Abstract
Few data on iodine status in Somalia are available, but it is assumed that deficiency is a public health problem due to the limited access to iodized salt. We aimed to describe the iodine status of the population of Somalia and to investigate possible determinants of iodine status. A national 2-stage, stratified household cluster survey was conducted in 2009 in the Northwest, Northeast, and South Central Zones of Somalia. Urinary iodine concentration (UIC) was determined in samples from women (aged 15–45 y) and children (aged 6–11 y), and examination for visible goiter was performed in the Northwest and South Central strata. A 24-h household food-frequency questionnaire was conducted, and salt samples were tested for iodization. The median UICs for nonpregnant women and children were 329 and 416 μg/L, respectively, indicating excessive iodine intake (>300 μg/L). The prevalence of visible goiter was <4%. The coverage of salt iodization was low, with a national average of 7.7% (95% CI: 3.2%, 17.4%). Spatial analysis revealed localized areas of relatively high and low iodine status. Variations could not be explained by food consumption or salt iodization but were associated with the main source of household drinking water, with consumers of borehole water having a higher UIC (569 vs. 385 μg/L; P < 0.001). Iodine intake in Somalia is among the highest in the world and excessive according to WHO criteria. Further work is required to investigate the geochemistry and safety of groundwater sources in Somalia and the impact on human nutrition and health.
Introduction
The relation between iodine intake and the risk of health problems can be described by a U-shaped curve, with extremes of both high and low intakes having adverse consequences on health (1). There is a narrow optimal range of iodine nutrition. Iodine deficiency is a global problem resulting in goiter, impaired cognitive and physical development, and cretinism, whereas excessive iodine intakes have been associated with goiter, hypothyroidism, and hyperthyroidism (2–4). The most severe consequences of excessive intakes include iodine-induced hyperthyroidism, which may sometimes lead to death from cardiac complications (5).
Somalia lies at the northeast tip of Africa and currently comprises the semiautonomous zones of the Northwest Zone (NWZ)10 (Somaliland), Northeast Zone (NEZ) (Puntland) and the war-ravaged South Central Zone (SCZ). Since the fall of the Siad Barre government in 1991, Somalia has lacked an effective central government. A high and persistent level of internal conflict and incursions by foreign governments has contributed to a series of health and nutrition crises (6, 7).
Nutrition survey data have been collected and analyzed by the Food Security and Nutrition Analysis Unit (FSNAU) of the United Nations (UN) FAO since 2000, and this has allowed a detailed monitoring of the anthropometric status of the child population (8). Information on micronutrient nutrition has been much scarcer due to the difficulties of sample collection and analysis in remote and insecure areas. A Multiple Indicator Cluster Survey was conducted in 2006 but did not collect data on iodine status (9).
To address this paucity of data, a national survey of micronutrient nutritional status was carried out in 2009 by the FSNAU and University College London, in collaboration with UNICEF, the World Food Programme, and WHO. Here, we describe the first national-level data on iodine status in Somalia, map variations in its geographic distribution, and explore factors that may explain the findings.
Participants and Methods
Survey design and population sampling.
Data were collected as part of a national micronutrient and anthropometric nutrition survey in Somalia. The survey field work was conducted between 19 March and 5 August 2009 and was conducted in 4 phases, starting in the northeast, then the northwest, central, and south.
The survey was a stratified, 2-stage, cluster, cross-sectional household survey. Survey strata consisted of the 3 zones of Somalia: the NEZ (Puntland), the NWZ (Somaliland), and the SCZ. At the time of writing, the political status of the zones of Somalia is in dispute and no opinion on their status is intended by the terminology used in this article.
First-stage sampling was performed by using population lists compiled from a combination of data obtained by the UN Development Program, WHO, and the UN High Commissioner for Refugees (UNHCR). Lists were compiled with the use of Excel (Microsoft) spreadsheets, and the most recent estimates were used for each area. Using these combined data sources allowed for detailed listings of settlements and population estimates. Data on internally displaced persons (IDPs) were obtained from a database maintained by UNHCR. To account for the substantial population displacements in the SCZ of Somalia, the consolidated UNHCR district estimate for IDPs from April 2009 was divided by the total number of settlements in each district. This was necessary because no data were available on the distribution between settlements within the districts.
Certain areas were excluded from the survey sampling frame due to prevailing insecurity. These areas were the Banadir and Hiran regions, Kismayo District, and Jowhar Town. Probability proportional to size was then used to allocate clusters to geographic enumeration areas. The survey design required the surveying of at least 30 clusters in each stratum. Additional clusters were planned, because due to the high levels of insecurity it was considered likely that UN security clearance for access to some of the planned sites could be withdrawn during the course of the survey. A total of 32 of 35, 31 of 35, and 33 of 40 clusters were therefore surveyed from the NWZ, NEZ, and SCZ, respectively.
Second-stage sampling involved the selection of 30 households in each cluster. This was done by using a combination of segmentation, Expanded Program on Immunization, and grid-based sampling using maps provided by the FAO Somalia Water and Land Information Management. Once the first house in each cluster was selected, subsequent households were selected by proximity sampling. A household was defined as persons who slept under the same roof during the previous night. Subsamples were selected by using quota sampling so that sampling would continue until the required sample size for each subsample in that cluster had been obtained. Within each selected household, all eligible individuals were included.
Sample size calculation.
For the measurement of iodine status, a subsample of school-aged children [SAC (6–11 y)] and women of reproductive age (15–49 y) was included. No data had been previously collected on iodine status in Somalia, so a formal sample size calculation was not possible. On the basis of previous survey experience, a target sample size of 300 participants was selected for the measurement of urinary iodine concentration (UIC) in each population group in each of the 3 strata, for a total of 1800. The sample size required per cluster was then determined by dividing the strata sample into the number of clusters (35 clusters). No sample size calculation was performed for the assessment of goiter, and all women between 15 and 49 y of age and children aged 6–11 y in all surveyed households were included. This procedure was omitted in the NEZ due to initial concerns about acceptability. Salt samples were tested by using a rapid test kit in all surveyed households that made a salt sample available on request.
Survey team training and supervision.
Data collection was performed by 6 teams in the Northwest and Northeast, by 3 teams in the Central region, and by 8 teams in South Central Somalia. The number of teams deployed was dependent on the proximity of the clusters, security, and the need to balance the clan affiliation of team members, especially in the South Central areas. Each team was composed of 8 people: 2 enumerators, 2 biologic sample collectors, 2 measurers, and 1 team leader, and an FSNAU Nutrition Field Analyst was allocated to each team to supervise the field work. Most of the survey teams were experienced in conducting surveys in Somalia. The biologic sample collectors were mainly laboratory staff who were taken from hospitals within the respective zones. In some situations in which laboratory staff were unavailable, experienced nurses were selected instead for this task.
A 7-d intensive training period was initially planned in all of the 3 zones; however, the number of days was increased to 9 and 8 in the NEZ and NWZ, respectively. This was mainly due to challenges experienced during the piloting, which necessitated an extra 1 to 2 d. Due to security concerns, it was only possible to provide minimal field-level supervision in the SCZ. Here, great attention was given to both training and pilot testing, and the latter was repeated in case of a team’s poor performance until satisfactory quality was achieved.
Pilot testing was performed in an area that had not been selected for inclusion in the survey. Approximately 3–4 households were sampled by each team, and the quality of the sample collection, including anthropometric measurements and questionnaire completion, were examined. Any teams that made major errors such as incorrect labeling, poorly filled questionnaires, or missing records were asked to repeat the pilot test after a review of the areas of concern.
During survey implementation, teams in the NEZ and NWZ were able to work in close proximity to each other and move systematically within each zone. Each team was allocated a preselected list of clusters, which eased both logistics and team supervision. All biologic samples and questionnaires were compiled by the team supervisors upon finishing a cluster and reviewed by the laboratory coordinators and the study coordinator, respectively. Continuous monitoring of errors and discussion of concerns helped to improve subsequent data collection in each cluster for the teams. However, this type of practice was not possible in the SCZ. Here, teams were allocated to areas on the basis of clan settlement patterns, and each team was sent to clusters around their home area. Due to security restrictions, team supervision was not possible in all of the clusters, and where possible, teams were supervised at the start of their first cluster. In the SCZ it proved to be impossible to provide any field-level senior supervision of the team surveying the 3 clusters in Middle Juba.
Household and individual questionnaires.
Because most of the survey staff were comfortable reading the questionnaires in English, translation of the questionnaires was not necessary. However, for some interviewers, it was necessary to clarify the specific meaning of certain questions during the training and, when necessary, interviewers carried copies of the questionnaires with their own handwritten annotations reminding them of the exact meaning of key terms. Questionnaires were divided into 6 modules. The 3 that were relevant to the data reported here were as follows: module 1, household-level questions (contained geographic, demographic, water source, and FFQ questions); module 4, questions on children aged 6–11 y (demographic); and module 5, questions on women aged 15–49 y (demographic and health questions).
Household FFQ.
The household FFQ with 16 food groups was designed on the basis of FAO Guidelines for Measuring Household and Individual Dietary Diversity (10). It was administered to all households included in the survey sample using a 24-h recall period, which was defined as the day before the survey team administered the questionnaire. Results were expressed as the percentage of households consuming each food group.
Goiter examination.
In all women and SAC surveyed in the NWZ and SCZ, assessment for the presence or absence of visible goiter was performed. Palpation of goiter was not performed due to the challenge of ensuring the correct identification of an enlarged thyroid and concerns about social acceptability.
To observe visible goiter, the participants were asked to raise their head to the normal position and the presence or absence of an enlarged thyroid (grade 2 goiter) was observed by a team member (11). Women were asked to remove the veil around the neck, and in some cases they were examined by fellow female staff from the team in private. In most cases, team supervisors cross-checked goiter cases and took a photograph of the case for cross-checking by the survey coordinator. Goiter assessment was not performed in the NEZ due to initial concerns about cultural acceptability.
Urine sample collection.
A 10-mL urine sample was collected from women (15–49 y old) and SAC (6–11 y old) for urinary iodine measurements. The participants were provided with a prelabeled 30-mL urine collection tube and asked to provide a urine sample. Upon receiving the urine sample, the tube was placed into a cool box and transported back to the base at the end of the day. Sheets of preprinted identification number labels were used for labeling of all biologic samples to enable robust linking of the laboratory results with questionnaire data.
Urinary iodine analysis.
The analysis of urinary iodine was performed in South Africa at the Nutritional Intervention Research Unit laboratory of the Medical Research Council. This laboratory is part of the International Resource Laboratory for Iodine network that participates in the CDC’s Ensuring the Quality of Iodine Procedures (EQUIP) program, which evaluates the accuracy of results.
The urine samples were analyzed by using a slightly modified quantitative method based on the Sandell-Kolthoff reaction. Approximately 0.25 mL of the urine sample was digested in 1.0 mL of ammonium persulfate at 91–95°C for 60 min to remove interfering substances. An aliquot of 0.05 mL of the digested urine sample was then pipetted into the wells of a 96-well microplate, followed by 0.1 mL of arsenic acid and 0.05 mL of ceric ammonium sulfate solution. The reaction was then determined spectrophometrically with a microplate reader at 405 nm after 25 min incubation at 30°C. Each of the plates had a unique standard curve (ranging from 0 to 320 μg/L) and internal controls (ranging from 80 to 600 μg/L). Urine samples with values above the standard curve were first diluted at 1 in 5, followed by dilutions of 1 in 10 and 1 in 20, if needed. As a quality-control procedure, triplicate samples from 2 volunteers were labeled identically to the survey samples and included in the samples shipped to the laboratory. The CVs for these triplicates were 1.23% and 0.71%. Population median UICs were used to categorize population iodine intakes as insufficient (<100 μg/L), adequate (100–199 μg/L), above requirements (200–299 μg/L), or excessive (≥300 μg/L) (11).
Salt sample collection and testing.
Salt samples were tested for iodine by using an improved field test kit (MBIK001) from MBI Kits International, India. The principle of the method is that salt samples are oxidized with an acidic solution to liberate free iodine, which then turns starch blue. The intensity of the color is proportional to the amount of iodine in the salt sample. The test provides semiquantitative results with categories given as 0, <15, and >15 mg/kg. A small amount of salt was put into the kit’s cup, 2 drops of the test solution were added, and the results were observed after 1 min. If no color change occurred, a separate sample of salt was taken and 5 drops of the recheck solution were added plus 2 drops of the test solution, and a change in color was observed for 1 min. The salt was only regarded as not containing iodine when both tests were negative. This reagent, however, does not detect the presence of iodine in salt samples fortified with potassium iodide.
Data management.
Data entry was performed with EpiData 3.0 (12). Three d of data cleaning were performed after the end of each data collection phase during which the data entry clerk, together with the study coordinator, reverted to the hard copies of the data collection forms to resolve any anomalies that were discovered.
Statistical analysis.
Analysis was conducted with SPSS 16.0 for Windows (IBM SPSS Statistics). Descriptive statistics for means, medians, and proportions were generated by using the Complex Samples module to allow for the design effect of the survey design, and national estimates were weighted to allow for unequal selection probability within the 3 strata. Differences in UIC associated with different potential predictors were assessed by using nonparametric tests (Kruskal-Wallis and Mann-Whitney), because UIC data are positively skewed. Significance was attributed at P < 0.05.
Mapping and spatial analysis.
The locations of the survey clusters were mapped by using ArcGIS 10 (ESRI). Because the use of global positioning system (GPS) handsets was considered inappropriate during the survey field work due to security risks, it was not possible to record precise coordinates for the locations. Instead, place names from shape files downloaded from the Somalia Water and Land Information Management GeoNetwork were used to manually geo-locate the cluster start points. Shape files containing the median UICs for women and SAC and the respective sample sizes were constructed, and the categories of UIC visualized by using graduated color symbols. All map layers used the World Geodetic System 1984 geographic coordinate system and were projected by using the Universal Transverse Mercator zone 38N before spatial analysis.
To investigate the possibility of spatial clustering, the ArcGIS Hot Spot tool (ArcGIS 10; ESRI) was used to identify areas with high or low UICs. This approach is appropriate for the identification of hot spots with skewed data (13). A range of distance bands was manually trialed, and 150 km was selected for the analysis because it provided the most discriminatory analysis suited to the distribution of the sampling points.
Ethics.
Ethical approval was provided by the Ministries of Health for the 3 zones of Somalia and the Research Ethics Committee of University College London. Access to survey sites was agreed upon with local authorities and community leaders in the districts where the clusters were sampled. It was made clear to survey participants that participation was entirely voluntary and refusal would not in any way affect entitlements to humanitarian assistance or other forms of support. The risks to members of the survey teams were minimized by adherence to the UN safety and security procedures that were in place at that time.
Results
Sampling.
A total of 1860 women were sampled from households in the NWZ and SCZ for visible goiter examination, and valid measures were obtained for 1838 (98.8% of total sample) of the women. A total of 897 women from all 3 zones were subsampled for urine iodine analysis, and 767 urine samples were obtained. Of these, 759 samples yielded valid results (84.2% of the subsample), 7 samples contained too little urine for analysis, and 1 sample was mislabeled and the analysis result could not be used. Within the subsample, 159 women reported being pregnant (17.7%) and status was unknown in a further 14 women (1.6%). After excluding the women known to be pregnant, the main analysis of UIC was conducted in the remaining 617 participants with valid measurements. These samples came from 536 households in 90 clusters.
A total of 868 SAC from all 3 zones were sampled for urine iodine analysis, and 788 urine samples were obtained (90.8% of total sample). Of these, 5 samples contained too little urine for analysis and 27 samples were mislabeled and the results could not be used. The analysis of UIC was conducted in the remaining 756 participants (87.1% of total sample) with valid measurements. These samples came from 507 households in 92 clusters.
Descriptive analysis.
The characteristics of the samples analyzed for UIC are shown in Tables 1 and 2. The average age of the sampled women was 30 y, with no differences between strata. Recalled school attendance among women was low in all zones of Somalia, with only 25.2% reporting having been exposed to any form of formal education. A history of formal education was highest in the NEZ and lowest in the NWZ. A breakdown of the levels of education exposure is given in Table 1. The average age of the sample of SAC was 7.9 y, with little variation by stratum. There was also no significant difference in the proportion of females by stratum.
TABLE 1.
Characteristics of nonpregnant women of reproductive age (15–49 y) sampled for urinary iodine concentration within the 3 zones of Somalia1
| Combined strata (n = 617) | NWZ (n = 196) | NEZ (n = 202) | SCZ (n = 219) | |
| Age, y | 29.8 (29.0, 30.7) | 30.6 (29.2, 32.0) | 29.5 (27.9, 31.2) | 29.6 (28.3, 30.9) |
| BMI, kg/m2 | 21.8 (21.2, 22.3) | 22.5 (21.8, 23.2) | 23.5 (22.5, 24.5) | 21.0 (20.2, 21.8) |
| No formal education, % | 74.8 (69.4, 79.5) | 81.6 (74.1, 87.3) | 60.4 (50.2, 69.7) | 75.8 (67.5, 82.5) |
| Internally displaced <2 y, % | 5.8 (3.1, 10.7) | 5.8 (2.3, 13.6) | 1.7 (1.0, 8.9) | 6.6 (2.8, 15.0) |
| Household size, n | 6.2 (5.9, 6.4) | 6.3 (5.7, 6.8) | 6.4 (5.7, 6.5) | 6.1 (5.7, 6.5) |
| Rural location, % | 75.1 (64.7, 83.2) | 62.8 (43.0, 79.0) | 55.0 (36.7, 71.9) | 85.4 (68.2, 94.1) |
| Type of dwelling,2 % | ||||
| Makeshift | 35.7 (27.1, 45.3) | 37.4 (24.3, 52.6) | 8.5 (3.3, 20.3) | 42.1 (29.0, 56.4) |
| Temporary (mud and/or sticks) | 25.0 (18.1, 33.4) | 18.9 (12.0, 28.7) | 7.5 (4.0, 13.7) | 31.9 (21.1, 45.2) |
| Semipermanent (mud/stone with metal sheet roofing) | 24.5 (17.3, 33.4) | 11.1 (4.4, 25.3) | 46.2 (33.9, 59.1) | 24.1 (13.9, 38.5) |
| Permanent (block houses with metal sheet roofing) | 14.5 (10.7, 19.2) | 32.6 (20.5, 47.7) | 36.7 (24.6, 50.8) | 1.4 (0.2, 9.4) |
| Other | 0.4 (0.1, 1.7) | 0.0 (-) | 0.5 (0.3, 3.9) | 0.5 (0.1, 3.3) |
Values are means or percentages (95% CIs). NEZ, Northeast Zone; NWZ, Northwest Zone; SCZ, South Central Zone.
For combined strata n = 605.
TABLE 2.
Characteristics of school-aged children (6–11 y) sampled for urinary iodine concentration within the 3 zones of Somalia1
| Combined strata (n = 756) | NWZ (n = 239) | NEZ2 (n = 249) | SCZ (n = 268) | |
| Age, y | 7.9 (7.8, 8.0) | 7.9 (7.7, 8.1) | 8.1 (7.9, 8.4) | 7.8 (7.7, 8.0) |
| Female, % | 50.1 (45.6, 54.5) | 50.2 (42.6, 57.4) | 45.8 (38.9, 52.8) | 51.1 (44.7, 57.5) |
Values are means or percentages (95% CIs). NEZ, Northeast Zone; NWZ, Northwest Zone; SCZ, South Central Zone.
One participant from the NEZ had age recorded as 12 y.
Table 3 shows the median UIC by population group and survey stratum. The national median UIC is indicative of excessive intake at the population level, exceeding the threshold concentration of 300μg/L in both population groups. Comparing results by zone showed that the NWZ had more than adequate intake, whereas the NEZ and SCZ had UICs indicative of excessive iodine intakes. In women of reproductive age, the UIC in the NEZ was significantly higher than in the NWZ or SCZ (Mann-Whitney; P < 0.001 and P = 0.045, respectively).
TABLE 3.
Urinary iodine concentration and prevalence of visible goiter in women (15–49 y) and school-aged children (6–11 y)
| Urinary iodine concentration |
Visible goiter |
||||
| Population group | Sample size | Median (IQR) | Range | Sample size | Prevalence1 |
| n | μg/L | μg/L | n | % | |
| Women | |||||
| National | 617 | 329 (152–721) | 24–9060 | —2 | — |
| Northwest Zone | 196 | 228 (115–701) | 24–7710 | 852 | 3.3 (2.2, 4.9) |
| Northeast Zone | 202 | 405 (202–766) | 49–4600 | — | — |
| South Central Zone | 219 | 316 (168–646) | 39–9060 | 986 | 1.4 (0.8, 2.6) |
| School-aged children | |||||
| National | 756 | 416 (200–1110) | 5–9920 | — | — |
| Northwest Zone | 239 | 288 (126–892) | 11–9920 | 300 | 1.3 (0.4, 4.3) |
| Northeast Zone | 249 | 619 (315–1420) | 8–6720 | — | — |
| South Central Zone | 268 | 398 (211–1070) | 5–5600 | 301 | 0.3 (0.0, 2.4) |
Values are percentages (95% CIs).
Dashes indicate that a measurement was not taken.
In SAC, the median UIC was also significantly higher in the NEZ compared with that in the other 2 zones (Mann-Whitney; P < 0.001 and P = 0.001, respectively). Not shown in Table 3 are the results for the 142 women who were pregnant at the time of sample collection. The median UIC in these women was 372 μg/L (IQR: 196–784) for the combined strata, which indicates an iodine intake above the requirement (11). Caution is needed in interpreting these data due to the small sample size.
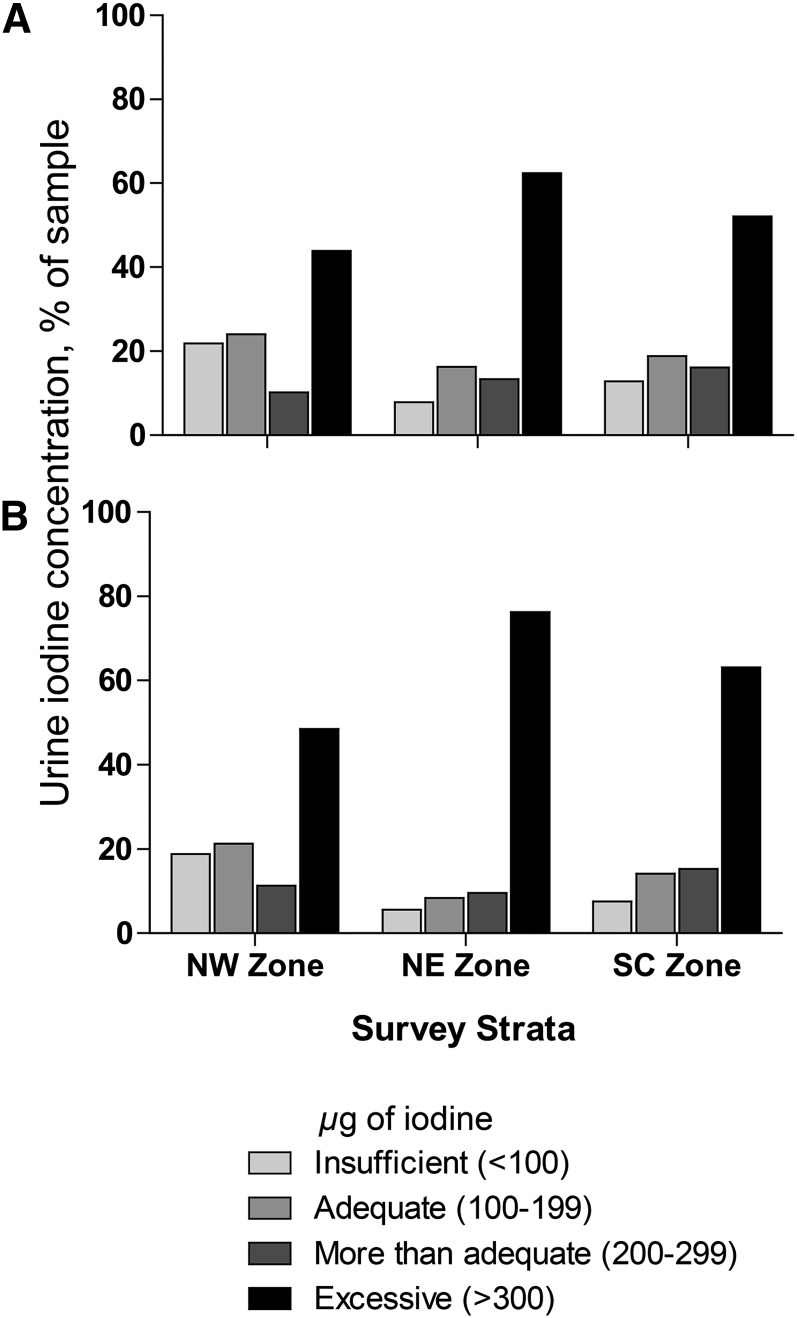
The close correlation between the categories of UIC in women and SAC is shown in Fig. 1, with the proportion showing a UIC indicative of excessive iodine intake being higher in SAC than in women in all 3 zones. Overall, a high proportion of participants showed a UIC indicative of excessive intake. This was seen in all 3 zones where 43.9%, 62.4%, and 52.1% of samples from women and 48.5%, 76.3%, and 63.1% of samples from SAC in the NWZ, NEZ, and SCZ, respectively, were categorized as having excessive intakes. In both population groups, the highest proportion of participants with a UIC >300 μg/L was found in the NEZ. In the weighted national estimate, 51.7% of women and 61.6% of SAC had a UIC indicating excessive intake.
FIGURE 1.
Distribution of urinary iodine concentrations for women (A; n = 617) and school-aged children (B; n = 756) in Somalia by geographic zone. NE, Northeast; NW, Northwest; SC, South Central.
Table 3 also shows the prevalence of visible (grade 2) goiter in the 2 zones in which these data were collected. In both zones where this was measured the prevalence was <5%.
To visualize the spatial distribution of UICs, the values for each cluster are shown in Supplemental Fig. 1. For both population samples, areas of relatively high concentration were observed. Statistical analysis of spatial hot spots (Supplemental Fig. 2) confirmed areas of particularly high iodine intakes in the central part of the NWZ and the southern part of the NEZ, whereas an area of relatively low intake was identified in the far west of the NWZ and in the Lower Shabelle region.
Investigation of iodine sources.
To investigate reasons for the high iodine intakes and their spatial distribution, we initially considered rural/urban location, displaced or resident status, household food consumption, and exposure to iodized salt. In women, an urban or rural location was not associated with a difference in median UIC at the national level (Mann-Whitney, P = 0.92). Likewise, having resident or internal displaced status did not affect UIC. In SAC, a rural location or IDP status was also not associated with a difference in median UIC at the national level (Mann-Whitney, P = 0.09; Kruskal-Wallis, P = 0.07).
Household consumption of individual food items is shown in Table 4. No association between UIC and the consumption of any food group, including fish, was found for either women or SAC at the national level (P > 0.05).
TABLE 4.
Frequency of food-group consumption by households of participants surveyed for urinary iodine concentration1
| Food group | Women (15–49 y)2 | Children (6–11 y)3 |
| % | % | |
| Cereals | 96.9 | 95.9 |
| Dark-green leafy vegetables | 16.1 | 15.7 |
| Eggs | 3.2 | 4.7 |
| Fish | 9.2 | 9.5 |
| Flesh meat4 | 44.5 | 48.7 |
| Legumes, nuts, and seeds | 26.6 | 25.8 |
| Milk and milk products | 64.9 | 64.4 |
| Oils or fats | 88.9 | 89.7 |
| Organ meat | 6.6 | 6.5 |
| Other fruits | 20.7 | 21.0 |
| Other vegetables | 36.5 | 33.0 |
| Spices or caffeine | 39.9 | 39.1 |
| Sweets | 82.9 | 84.5 |
| Vitamin A–rich fruits | 8.9 | 10.0 |
| Vitamin A–rich vegetables | 11.1 | 12.3 |
| White tubers or roots | 28.4 | 29.8 |
Values shown represent frequency of household food-group consumption in the day before the survey.
Sample size varied between n = 587 and n = 604 for each food group.
Sample size varied between n = 717 and n = 734 for each food group.
Flesh meat is any meat that does not include organs.
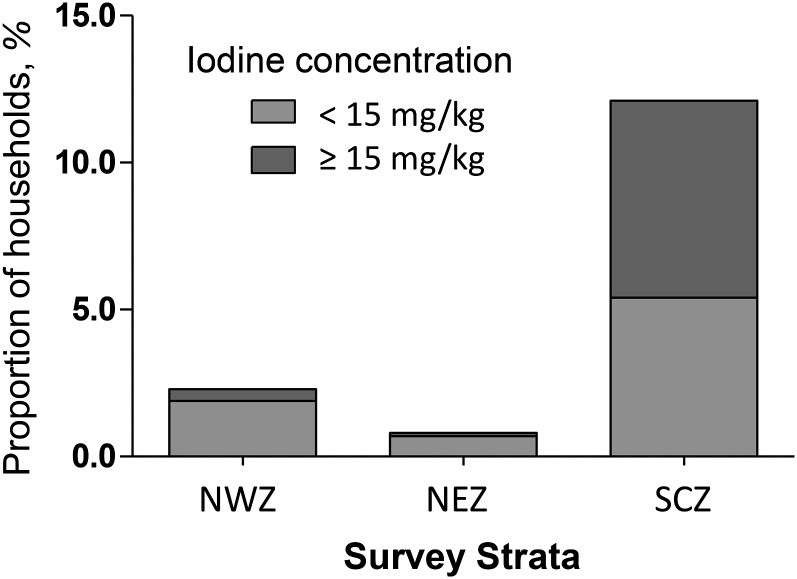
To assess exposure to iodized salt, samples from 2345 households were tested by using a rapid test kit. Fig. 2 shows the percentage of households in which the tested salt sampled contained iodine. It can be seen that the overall coverage of salt iodization was low at 7.7% (95% CI: 3.2%, 17.4%), and where it does occur a large proportion was inadequately iodized, with concentrations <15 mg/kg. Household salt iodization was only recorded with any frequency in the SCZ, where 6.7% of samples were fortified at concentrations ≥15 mg/kg and 5.4% of samples were fortified at concentrations <15 mg/kg. In this zone, exposure to iodized salt was associated with a significantly increased median UIC in women (770 vs. 281 μg/L; Mann-Whitney, P = 0.001) and in SAC (1260 vs. 366 μg/L; Mann-Whitney, P < 0.001). The 4 clusters where salt iodization exceeded 20% coverage were all located adjacent to the Kenyan border in the area of El Wak.
FIGURE 2.
Proportion of households in Somalia possessing iodized salt (n = 2345). NEZ, Northeast Zone; NWZ, Northwest Zone; SCZ, South Central Zone.
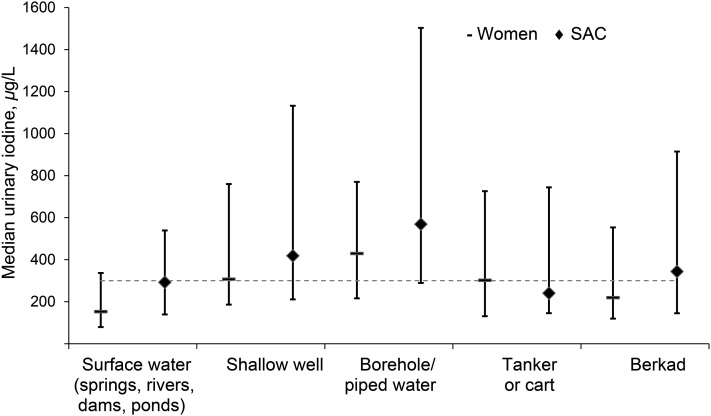
Because exposure to iodized salt was only localized to small areas in the SCZ and could not explain the occurrence of the spatial hot spots that were observed, we next analyzed the association between iodine intake and the main source of household drinking water for sources utilized by at least 50 households. Fig. 3 shows the main household water source ordered by water source, with surface water sources followed by shallow dug wells, deeper drilled boreholes, tanker/cart, and berkad (a water storage construction widely used in Somalia). The data show the relation between the main source of household water and the UIC of household members. Consumption of water sources of unknown origin, that is, water stored in berkads or from donkey carts or tankers, was associated with an intermediate UIC. A comparison of UICs of individuals from households that use water from boreholes against those that use any other main water source revealed a higher iodine intake in SAC (569 vs. 385 μg/L; Mann-Whitney, P = 0.001) and in women (430 vs. 282 μg/L; Mann-Whitney, P < 0.001).
FIGURE 3.
Median (IQR) urinary iodine concentrations in women (n = 617) and SAC (n = 756) by main household water source. The dashed reference line indicates the WHO cutoff above which median urinary iodine is considered indicative of population iodine excess (11). SAC, school-aged children.
Discussion
Somalia remains a country afflicted by instability, conflict, and humanitarian crisis, and this article is the first, to our knowledge, to describe national-level data on iodine status. The overall amount of iodine intake was highest in the NEZ, but it exceeded WHO criteria for adequate intakes for both women and SAC in all 3 zones of Somalia (11). The UIC for pregnant women was also above requirements. The finding of high iodine intake was interesting and unexpected, especially given the low exposure to fish, seafood, iodized salt, or other fortified foods. Our data suggest that the iodine content of surface and groundwater sources is likely to be a major determinant of dietary iodine intake at the national level, and spatial analysis allowed for the identification of geographic areas with particularly high intakes.
Our findings were strengthened by the use of household, rather than school-based, sampling for investigating the determinants of iodine status in children, because water sources may be specific to households rather than a school catchment area. Even so, some caution is needed in associating household water source with iodine status, because water may be consumed by household members from a variety of sources outside of the household and households may have multiple sources as well as the “main” source that was captured in the questionnaire.
The survey design was based on standard multistage cluster sampling methods, and field work procedures were made as robust as possible. Assessment of iodine status was based on the use of UIC, which is a well-established tool for monitoring iodine status in national and regional surveys (14). Although we found no evidence of widespread goiter in the 2 zones in which we carried out the examinations, caution is needed in the comparison of visible goiter with other prevalence data, which is usually based on total, rather than grade 2, goiter (11).
Given the situation that existed in Somalia in 2009, a number of limitations were encountered in survey implementation. Accurate population lists were absent and the estimates were compiled by using the best available data from a variety of UN and other sources. Uncertainty around the relative sizes of zonal, regional, and district populations may have affected the allocation of survey clusters and population weighting, but these effects are likely to have been small. Certain geographic areas had to be excluded due to security concerns, as indicated in the map figures, so the survey results need to be interpreted with these exclusions in mind. Although the achieved sample size was slightly smaller than the target value for both women and SAC, it exceeded 80% in both cases.
A small number of visible goiter cases were found, and the low prevalence prevented the measurement of this variable with a high level of precision. Because iodine intake was found to be highest in the NEZ, the lack of data on goiter from this area leaves open the question of whether iodine-induced visible goiter may exist at significant levels. A future assessment of total goiter, preferably using ultrasound, should be performed to establish whether any changes in thyroid morphology are associated with the high iodine intakes that were measured.
The cluster survey design used probability proportional to size to select enumeration areas for inclusion in the survey and is a nonspatial sampling method. Nonetheless, we used spatial analysis to explore the distribution of iodine intake and its potential determinants. If this work were to be repeated or if extended alternative sampling designs were considered, that would allow more fine-grained spatial analysis and mapping with higher resolution.
Comparing our results from Somalia with other national estimates compiled by WHO reveals that the UIC for SAC (416 μg/L) is the fifth highest globally, below that of Chile (984 μg/L), the Democratic Republic of Congo (495 μg/L), Uganda (464 μg/L), and Ecuador (420 μg/L) (15). A more recent analysis of global UIC data for 2011 revealed that between 2003 and 2011 there was a decrease in the number of countries with insufficient intakes and an increase in countries with adequate or more than adequate intakes.
By 2011, there were 11 countries in which the national median UIC was >300 μg/L, compared with only 5 in 2003 and 7 in 2007 (16). The excessive intakes described in Somalia in 2009 are therefore becoming more common at a global level. With regard to our regional estimates for Somalia, the highest UIC (619 μg/L) was found in SAC in the NEZ. This concentration is similar to the UIC reported for a high-iodine-intake region in China (651 μg/L), which was associated with an elevated incidence of thyroid disease (2).
Globally, the iodine concentration in drinking water has been shown to vary greatly, both between and within countries. For example, in Denmark, a study of water from 55 locations showed iodine concentrations between <1.0 and 139 μg/L (17). In borehole water in southwestern Algeria, concentrations varied between 55 and 545 μg/L, whereas in China, average iodine concentrations varied between iodine-deficient areas and areas with excessive intakes where the median concentration in water was >300 μg/L (18). In contrast, in the United States, the mean concentration has been reported as 4 μg/L, with a maximum of only 18 μg/L (19). Within groundwater, iodine may exist as iodine or iodide and may be integrated within humic substances, most of which remain biologically available (20).
Evidence that water can be an important contributor to total iodine intake comes from studies in Denmark, South Africa, China, and refugee populations in Algeria (20–24). Although iodine intake from water sources may be important in preventing deficiency, it may also contribute to excessive intakes and adverse effects (1, 2). An association has also been found between high iodine concentrations in drinking water and reduced intelligence quotient in China (25). Despite these data, WHO has not yet defined a guideline value for iodine in drinking water (19).
There is an urgent need to conduct further studies if and when security conditions within Somalia permit. The measurement of iodine concentrations in household water sources is required to confirm our observation that household water sources are the major contributor to excessive iodine intake. Correlation studies are required to establish the relation among the mineral content of water sources, the depth of extraction of groundwater, and the underlying lithology. These studies should be extended to other countries in the region, such as Kenya, where evidence suggests that some areas may be similarly affected (26). Detailed studies on thyroid physiology and morphology, using ultrasound, are also needed to investigate the physiologic effects of the very high intakes observed in this population. Iodine excess is known to be associated with thyroid pathology, and a full assessment of the possible health impact is needed (2). In addition, the concentration of other minerals and trace elements in the water sources should be investigated.
With an increasing global population, climate change, and persistent conflict and population migration, there may be increasing pressure on water resources and an increasing reliance on deep-level groundwater. Aquifer depletion, associated with increasing extraction rates, has already been seen in arid areas bordering Somalia that host refugee populations (27). This may become more common and, as water tables fall, populations may be forced to consume more highly mineralized water. The effects of this, both beneficial and adverse, are currently poorly understood and require urgent investigation due to their profound public health and environmental importance.
Supplementary Material
Acknowledgments
I.A.R.K. organized and conducted the field work and data collection; A.J.S. designed the survey, conducted the main analysis, and drafted the manuscript; P.P. supported the field work and spatial analysis; G.M. initiated and oversaw the project implementation; A.B. and H.G. supported project implementation; A.Y.N. supported the field work and data collection; and P.J. conducted the analysis of the urinary iodine samples. All authors contributed to, read, and approved the final version of the manuscript.
Footnotes
Abbreviations used: FSNAU, Food Security and Nutrition Analysis Unit; IDP, internally displaced person; NEZ, Northeast Zone; NWZ, Northwest Zone; SAC, school-aged children; SCZ, South Central Zone; UIC, urinary iodine concentration; UN, United Nations; UNHCR, United Nations High Commissioner for Refugees.
Literature Cited
- 1.Laurberg P, Bulow Pedersen I, Knudsen N, Ovesen L, Andersen S. Environmental iodine intake affects the type of nonmalignant thyroid disease. Thyroid. 2001;11:457–69. [DOI] [PubMed] [Google Scholar]
- 2.Teng W, Shan Z, Teng X, Guan H, Li Y, Teng D, Jin Y, Yu X, Fan C, Chong W, et al. Effect of iodine intake on thyroid diseases in China. N Engl J Med. 2006;354:2783–93. [DOI] [PubMed] [Google Scholar]
- 3.Zhao J, Wang P, Shang L, Sullivan KM, Haar Fvd, Maberly G. Endemic goiter associated with high iodine intake. Am J Public Health. 2000;90:1633–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zimmermann MB, Jooste PL, Pandav CS. Iodine-deficiency disorders. Lancet. 2008;372:1251–62. [DOI] [PubMed] [Google Scholar]
- 5.Dunn JT, Semigran MJ, Delange F. The prevention and management of iodine-induced hyperthyroidism and its cardiac features. Thyroid. 1998;8:101–6. [DOI] [PubMed] [Google Scholar]
- 6.Menkhaus K. Stabilisation and humanitarian access in a collapsed state: the Somali case. Disasters. 2010;34:S320–41. [DOI] [PubMed] [Google Scholar]
- 7.ReliefWeb. Somalia: humanitarian profile#x2014June 2008. Nairobi (Kenya): United Nations Office for the Coordination of Humanitarian Affairs; 2008.
- 8.Food Security and Nutrition Analysis Unit. Nairobi (Kenya): UN FAO; 2012 [cited 2011 Mar 1]. Available from: http://www.fsnau.org/.
- 9.Somali 2005 Multiple Indicator Cluster Survey. Unicef; 2006 [cited 2011 Mar 1]. Available from: http://www.childinfo.org/index.html.
- 10.Kennedy G, Ballard T, Dop M. Guidelines for measuring household and individual dietary diversity. Rome: FAO; 2011. [Google Scholar]
- 11.WHO; UNICEF; International Council for the Control of Iodine Deficiency Disorders. Assessment of iodine deficiency disorders and monitoring their elimination: a guide for programme managers. 3rd ed. Geneva: WHO; 2007.
- 12.EpiData. Version 3. A comprehensive tool for validated entry and documentation of data. [software]. Odense (Denmark): The EpiData Association; 2003.
- 13.Mitchell A. The ESRI guide to GIS analysis. Redlands (CA): ESRI Press; 2005. [Google Scholar]
- 14.Zimmermann MB. Methods to assess iron and iodine status. Br J Nutr. 2008;99:S2–9. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization. Vitamin and Mineral Nutrition Information System (VMNIS) database on iodine deficiency. Geneva: WHO; 2013. [Google Scholar]
- 16.Andersson M, Karumbunathan V, Zimmermann MB. Global iodine status in 2011 and trends over the past decade. J Nutr. 2012;142:744–50. [DOI] [PubMed] [Google Scholar]
- 17.Pedersen KM, Laurberg P, Nohr S, Jorgensen A, Andersen S. Iodine in drinking water varies by more than 100-fold in Denmark: importance for iodine content of infant formulas. Eur J Endocrinol. 1999;140:400–3. [DOI] [PubMed] [Google Scholar]
- 18.Zhao J, Chen ZP, Maberly G. Iodine-rich drinking water of natural origin in China. Lancet. 1998;352:2024. [DOI] [PubMed] [Google Scholar]
- 19.WHO. Iodine in drinking water. Geneva: WHO; 2003. Report No.: WHO/SDE/WSH/03.04/46. [Google Scholar]
- 20.Andersen S, Pedersen KM, Iversen F, Terpling S, Gustenhoff P, Petersen SB, Laurberg P. Naturally occurring iodine in humic substances in drinking water in Denmark is bioavailable and determines population iodine intake. Br J Nutr. 2008;99:319–25. [DOI] [PubMed] [Google Scholar]
- 21.Andersen S, Guan HX, Teng WP, Laurberg P. Speciation of iodine in high iodine groundwater in China associated with goitre and hypothyroidism. Biol Trace Elem Res. 2009;128:95–103. [DOI] [PubMed] [Google Scholar]
- 22.Barikmo I, Henjum S, Dahl L, Oshaug A, Torheim LE. Environmental implication of iodine in water, milk and other foods used in Saharawi refugees camps in Tindouf, Algeria. J Food Compost Anal. 2011;24:637–41. [Google Scholar]
- 23.Jooste PL, Weight MJ, Kriek JA, Louw AJ. Endemic goitre in the absence of iodine deficiency in schoolchildren of the Northern Cape Province of South Africa. Eur J Clin Nutr. 1999;53:8–12. [DOI] [PubMed] [Google Scholar]
- 24.Shen H, Liu SJ, Sun DJ, Zhang SB, Su XH, Shen YF, Han HP. Geographical distribution of drinking-water with high iodine level and association between high iodine level in drinking-water and goitre: a Chinese national investigation. Br J Nutr. 2011;106:243–7. [DOI] [PubMed] [Google Scholar]
- 25.Liu HL, Lam LT, Zeng Q, Han SQ, Fu G, Hou CC. Effects of drinking water with high iodine concentration on the intelligence of children in Tianjin, China. J Public Health (Oxf). 2009;31:32–8. [DOI] [PubMed] [Google Scholar]
- 26.Kassim IA, Ruth LJ, Creeke PI, Gnat D, Abdalla F, Seal AJ. Excessive iodine intake during pregnancy in Somali refugees. Matern Child Nutr. 2012;1:49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Enghoff M, Hansen B, Umar A, Gildestad B, Owen M, Obara A. In search of protection and livelihoods: socio-economic and environmental impacts of Dadaab refugee camps on host communities. Nairobi: Royal Danish Embassy; 2010. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.