in populations, Panama City, Panama, 15-17 September 2010. Geneva: WHO; 2012. p. 63#x201380.
- 49.Lee D-H, Zacharski LR, Jacobs DR., Jr Comparison of the serum ferritin and percentage of transferrin saturation as exposure markers of iron-driven oxidative stress–related disease outcomes. Am Heart J. 2006;151:1247.e1–7. [DOI] [PubMed] [Google Scholar]
- 50.Thurnham DI, McCabe LD, Haldar S, Wieringa FT, Northrop-Clewes CA, McCabe GP. Adjusting plasma ferritin concentrations to remove the effects of subclinical inflammation in the assessment of iron deficiency: a meta-analysis. Am J Clin Nutr. 2010;92:546–55. [DOI] [PubMed] [Google Scholar]
- 51.Yip R, Dallman P. The roles of inflammation and iron deficiency as causes of anemia. Am J Clin Nutr. 1988;48:1295–300. [DOI] [PubMed] [Google Scholar]
- 52.Ahluwalia N, Genoux A, Ferrieres J, Perret B, Carayol M, Drouet L, Ruidavets JB. Iron status is associated with carotid atherosclerotic plaques in middle-aged adults. J Nutr. 2010;140:812–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kiechl S, Willeit J, Egger G, Poewe W, Oberhollenzer F. Body iron stores and the risk of carotid atherosclerosis: prospective results from the Bruneck study. Circulation. 1997;96:3300–7. [DOI] [PubMed] [Google Scholar]
- 54.Coleman M, Forman D, Bryant H, Butler J, Rachet B, Maringe C. Trends in cancer incidence, mortality, risk factors, and health behaviours in California. Sacramento (CA): Department of Public Health, Cancer Surveillance Section; 2010. [Google Scholar]
- 55.Levi F, Lucchini F, Negri E, La Vecchia C. Trends in mortality from cardiovascular and cerebrovascular diseases in Europe and other areas of the world. Heart. 2002;88:119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.