Abstract
Infant iron status at birth is influenced by maternal iron status during pregnancy; however, there are limited data on the extent to which maternal iron status is associated with infant iron status during exclusive breastfeeding. We evaluated how maternal and infant hemoglobin and iron status [soluble transferrin receptors (TfR) and ferritin] were related during exclusive breastfeeding in HIV-infected women and their infants. The Breastfeeding, Antiretrovirals, and Nutrition Study was a randomized controlled trial in Lilongwe, Malawi, in which HIV-infected women were assigned with a 2 × 3 factorial design to a lipid-based nutrient supplement (LNS), or no LNS, and maternal, infant, or no antiretroviral drug, and followed for 24 wk. Longitudinal models were used to relate postpartum maternal hemoglobin (n = 1926) to concurrently measured infant hemoglobin, adjusting for initial infant hemoglobin values. In a subsample, change in infant iron status (hemoglobin, log ferritin, log TfR) between 2 (n = 352) or 6 wk (n = 167) and 24 wk (n = 519) was regressed on corresponding change in the maternal indicator, adjusting for 2 or 6 wk values. A 1 g/L higher maternal hemoglobin at 12, 18, and 24 wk was associated with a 0.06 g/L (P = 0.01), 0.10 g/L (P < 0.001), and 0.06 g/L (P = 0.01), respectively, higher infant hemoglobin. In the subsample, a reduction in maternal log TfR and an increase in hemoglobin from initial measurement to 24 wk were associated with the same pattern in infant values (log TfR β = −0.18 mg/L, P < 0.001; hemoglobin β = 0.13 g/L, P = 0.01). Given the observed influence of maternal and initial infant values, optimizing maternal iron status in pregnancy and postpartum is important to protect infant iron status. This trial was registered at clinicaltrials.gov as NCT00164736.
Introduction
Iron deficiency is believed to be the most common nutrient deficiency in low-income countries (1) and is associated with impaired neurodevelopment and immune function (2–4). Given the iron endowment at birth, the predominant opinion has been that infant iron stores are sufficient for 6 mo during exclusive breastfeeding (5, 6). However, infants in resource-poor settings are prone to early depletion of iron stores, especially if maternal iron status was poor before or during pregnancy (7), they had shorter gestational age (7) or low birth weight (8, 9), they were male (8), or they had rapid growth from 2 to 6 mo of age (10, 11).
Whether maternal iron status is associated with infant iron status during breastfeeding, independently of infant iron stores at birth, is unclear. Understanding this relation is especially important in the context of HIV. HIV-infected women are at high risk of anemia during pregnancy and depleted iron stores postpartum (12, 13). In resource-poor settings, 6 mo of exclusive breastfeeding by HIV-infected women coupled with antiretroviral drug (ARV)13 prophylaxis to the mother or infant is recommended to promote child survival and prevent mother-to-child transmission of HIV if replacement feedings are not acceptable, feasible, affordable, sustainable, and safe (14). Together, impaired maternal iron status, ARV prophylaxis (15), and exclusive breastfeeding may result in early depletion of infant iron stores.
Our objective was to examine the relation between maternal and infant hemoglobin and iron status [ferritin and soluble transferrin receptors (TfR)] in participants of the Breastfeeding, Antiretrovirals, and Nutrition (BAN) Study and in a subsample of BAN Study participants with additional biomarker assays.
Methods
Study population.
Data are from the BAN Study, whose design (16) and primary intervention findings (17–20) have been reported elsewhere. The BAN Study was a randomized controlled trial conducted in Lilongwe, Malawi, from April 2004 to February 2010. Briefly, HIV-1-positive pregnant women (n = 3572) were recruited from 4 antenatal clinics and screened for initial eligibility criteria including: cluster of differentiation 4 count ≥ 250 cells/mm3, hemoglobin ≥ 70 g/L, and gestational age < 30 wk. As part of routine antenatal care, mothers received daily iron-folic acid supplementation containing 200 mg ferrous sulfate (40 mg elemental iron) and 0.25 mg folic acid. At delivery, eligible dyads (n = 2791) received peripartum single dose nevirapine and twice-daily zidovudine and lamivudine for 7 d. Within 36 h of delivery, dyads had to meet secondary eligibility criteria for random assignment (n = 2382). Of these dyads, 13 declined further participation (17).
Mother-infant dyads (n = 2369) were randomly assigned using a permuted-block method to 1 of 6 28-wk treatment arms according to a 2-arm nutritional and 3-arm antiretroviral factorial design. Half of the mothers received a daily lipid-based nutrient supplement [LNS (Nutriset)], which included 15 mg of elemental iron (Supplemental Table 1) (18). There was further randomization to maternal ARVs (a 3-drug, highly-active regimen), infant ARV (daily oral Nevirapine), or standard of care (17). On March 26, 2008, the data safety monitoring board halted enrollment in the no-ARV arms because there was evidence that HIV transmission through breast milk was higher in these groups (21). Mothers enrolled in these arms who were <21 wk postpartum (n = 67) were allowed to change to the maternal or infant drug regimen or remain in the control arm (n = 32) until 28 wk (21). Control mothers who switched to the maternal arm (n = 30) or infant arm (n = 5) received ARVs for an average of 37 ± 34 d and 27 ± 19 d, respectively. Intensive exclusive breastfeeding counseling was provided in accordance with WHO guidelines when the BAN Study was designed (16, 22): exclusive breastfeeding for 24 wk and weaning between 24 and 28 wk (23).
Informed consent was obtained from all mothers. The Malawi National Health Science Research Committee and the Institutional Review Boards at the University of North Carolina at Chapel Hill and the CDC approved the study, and the Institutional Review Board at the University of California, Davis, approved the laboratory analyses at the Western Human Nutrition Research Center.
Anthropometrics, study procedures, and laboratory analyses.
Participant visits were conducted at the BAN Study clinic at Bwaila Hospital in Lilongwe at screening, birth, and 1, 2, 4, 6, 8, 12, 18, 21, and 24 wk postpartum (17). Screening visits were conducted at mean 24 ± 5 wk gestation based on last menstrual period or fundal height (66% of sample). Because of missing gestational age (34% of sample), we also determined that the screening visit was conducted at mean 14 ± 6 wk before birth for the full analytic sample. Birth visits were conducted on the day of birth (27%), 1 d (65%), 2 d (7%), and 3–7 d (1%) following delivery. Maternal hemoglobin was measured at screening, and maternal and infant hemoglobin was analyzed from venous blood samples obtained at birth and 2, 6, 12, 18, and 24 wk using a Beckman Coulter AcT or AcT 5-part Differential Analyzer (Beckman Coulter). Infant HIV status was tested with Amplicor 1.5 DNA PCR (Roche Diagnostics). Plasma was separated from red blood cells, aliquoted to 1-mL plastic storage tubes, and stored at −70°C. Maternal and infant weights were measured with Tanita digital electronic scales. Maternal height was measured with a wall-mounted stadiometer and infant recumbent length with a wooden length board made to UNICEF specifications (24). Maternal report of parity, marital status, and education was obtained at the screening visit. At follow-up visits, maternal report of infant breastfeeding status was obtained and included questions regarding foods or liquids provided.
To identify subsample dyads (n = 533), samples were drawn from the LNS and no-LNS groups, excluding multiples (n = 49) and infants who became HIV-infected (n = 58), and prioritizing those with complete anthropometric and dietary data. The subsample size was powered to detect intervention effects. A sample size of 518 was required for 80% power to detect a 0.25 difference in mean infant ferritin at an α of 0.05. Initial assays were conducted with 2-wk plasma in infants with sufficient plasma at that time; otherwise, initial assays were conducted with 6-wk plasma. Subsequent assays used 24-wk plasma. TfR and markers of inflammation [C-reactive protein (CRP) and α-1-acid glycoprotein (AGP)] concentrations were measured using a Cobas Integra 400 (Roche Diagnostics). Ferritin was measured with the IRMA Ferritin Coat-a-Count radioimmunoassay (Siemens Health Care Diagnostics Inc.).
Statistical analysis.
This paper focuses on 2 groups of dyads: those with longitudinal hemoglobin data and the subsample selected for TfR, ferritin, CRP, and AGP analyses. Infants weaned before 24 wk (n = 277) were excluded from longitudinal analyses following exclusive breastfeeding cessation, defined as receiving anything to eat or drink other than breast milk.
Dyads in the longitudinal analysis (n = 1926) had measurements of infant birth hemoglobin, birth weight, and concurrent maternal and infant hemoglobin at subsequent visits. Characteristics of this sample were compared with those of randomly assigned mothers excluded from the analysis because of insufficient data (n = 443) using t tests for continuous normally distributed variables and nonparametric tests for skewed continuous variables. These tests were also used to compare 1) subsample dyads (n = 519) with excluded dyads (n = 1850), and 2) longitudinal analysis subsample dyads (n = 498) with other longitudinal analysis dyads (n = 1428). Twenty-one subsample dyads were not included in the longitudinal analysis due to missing birth hemoglobin data.
In the longitudinal sample, linear regression was used to evaluate the association between maternal anemia during pregnancy [hemoglobin < 110 g/L (3)] and infant birth hemoglobin, adjusting for birth weight. Longitudinal random-effects models were used to evaluate the association between maternal hemoglobin and concurrently measured infant hemoglobin from 2 to 24 wk, adjusting for infant birth hemoglobin, sex, birth weight, ARV arms, and rate of weight gain since preceding visit. Study visit was used to model time. Because the iron in a maternal LNS is metabolized by the mother and then “theoretically” reflected in her iron status values, and does not affect the iron concentration in breast milk, it therefore does not exert an independent effect on infant iron status. Thus, maternal LNS is not a confounder of this association and was not included in the models (25). Interactions of visits with rate of weight gain, infant birth hemoglobin, and ARV arms were evaluated using Wald tests for joint significance. No significant interactions were observed for the maternal ARV arm and visits [Wald χ2 (4) = 2.01, P = 0.73], thus the interaction terms were not retained. Because some control dyads switched treatment arms, a sensitivity analysis was conducted to evaluate whether exclusion of these dyads after treatment changes (n = 35) influenced the longitudinal model estimates.
Infection and inflammation reduce hemoglobin and increase ferritin concentrations (26). Although TfR is thought to be less sensitive to inflammation and infection than ferritin (26), associations were observed between TfR and CRP and/or AGP. Therefore, TfR, hemoglobin, and ferritin in the subsample were adjusted for infection and inflammation using methods proposed by Thurnham and colleagues (27, 28). Briefly, cut points defined elevated CRP (>5 mg/L) and AGP (>1 g/L) and stage of inflammation [healthy (normal CRP and AGP), incubation (elevated CRP), early convalescence (CRP and AGP elevated), and late convalescence (elevated AGP)] (28). Correction factors for each inflammation group were determined by dividing the median value of the healthy inflammation group by the median value of the other groups, and hemoglobin and indicators were multiplied by the group-specific correction factor. Plots and Shapiro-Wilk tests indicated that plasma ferritin, TfR, CRP, and AGP had a non-Gaussian distribution, so these variables were log transformed.
In the subsample, linear regression was used to evaluate the association between change in inflammation-adjusted maternal iron status (hemoglobin, ferritin, and TfR) and change in inflammation-adjusted infant iron status (hemoglobin, ferritin, or TfR) from 2 wk (n = 355) or 6 wk (n = 167) to 24 wk (n = 522), controlling for initial maternal and infant values, birth weight, sex, ARV arms, and whether the initial values were at 2 or 6 wk. A sensitivity analysis was conducted to evaluate how adjustment for infection and inflammation influenced model estimates by evaluating models with inflammation-uncorrected markers. Logistic regression was used to evaluate whether depleted maternal iron stores (ferritin < 15 μg/L) at 2 or 6 wk were associated with increased odds of depleted infant iron stores (ferritin < 30 μg/L) at 2 or 6 wk and 24 wk (<12 μg/L), adjusting for birth weight, ARV arm, timing of initial measurement, sex, and for 24-wk models, initial infant value.
STATA 12.0 (StataCorp) was used for all statistical analyses. An α of 0.10 was used for all statistical tests of interaction (29); an α of 0.05 was used for all other statistical tests.
Results
Compared to those excluded because of incomplete data, HIV infection, or multiple birth, mothers (n = 1926) in the longitudinal analysis were older, had a lower prevalence of low cluster of differentiation 4, had higher pregnancy hemoglobin and less anemia during pregnancy, and fewer were primiparous (Supplemental Table 2). Infants in the longitudinal analysis had higher birth weight and length, and were more likely to be in the ARV arm (Supplemental Table 2). Compared to randomly assigned mothers, subsample mothers were older, had lower BMI at delivery, and were less likely to be married (Supplemental Table 2). A smaller proportion of subsample infants had low birth weight, and a larger proportion of infants were in the ARV arm (Supplemental Table 2). Of the 1926 dyads in the longitudinal analysis, 498 were in the subsample. Compared to mothers only in the longitudinal analysis (n = 1498), subsample mothers (n = 498) were older, had lower BMI at delivery, and were less likely to be in the ARV arm. In addition, a smaller proportion of infants had low birth weight and a larger proportion received the ARV intervention (Supplemental Table 2).
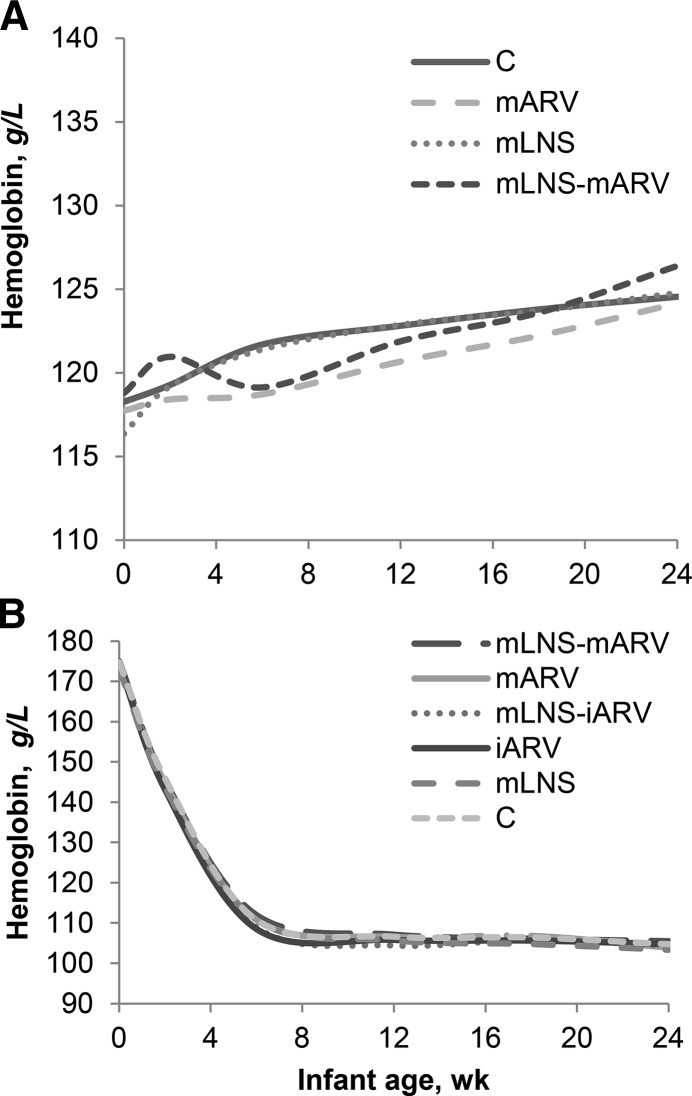
In general, mothers were young, multiparous, married, and had a low prevalence of under- or overweight at delivery, but more than half had anemia [hemoglobin < 110 g/L (3)] during pregnancy (Table 1). From delivery to 24 wk, maternal hemoglobin increased in the longitudinal sample (Figure 1) and prevalence of anemia decreased from 50% to 31%. In the subsample where we were able to adjust for inflammation, inflammation-adjusted maternal hemoglobin remained relatively stable from initial postpartum measurement to 24 wk, whereas maternal TfR declined (Table 2). Prevalence of depleted maternal iron stores (ferritin < 15 μg/L) declined from 2 to 24 wk, but in women measured at 6 wk, prevalence of depleted iron stores remained stable at 24 wk. Prevalence of tissue iron depletion (TfR > 8.3 mg/L) decreased from initial measurement to 24 wk.
TABLE 1.
Characteristics of BAN Study mother–infant dyads in each analysis sample1
| Longitudinal (n = 1926) | Subsample (n = 519) | |
| Mother | ||
| Age, y | 26 ± 5 | 27 ± 5 |
| Height, cm | 157 ± 6 | 157 ± 5 |
| BMI at 2 wk postpartum, kg/m2 | 24 ± 3 | 23 ± 3 |
| CD4+ count,2 cells/mm3 | 481 ± 198 | 483 ± 222 |
| Low CD4+ count,3 % | 28.6 | 30.1 |
| Hb,2 g/L | 108 ± 12 | 108 ± 12 |
| Anemia,24 % | 52.0 | 53.8 |
| Primiparous, % | 11.6 | 10.2 |
| Married, % | 92.3 | 90.2 |
| Education beyond primary school, % | 35.0 | 36.8 |
| Infant | ||
| Female, % | 49.3 | 47.6 |
| Birth weight, kg | 3.0 ± 0.4 | 3.1 ± 0.4 |
| Low birth weight,5 % | 6.2 | 4.6 |
| Birth length, cm | 48.2 ± 1.9 | 48.2 ± 2.0 |
| Hb at birth, g/L | 174 ± 20 | 173 ± 21 |
| Study intervention, % | ||
| mLNS-mARV | 17.7 | 16.8 |
| mARV | 17.6 | 15.8 |
| mLNS-iARV | 19.2 | 20.0 |
| iARV | 18.5 | 20.4 |
| mLNS | 13.2 | 14.5 |
| Control | 13.9 | 12.5 |
Values are means ± SDs or percentages. Although 533 mother–infant pairs were included in the subsample, 14 of these pairs were missing mother or infant inflammatory marker measurements and were not included in the analysis. BAN, Breastfeeding, Antiretroviral and Nutrition; CD4, cluster of differentiation 4; Hb, hemoglobin; iARV, infant antiretroviral drug; mARV, Maternal antiretroviral drug; mLNS, maternal lipid-based nutrient supplement; mLNS-iARV, maternal lipid-based nutrient supplement and infant antiretroviral drug; mLNS-mARV, maternal lipid-based nutrient supplement and maternal antiretroviral drug.
Measured during pregnancy at a mean of 24 ± 5 wk gestation for those with gestational age data or 14 ± 6 wk prior to delivery for the full sample.
Defined as <350 cells/mm3.
Defined as Hb <110 g/L (3).
Defined as <2500 g (7).
FIGURE 1.
Mean maternal Hb from birth to 24 wk (A) in the mLNS (n = 624), mLNS-mARV (n = 341), mARV (n = 338), and control (n = 623) arms of the longitudinal sample of BAN Study mother–infant dyads with at least 1 concurrent mother and infant Hb measurement. Mean infant Hb from birth to 24 wk (B) in the mLNS (n = 255), mLNS-mARV (n = 341), mARV (n = 338), iARV (n = 356), mLNS-iARV (n = 369), and control (n = 267) arms of the longitudinal sample with at least 1 concurrent mother and infant Hb measurement. BAN, Breastfeeding, Antiretrovirals, and Nutrition; C, control; Hb, hemoglobin; iARV, infant antiretroviral drug; LNS, lipid-based nutrient supplement; mARV, maternal antiretroviral drug; mLNS, maternal lipid-based nutrient supplement; mLNS-iARV, maternal lipid-based nutrient supplement and infant antiretroviral drug; mLNS-mARV, maternal lipid-based nutrient supplement and maternal antiretroviral drug.
TABLE 2.
Inflammation-adjusted Hb and markers of iron status in the subsample BAN Study mother–infant dyads1
| Initial measurement2 |
|||
| 2 wk | 6 wk | 24 wk | |
| Mother | |||
| Hb, g/L | 125 ± 16 (337) | 122 ± 14 (159) | 126 ± 12 (496) |
| Adverse event3 (<100 g/L), % | 6.5 | 6.9 | 1.6 |
| Anemic (<120 g/L), % | 35.6 | 40.25 | 29.6 |
| Plasma ferritin, μg/L | 29.8 ± 37.7 (351) | 43.5 ± 64.1 (167) | 32.6 ± 43.7 (518) |
| Deficient (<15 μg/L), % | 40.2 | 31.7 | 33.8 |
| Plasma TfR, mg/L | 5.7 ± 2.8 (348) | 5.7 ± 3.5(165) | 4.9 ± 2.3 (513) |
| Elevated (>8.3 mg/L), % | 12.9 | 16.4 | 7.2 |
| Elevated inflammatory markers, % | |||
| CRP (>5 mg/L) | 42.9 | 22.2 | 16.4 |
| AGP (>1 g/L) | 77.0 | 46.7 | 33.0 |
| Infant | |||
| Hb, g/L | 143 ± 18 (337) | 111 ± 13 (159) | 105 ± 11 (496) |
| Adverse event,4 % | 23.4 | 10.6 | 70.0 |
| Anemic (<105 g/L), % | —5 | —5 | 51.6 |
| Plasma ferritin, μg/L | 461 ± 340 (351) | 315 ± 229 (167) | 42 ± 103 (518) |
| Deficient,6 % | 2.0 | 6.0 | 31.1 |
| PlasmaTfR, mg/L | 3.5 ± 1.9 (348) | 3.3 ± 1.9 (165) | 6.7 ± 3.4 (513) |
| Elevated (>8.3 mg/L), % | 1.7 | 3.0 | 22.2 |
| Elevated inflammatory markers, % | |||
| CRP (>5 mg/L) | 8.8 | 16.2 | 28.9 |
| AGP (>1 g/L) | 8.2 | 15.6 | 47.0 |
Values are means ± SDs (n) or percentages. Values were adjusted for inflammation using group-specific correction factors estimated from ratios of medians for the various iron indicators (27). AGP, plasma α1-acid glycoprotein; BAN, Breastfeeding, Antiretroviral and Nutrition; CRP, plasma C-reactive protein; Hb, hemoglobin; TfR, soluble transferrin receptor.
Initial values were obtained at 2 or 6 wk due to insufficient plasma availability at 2 wk.
Defined as greater than or equal to a mild adverse event as described in the DAIDS toxicity tables for severe adverse events (30).
Defined as greater than or equal to the mild adverse event cut points given in the DAIDS toxicity tables for severe adverse events (30): 1–21 d, Hb <130 g/L; 22–35 d, Hb <105 g/L; 36–56 d, Hb <94 g/L; ≥56 d, Hb <109 g/L.
Cut points for Hb are not available for infants <3 mo of age.
Ferritin <30 μg/L at 2–6 wk and <12 μg/L at 24 wk.
Few infants had low birth weight, and mean infant hemoglobin concentrations at birth were within normal reference ranges based on a sample of infants from the United States (25). In the longitudinal sample, mean infant hemoglobin decreased from birth to 24 wk (Figure 1) and from 12–24 wk, and prevalence of low infant hemoglobin [<105 g/L (6)] increased from 43% to 50%. Infant ferritin declined markedly from baseline to 24 wk, consistent with normalization of values after erythrocyte breakdown, whereas TfR increased (Table 2). Few infants had abnormal initial indicators, but by 24 wk many infants began to show signs of poor iron status.
In the longitudinal sample, maternal anemia during pregnancy [hemoglobin < 110 g/L (3)] was associated with lower infant hemoglobin at birth [β = −2.33 g/L (95% CI: −4.13, −0.54), P = 0.01], adjusting for birth weight. In the longitudinal random-effects model (Supplemental Table 3), significant interactions were observed between study visits and maternal hemoglobin [Wald χ2 (4) = 9.08, P = 0.06)] (Figure 2). The strongest association was observed at 18 wk, where a 1 g/L increase in maternal hemoglobin was associated with 0.1 g/L higher infant hemoglobin. Female sex, higher birth weight, and infant birth hemoglobin were associated with higher infant hemoglobin at all visits. Faster infant growth, after adjusting for birth weight, was associated with lower hemoglobin at 6 wk [β = −3.64 g/L (95% CI: −5.19, −2.09), P < 0.001], but not at subsequent visits. Compared to controls, maternal ARVs were not associated with infant hemoglobin, whereas infant ARVs were associated with lower hemoglobin at 6 and 12 wk, but not at later visits. In the sensitivity analysis, exclusion of control participants who switched to an ARV treatment arm increased the precision of the maternal hemoglobin estimates without changing the β coefficients, and did not markedly influence the ARV arm results.
FIGURE 2.
Estimated infant Hb coefficients (β ± 95% CI, g/L) for each age in the longitudinal sample of BAN Study mother–infant dyads at 6 (n = 1644), 12 (n = 1635), 18 (n = 1575), and 24 (n = 1329) wk. Predicted Hb values from a longitudinal random-effects model relating maternal Hb to infant Hb. Predicted values represent at each age the linear combination of the maternal Hb coefficient and the maternal Hb interactions with study visit. The model also contained infant birth weight, rate of weight gain since previous visit, infant Hb at birth, infant sex, and the mARV and iARV arms, which were compared with the control arm. Interactions between study visits included infant birth weight, rate of weight gain since previous visit, infant Hb at birth, infant sex, and iARV arm. Data from HIV-negative infants and their mothers with at least 1 concurrent mother and infant Hb measurement and birth Hb were included until 24 wk or cessation of exclusive breastfeeding: n = 341 in the mLNS-mARV arm; n = 338 in the mARV arm; n = 369 in the mLNS-iARV arm; n = 356 in the iARV arm; n = 255 in the mLNS arm; and n = 267 in the control arm. BAN, Breastfeeding, Antiretrovirals, and Nutrition; Hb, hemoglobin; iARV, infant antiretroviral drug; mARV, maternal antiretroviral drug; mLNS, maternal lipid-based nutrient supplement; mLNS-iARV, maternal lipid-based nutrient supplement and infant antiretroviral drug; mLNS-mARV, maternal lipid-based nutrient supplement and maternal antiretroviral drug.
In the subsample, change in inflammation-adjusted maternal TfR and hemoglobin, but not ferritin, was associated with change in infant values (Table 3). An increase in maternal TfR was associated with an increase in infant TfR from 2 or 6 to 24 wk, suggesting that worsening maternal iron status was related to worsening infant iron status. In all models, initial infant values were strong predictors of change in iron markers; higher values initially were associated with a smaller change in values from 2 or 6 to 24 wk. Maternal and infant ARV regimens were not associated with infant TfR, hemoglobin, or ferritin. Models unadjusted for inflammation depicted similar associations, but there was a strengthening of the sex association in the unadjusted hemoglobin models compared with the inflammation-adjusted model. Although associations were observed between some continuous maternal and infant markers, no associations were observed between depleted maternal iron stores at 2 or 6 wk (ferritin < 15 μg/L) and risk of depleted infant iron stores at either 2 or 6 and 24 wk (2 or 6 wk: ferritin < 30 μg/L; 12 wk: < 12 μg/L at 24 wk) (2 or 6 wk: OR = 2.06; 95% CI: 0.64, 6.59; P = 0.22; 24 wk: OR = 0.99; 95% CI: 0.65, 1.50; P = 0.95).
TABLE 3.
Linear regression models of the association between change in maternal iron status and change in infant iron status from 2 or 6 wk to 24 wk in the subsample of BAN Study mother–infant dyads1
| Log plasma TfR (n = 513) |
Hb (g/L; n = 496) |
Log plasma ferritin (n = 518) |
||||
| β (95% CI) | P | β (95% CI) | P | β (95% CI) | P | |
| Inflammation-adjusted | ||||||
| Change in maternal value | 0.18 (0.06, 0.29) | <0.001 | 0.13 (0.03, 0.23) | 0.01 | 0.06 (−0.06, 0.17) | 0.34 |
| Infant initial value | −0.66 (−0.72, −0.60) | <0.001 | −0.89 (−0.94, −0.83) | <0.001 | −0.92 (−1.01, −0.83) | <0.001 |
| Maternal initial value | 0.23 (0.14, 0.33) | <0.001 | 0.10 (0.02, 0.18) | 0.02 | 0.03 (−0.08, 0.14) | 0.61 |
| Infant birth weight | −0.23 (−0.32, −0.14) | <0.001 | 4.58 (2.17, 7.00) | <0.001 | 0.34 (0.13, 0.56) | <0.001 |
| Maternal ARV intervention | −0.01 (−0.10, −0.09) | 0.85 | 1.01 (−1.41, 3.43) | 0.41 | 0.14 (−0.08, 0.35) | 0.22 |
| Infant ARV intervention | 0.03 (−0.05, 0.12) | 0.43 | −1.14 (−3.47, 1.19) | 0.34 | −0.10 (−0.30, 0.11) | 0.35 |
| Initial measure at 6 wk | 0.21 (0.13, 0.28) | <0.001 | 3.19 (0.49, 5.89) | 0.02 | 0.13 (−0.06, 0.31) | 0.18 |
| Female gender | −0.19 (−0.26, −0.12) | <0.001 | 1.52 (−0.36, 3.41) | 0.11 | 0.45 (0.28, 0.62) | <0.001 |
| Inflammation-unadjusted | ||||||
| Change in maternal value | 0.17 (0.06, 0.29) | 0.004 | 0.15 (0.04, 0.25) | 0.01 | 0.07 (−0.04, 0.18) | 0.23 |
| Infant initial value | −0.65 (−0.71, −0.58) | <0.001 | −0.88 (−0.94, 0.83) | <0.001 | −0.89 (−0.98, −0.81) | <0.001 |
| Maternal initial value | 0.24 (0.14, 0.33) | <0.001 | 0.09 (0.01, 0.17) | 0.03 | 0.04 (−0.07, 0.15) | 0.46 |
| Infant birth weight | −0.23 (−0.33, −0.14) | <0.001 | 4.33 (1.93, 6.74) | <0.001 | 0.35 (0.13, 0.56) | 0.002 |
| Maternal ARV intervention | −0.01 (−0.11, 0.08) | 0.81 | 1.28 (−1.14, 3.69) | 0.30 | 0.14 (−0.07, 0.36) | 0.19 |
| Infant ARV intervention | 0.04 (−0.05, 0.13) | 0.41 | −1.15 (−3.47, 1.17) | 0.33 | −0.10 (−0.30, 0.10) | 0.33 |
| Initial measure at 6 wk | 0.19 (0.11, 0.27) | <0.001 | 3.50 (0.84, 6.17) | 0.01 | 0.14 (−0.04, 0.32) | 0.13 |
| Female gender | −0.20 (−0.27, −0.12) | <0.001 | 4.12 (2.24, 6.00) | <0.001 | 0.41 (−0.24, 0.58) | <0.001 |
The LNS arm was not included in the model to isolate the effects of maternal iron status independent of the study supplement. The maternal ARV and infant ARV intervention groups were compared with the groups that received no ARV intervention. Mother and infant values were adjusted for inflammation using group-specific correction factors estimated from ratios of medians for the various iron indicators (28). ARV, antiretroviral drug; BAN, Breastfeeding, Antiretroviral and Nutrition; Hb, hemoglobin; LNS, lipid-based nutrient supplement; TfR, soluble transferrin receptor.
Discussion
In our study of HIV-infected Malawian mothers and their infants, maternal hemoglobin and TfR were associated with infant values during exclusive breastfeeding. Maternal iron status during pregnancy was associated with subsequent infant iron status, as described by others (7, 31–33); more than half of the mothers in the longitudinal sample had mild anemia during pregnancy, which was associated with lower infant hemoglobin at birth. Few studies, however, have investigated an association between maternal iron status during lactation and infant iron status postpartum in exclusively breastfed infants, taking into account the mother’s initial influence on infant iron status.
Initial maternal iron concentrations at birth and at 2 or 6 wk indicate that despite reported provision of antenatal iron supplementation, iron stores were depleted in many women during pregnancy, predisposing them and their infants (via fetal iron acquisition during pregnancy) to poor iron status. Although many women had impaired iron status, initial infant hemoglobin and iron concentrations at birth and/or infant iron stores at 2 or 6 wk were adequate; mean infant hemoglobin was within normal levels and few infants had depleted iron stores or tissue iron depletion. Between birth and 24 wk, infant iron indicators changed dramatically. During this period, senescent fetal hemoglobin lyses, erythropoiesis slows, and vascular volume increases, leading to a decrease in hemoglobin. In addition, there is a small increase in ferritin and subsequent decline thereafter as stores are used (10). Although few infants were deficient initially, 24-wk values indicate worsening iron status, suggesting that infant stores at birth were insufficient for this period and that breast milk iron concentrations may have been inadequate to supply the iron needs of the infant.
In the longitudinal model, higher maternal hemoglobin was associated with higher infant hemoglobin at 12, 18, and 24 wk. Similarly, in the subsample, after adjustment for inflammation, an increase in inflammation-adjusted maternal hemoglobin from 2 or 6 to 24 wk was associated with an increase in infant hemoglobin. We are unaware of a study sample similar to ours with concurrent maternal and infant hemoglobin concentrations measured longitudinally. However, a previous study found that maternal hemoglobin at delivery was positively linearly associated with newborn total body iron and hemoglobin in HIV-positive and HIV-negative Zimbabwean dyads (6). Because we controlled for initial hemoglobin, our findings suggest that this association may extend postpartum. Larger increases in maternal TfR also were associated with larger increases in infant TfR during breastfeeding, suggesting that worsening maternal iron status contributed to worsening infant status. Maternal iron stores must be depleted for tissue iron depletion to manifest as increased TfR concentrations; thus, our observed association between maternal and infant TfR suggests that mothers with severely impaired iron status may have impaired breast milk iron concentrations or some other unmeasured factor, or that controlling for initial infant hemoglobin does not adequately account for their initial iron stores.
We did not observe an association between maternal and infant ferritin, which is similar to previous reports where maternal ferritin was measured in late pregnancy. In 26 term infants, maternal ferritin measured prior to delivery was not associated with infant ferritin at 1.5 mo postpartum (33). A small positive correlation (r = 0.07, P < 0.0001) between maternal serum, measured ≥37 wk gestation, and cord blood ferritin was observed in Chinese mothers and their neonates (n = 3247); however, this was attributed to a large sample size and may not be comparable to our study, as we have postpartum plasma infant ferritin measurements (35). We are unaware of other studies reporting associations between postpartum maternal and infant ferritin. Given the underlying mechanisms to protect and maximize iron transfer to the fetus even with the presence of maternal anemia (evident from high infant ferritin concentrations at 2 or 6 wk) (11), coupled with the many factors that influence infant iron endowment at birth, including gestational age (7) and timing of cord clamping (36), as well as senescent fetal hemoglobin lysis (10), the lack of an association between maternal and infant ferritin is not surprising. This is further supported by the absence of an association between the maternal ferritin measurement at 2 or 6 wk postpartum and change in infant ferritin from initial measure to 24 wk in the regression model.
This research has some limitations. We do not know whether all mothers received and took iron supplements during pregnancy, but by controlling for the mother’s initial measurement this may not have biased our findings. Maternal and infant hemoglobin measurements at birth were conducted on average about 1 d postpartum. Because delivery is an inflammatory process (37), it is possible that infant hemoglobin concentrations were lowered at this time; but an association between inflammation at delivery and infant hemoglobin concentrations has not been reported. Infant hemoglobin values at birth are included in the longitudinal model; therefore, the inflammatory processes at delivery possibly could have attenuated the association between birth hemoglobin and later concentrations. Furthermore, we do not know if the observed maternal and infant hemoglobin associations are due to some other unmeasured factor or are residual effects from pregnancy. Because of insufficient plasma availability at 2 wk, we have initial iron indicators at 2 times in the subsample. During this period, infant iron status, particularly hemoglobin, changes dramatically; thus, we accounted for timing of measurement using a dummy variable. Selection bias may have influenced our findings. In the longitudinal sample, we excluded about 19% of randomly assigned dyads who lacked the measurements required for inclusion in the analysis. Compared with the randomly assigned dyads in the longitudinal sample, worse-off infants and mothers were lost to follow-up or excluded. Similarly, subsample dyads were healthier than randomly assigned infants. Therefore, we may have underestimated the observed relations between maternal and infant iron markers. Furthermore, the longitudinal sample size was smaller at 24 wk due to attrition and weaning, therefore 24 wk effects may have been attenuated. Finally, we also do not know whether the method we used to correct iron indicators for inflammation (27) is valid from 2 to 24 wk; however, the corrections did not have much influence on the interpretation of the data, which may indicate that this correction may not be indicated in this period.
This study also has many strengths. Importantly, detailed exclusive breastfeeding reports allowed for the exclusion of infants who potentially received other sources of iron in complementary foods, strengthening our findings. This is the first study to characterize the relation between maternal and infant iron status using multiple iron indicators during exclusive breastfeeding. Understanding these relations is especially important in the context of HIV to guide future interventions and programs to promote maternal and infant health. We focused on the early postpartum period, a period where infant iron status is not well characterized, which improved our understanding of hematologic dynamics during this time.
Because we controlled for the infant’s iron status at birth, these results might be explained by an effect of breast milk iron because breast milk was the sole source of nutrition for infants at this time. Previous studies evaluating the association between maternal iron status during lactation and breast milk iron content have been inconsistent (38–42). Several studies have shown that maternal hemoglobin and serum iron concentrations are related to breast milk iron concentrations, especially in anemic women or women with impaired iron status markers (41–43); however, whether breast milk iron concentrations subsequently are associated with infant iron status is still equivocal (43). Further research to understand how iron status markers relate to breast milk iron concentrations is needed, particularly in light of the high prevalence of infant anemia at 6 mo in this population.
Although poor maternal iron status and exclusive breastfeeding are likely to occur in other populations, we do not know if our findings are generalizable to other settings in HIV-infected and uninfected populations with varying concentrations of maternal iron deficiency or anemia. Because of study inclusion criteria, BAN Study mothers were healthier than other women in Malawi and low birth weight infants (<2 kg) were excluded. Furthermore, dyads were provided high-quality health care for the duration of the study. As such, the association between changes in maternal and infant iron status among dyads with very poor maternal iron status and low infant birth weight is unknown. However, given the observed association between maternal and infant hemoglobin and TfR, and the strong sustained effects of initial infant iron markers on later status, optimizing maternal iron status in pregnancy and postpartum is important for reducing risk of iron depletion in infants.
Supplementary Material
Acknowledgments
We thank the UNC project team in Lilongwe, including: Yusuf Ahmed, Mounir Ait-Khaled, Sandra Albrecht, Shrikant Bangdiwala, Ronald Bayer, Brian Bramson, Emily Bobrow, Nicola Boyle, Sal Butera, Charity Chavula, Joseph Chimerang’ambe, Maggie Chigwenembe, Maria Chikasema, Norah Chikhungu, David Chilongozi, Grace Chiudzu, Lenesi Chome, Anne Cole, Amanda Corbett, Amy Corneli, Anna Dow, Ann Duerr, Henry Eliya, Sascha Ellington, Joseph Eron, Sherry Farr, Yvonne Owens Ferguson, Susan Fiscus, Valerie Flax, Ali Fokar, Shannon Galvin, Laura Guay, Chad Heilig, Irving Hoffman, Elizabeth Hooten, Mina Hosseinipour, Michael Hudgens, Stacy Hurst, Lisa Hyde, George Joaki (deceased), David Jones, Elizabeth Jordan-Bell, Esmie Kamanga, Gift Kamanga, Coxcilly Kampani, Portia Kamthunzi, Deborah Kamwendo, Cecilia Kanyama, Angela Kashuba, Damson Kathyola, Peter Kazembe, Caroline C. King, Rodney Knight, Robert Krysiak, Jacob Kumwenda, Hana Lee, Edde Loeliger, Dustin Long, Misheck Luhanga, Victor Madhlopa, Maganizo Majawa, Alice Maida, Cheryl Marcus, Francis Martinson, Navdeep Thoofer, Chrissie Matiki (deceased), Douglas Mayers, Isabel Mayuni, Marita McDonough, Joyce Meme, Ceppie Merry, Khama Mita, Chimwemwe Mkomawanthu, Gertrude Mndala, Ibrahim Mndala, Agnes Moses, Albans Msika, Wezi Msungama, Beatrice Mtimuni, Jane Muita, Noel Mumba, Bonface Musis, Charles Mwansambo, Gerald Mwapasa, Jacqueline Nkhoma, Megan Parker, Richard Pendame, Ellen Piwoz, Byron Raines, Zane Ramdas, John Rublein, Mairin Ryan, Ian Sanne, Christopher Sellers, Diane Shugars, Dorothy Sichali, Wendy Snowden, Alice Soko, Allison Spensley, Jean-Marc Steens, Martin Tembo, Roshan Thomas, Hsiao-Chuan Tien, Beth Tohill, Esther Waalberg, Jeffrey Wiener, Cathy Wilfert, Patricia Wiyo, Innocent Zgambo, and Chifundo Zimba. We also thank Anna Maria Siega-Riz, Michael Hudgens, Catherine Zimmer, Valerie Flax, and Lindsay Jaacks for their input on the manuscript.
M.E.B., C.S.C., A.P.K., D.J.J., C.M.v.d.H., and L.S.A. contributed to the trial design; D.K., C.S.C., Z.K.K., and G.T. contributed to the data collection; L.H.A. supervised and S.S.-F. conducted biomarker assays for the subsample; E.M.W., E.J.D., and L.S.A. contributed to interpretation of data analysis; E.M.W. performed the data analysis, wrote the manuscript, and had primary responsibility for the final content; and M.E.B., L.S.A., L.H.A., and E.M.W. contributed to manuscript revisions. All authors read and approved the final version of the paper as well as contributed to data interpretation and the intellectual content of the manuscript.
Footnotes
Abbreviations used: AGP, α-1-acid glycoprotein; ARV, antiretroviral drug; BAN, Breastfeeding, Antiretrovirals, and Nutrition; CRP, C-reactive protein; LNS, lipid-based nutrient supplement; TfR, soluble transferrin receptor.
Literature Cited
- 1.World Health Organization. Worldwide prevalence of anemia, 1993–2005: WHO Global Database on Anemia. Geneva, Switzerland: World Health Organization Press; 2008. [Google Scholar]
- 2.Beard JL. Why iron deficiency is important in infant development. J Nutr. 2008;138:2534–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Iron deficiency anemia: assessment, prevention and control. Geneva, Switzerland: World Health Organization Press; 2001.
- 4.Stoltzfus RJ. Iron interventions for women and children in low-income countries. J Nutr. 2011;141:756S–62S. [DOI] [PubMed] [Google Scholar]
- 5.Dewey KG, Cohen RJ, Rivera LL, Brown KH. Effects of age of introduction of complementary foods on iron status of breast-fed infants in Honduras. Am J Clin Nutr. 1998;67:878–84. [DOI] [PubMed] [Google Scholar]
- 6.Miller MF, Stoltzfus RJ, Mbuya NV, Malaba LC, Iliff PJ, Humphrey JH, Group ZS. Total body iron in HIV-positive and HIV-negative Zimbabwean newborns strongly predicts anemia throughout infancy and is predicted by maternal hemoglobin concentration. J Nutr. 2003;133:3461–8. [DOI] [PubMed] [Google Scholar]
- 7.Dewey KG, Chaparro CM. Session 4: mineral metabolism and body composition iron status of breast-fed infants. Proc Nutr Soc. 2007;66:412–22. [DOI] [PubMed] [Google Scholar]
- 8.Yang Z, Lonnerdal B, Adu-Afarwuah S, Brown KH, Chaparro CM, Cohen RJ, Domellof M, Hernell O, Lartey A, Dewey KG. Prevalence and predictors of iron deficiency in fully breastfed infants at 6 mo of age: comparison of data from 6 studies. Am J Clin Nutr. 2009;89:1433–40. [DOI] [PubMed] [Google Scholar]
- 9.Eneroth H, Persson LA, El Arifeen S, Ekstrom EC. Infant anaemia is associated with infection, low birthweight and iron deficiency in rural Bangladesh. Acta Paediatr. 2011;100:220–5. [DOI] [PubMed] [Google Scholar]
- 10.Rao R, Georgieff MK. Iron in fetal and neonatal nutrition. Semin Fetal Neonatal Med. 2007;12:54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaparro CM. Setting the stage for child health and development: prevention of iron deficiency in early infancy. J Nutr. 2008;138:2529–33. [DOI] [PubMed] [Google Scholar]
- 12.Mehta S, Manji KP, Young AM, Brown ER, Chasela C, Taha TE, Read JS, Goldenberg RL, Fawzi WW. Nutritional indicators of adverse pregnancy outcomes and mother-to-child transmission of HIV among HIV-infected women. Am J Clin Nutr. 2008;87:1639–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Papathakis PC, Rollins NC, Chantry CJ, Bennish ML, Brown KH. Micronutrient status during lactation in HIV-infected and HIV-uninfected South African women during the first 6 mo after delivery. Am J Clin Nutr. 2007;85:182–92. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization. Guidelines on HIV and infant feeding: principles and recommendations for infant feeding in the context of HIV and a summary of evidence. Geneva, Switzerland: World Health Organization Press; 2010. [PubMed]
- 15.Dryden-Peterson S, Shapiro RL, Hughes MD, Powis K, Ogwu A, Moffat C, Moyo S, Makhema J, Essex M, Lockman S. Increased risk of severe infant anemia after exposure to maternal HAART, Botswana. J Acquir Immune Defic Syndr. 2011;56:428–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van der Horst C, Chasela C, Ahmed Y, Hoffman I, Hosseinipour M, Knight R, Fiscus S, Hudgens M, Kazembe P, Bentley M, et al. Modifications of a large HIV prevention clinical trial to fit changing realities: a case study of the Breastfeeding, Antiretroviral, and Nutrition (BAN) protocol in Lilongwe, Malawi. Contemp Clin Trials. 2009;30:24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chasela CS, Hudgens MG, Jamieson DJ, Kayira D, Hosseinipour MC, Kourtis AP, Martinson F, Tegha G, Knight RJ, Ahmed YI, et al. Maternal or infant antiretroviral drugs to reduce HIV-1 transmission. N Engl J Med. 2010;362:2271–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kayira D, Bentley ME, Wiener J, Mkhomawanthu C, King CC, Chitsulo P, Chigwenembe M, Ellington S, Hosseinipour MC, Kourtis AP, et al. A lipid-based nutrient supplement mitigates weight loss among HIV-infected women in a factorial randomized trial to prevent mother-to-child transmission during exclusive breastfeeding. Am J Clin Nutr. 2012;95:759–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flax VL, Bentley ME, Chasela CS, Kayira D, Hudgens MG, Knight RJ, Soko A, Jamieson DJ, van der Horst CM, Adair LS. Use of lipid-based nutrient supplements by HIV-infected Malawian women during lactation has no effect on infant growth from 0 to 24 weeks. J Nutr. 2012;142:1350–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jamieson DJ, Chasela CS, Hudgens MG, King CC, Kourtis AP, Kayira D, Hosseinipour MC, Kamwendo DD, Ellington SR, Wiener JB, et al. Maternal and infant antiretroviral regimens to prevent postnatal HIV-1 transmission: 48-week follow-up of the BAN randomised controlled trial. Lancet. 2012;379:2449–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chavula C, Long D, Mzembe E, Kayira D, Chasela C, Hudgens MG, Hosseinipour M, King CC, Ellington S, Chigwenembe M, et al. Stopping the control arm in response to the DSMB: mother's choice of HIV prophylaxis during breastfeeding in the BAN Study. Contemp Clin Trials. 2012;33:55–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization. WHO HIV and infant feeding technical consultation consensus statement. Geneva, Switzerland: World Health Organization; 2006.
- 23.Ferguson YO, Eng E, Bentley M, Sandelowski M, Steckler A, Randall-David E, Piwoz EG, Zulu C, Chasela C, Soko A, et al. Evaluating nurses’ implementation of an infant-feeding counseling protocol for HIV-infected mothers: The Ban Study in Lilongwe, Malawi. AIDS Educ Prev. 2009;21:141–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cogill B. Anthropometric indicators measurement guide. Washington: Food and Nutrition Technical Assistance Project and Academy for Educational Development;2003. [Google Scholar]
- 25.Hernán MA, Hernandez-Diaz S, Werler MM, Mitchell AA. Causal knowledge as a prerequisite for confounding evaluation: an application to birth defects epidemiology. Am J Epidemiol. 2002;155:176–84. [DOI] [PubMed] [Google Scholar]
- 26.Northrop-Clewes CA. Interpreting indicators of iron status during an acute phase response–lessons from malaria and human immunodeficiency virus. Ann Clin Biochem. 2008;45:18–32. [DOI] [PubMed] [Google Scholar]
- 27.Thurnham DI, Mburu AS, Mwaniki DL, Muniu EM, Alumasa F, de Wagt A. Using plasma acute-phase protein concentrations to interpret nutritional biomarkers in apparently healthy HIV-1-seropositive Kenyan adults. Br J Nutr. 2008;100:174–82. [DOI] [PubMed] [Google Scholar]
- 28.Thurnham DI, McCabe LD, Haldar S, Wieringa FT, Northrop-Clewes CA, McCabe GP. Adjusting plasma ferritin concentrations to remove the effects of subclinical inflammation in the assessment of iron deficiency: a meta-analysis. Am J Clin Nutr. 2010;92:546–55. [DOI] [PubMed] [Google Scholar]
- 29.Marshall SW. Power for tests of interaction: effect of raising the Type I error rate. Epidemiol Perspect Innov. 2007;4:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. National Institute of Allergy and Infectious Diseases Division of AIDS. DAIDS/toxicity tables for grading severity of adult and pediatric adverse events, version 1; December, 2004. Available from: http://www.niaid.nih.gov/LabsAndResources/resources/DAIDSClinRsrch/Documents/daidsaegradingtable.pdf.
- 31.De Pee S, Bloem MW, Sari M, Kiess L, Yip R, Kosen S. The high prevalence of low hemoglobin concentration among Indonesian infants aged 3–5 months is related to maternal anemia. J Nutr. 2002;132:2215–21. [DOI] [PubMed] [Google Scholar]
- 32.Kilbride J, Baker TG, Parapia LA, Khoury SA, Shuqaidef SW, Jerwood D. Anaemia during pregnancy as a risk factor for iron-deficiency anaemia in infancy: a case-control study in Jordan. Int J Epidemiol. 1999;28:461–8. [DOI] [PubMed] [Google Scholar]
- 33.Colomer J, Colomer C, Gutierrez D, Jubert A, Nolasco A, Donat J, Fernandez-Delgado R, Donat F, Alvarez-Dardet C. Anaemia during pregnancy as a risk factor for infant iron deficiency: report from the Valencia Infant Anaemia Cohort (VIAC) study. Paediatr Perinat Epidemiol. 1990;4:196–204. [DOI] [PubMed] [Google Scholar]
- 34.Rios E, Lipschitz DA, Cook JD, Smith NJ. Relationship of maternal and infant iron stores as assessed by determination of plasma ferritin. Pediatrics. 1975;55:694–9. [PubMed] [Google Scholar]
- 35.Shao J, Lou J, Rao R, Georgieff MK, Kaciroti N, Felt BT, Zhao ZY, Lozoff B. Maternal serum ferritin concentration is positively associated with newborn iron stores in women with low ferritin status in late pregnancy. J Nutr. 2012;142:2004–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andersson O, Hellstrom-Westas L, Andersson D, Domellof M. Effect of delayed versus early umbilical cord clamping on neonatal outcomes and iron status at 4 months: a randomised controlled trial. BMJ. 2011;343:d7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Golightly E, Jabbour HN, Norman JE. Endocrine immune interactions in human parturition. Mol Cell Endocrinol. 2011;335:52–9. [DOI] [PubMed] [Google Scholar]
- 38.Domellöf M, Lonnerdal B, Dewey KG, Cohen RJ, Hernell O. Iron, zinc, and copper concentrations in breast milk are independent of maternal mineral status. Am J Clin Nutr. 2004;79:111–5. [DOI] [PubMed] [Google Scholar]
- 39.Celada A, Busset R, Gutierrez J, Herreros V. No correlation between iron concentration in breast milk and maternal iron stores. Helv Paediatr Acta. 1982;37:11–6. [PubMed] [Google Scholar]
- 40.Murray MJ, Murray AB, Murray NJ, Murray MB. The effect of iron status of Nigerian mothers on that of their infants at birth and 6 months, and on the concentration of Fe in breast milk. Br J Nutr. 1978;39:627–30. [DOI] [PubMed] [Google Scholar]
- 41.El-Farrash RA, Ismail EA, Nada AS. Cord blood iron profile and breast milk micronutrients in maternal iron deficiency anemia. Pediatr Blood Cancer. 2012;58:233–8. [DOI] [PubMed] [Google Scholar]
- 42.Yalçin SS, Baykan A, Yurdakok K, Yalcin S, Gucus AI. The factors that affect milk-to-serum ratio for iron during early lactation. J Pediatr Hematol Oncol. 2009;31:85–90. [DOI] [PubMed] [Google Scholar]
- 43.Baykan A, Yalcin SS, Yurdakok K. Does maternal iron supplementation during the lactation period affect iron status of exclusively breast-fed infants? Turk J Pediatr. 2006;48:301–7. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.